Full Length Research Paper
ABSTRACT
Identification of resistant germplasms is crucial for crop breeding. The aim of this study was to determine the reaction of accessions of watermelon plants to Meloidogine enterolobii using two experiments. In the first experiment, 20 accessions were evaluated, and in the second, the four most promising accessions were selected in the first experiment and two controls. Both experiments were implemented under a completely randomized design with ten replications. Each parcel consisted of a plastic pot containing autoclaved soil inoculated with 2,200 eggs of M. enterolobii. Among the variables studied, the egg number and reproduction factor showed a high positive correlation. The subsamples indicated a wide variation among and within each accession (p < 0.01). At the end, variation in the response among and within the accessions was observed regarding the reproduction factor. Of the accessions evaluated, accessions 9 and 10 exhibited potential for the implementation of breeding programs.
Key words: Citrullus lanatus, resistance, vegetable genetic capabilities
INTRODUCTION
Watermelon [Citrullus lanatus (Thunb.) Matsum and Nakai] is a species of the family Cucurbitaceae, being cultivated in almost the entire national territory. The Brazilian northeastern region has an estimated average production of 54,117 tons (IBGE, 2014), and Bahia, Rio Grande do Norte and Ceará, Brazil are the largest producing states, with respectively 122,320, 121,688 and 82,424 tons of the fruit. Among the largest producers in the Northeast region, the State of Rio Grande do Norte, Brazil, is considered an excellent choice for growing this vegetable due to favorable climatic conditions, besides being a potential market for melon exports.
However, nematode problems caused by M. enterolobii already represent high instability for cultivation. The damages caused by this root-knot nematode were reported by the first time for guava culture in the sub-middle region of the São Francisco basin in 1988, in the municipalities of Petrolina-PE and Juazeiro-BA, Brazil (Moura and Moura, 1989). It was also observed in the watermelon culture in the municipalities of Mossoró and Assu, State of Rio Grande do Norte, Brazil (Moura et al., 2002).
Effective control of M. enterolobii is one of the major issues to be overcome for an economically sustainable cultivation of irrigated watermelon crops. Even in the case of inexistent susceptible crops, nematodes can survive and reproduce in many weed species (Terao et al., 2012). Difficulties for the parasite control is increased by the reduced number of active ingredients available in the market and the high cost they add to production. Thus, an investigation of the genetic variability present in the species’ germplasm, aiming to develop resistant varieties, could be the most effective and economic method of control for the implementation of integrated management practices (Pontes, 2009).
Taking into account that family farmers usually manage their seeds and grow crops without chemicals, especially pesticides, the populations they use are probably resistant to some biotic stresses. Thus, this study aimed to evaluate the reaction of watermelon accessions collected in the Rio Grande do Norte State, Brazil, to M. enterolobii parasitism, to verify the hypothesis probable resistant genotypes.
MATERIALS AND METHODS
The germplasm was obtained by collecting samples of seeds stored by farmers of traditional agriculture in the Rio Grande do Norte State (12 accessions), Brazil, and from spontaneously-growing plants in the Experimental Stations (seven accessions) and other areas (one accession). The samples were collected in the towns of Apodi (Latitude: 5° 38' 58'' South and Longitude: 37° 47' 45'' West), Cerro Corá (Latitude: 6° 2' 44'' South and Longitude: 36° 20' 52'' West) and Tibau do Sul (Latitude: 6° 11' 23'' South and Longitude: 35° 5' 29'' West).
Two experiments were carried out to determine the response to M. enterolobii nematodes. The first experiment was conducted from March to May, 2011 and the second one from April to June, 2012. The second one was conducted for reaffirmation of the results identified in the accessions that showed parcels with resistance reaction.
Both experiments were carried out and evaluated in a greenhouse and in the Embrapa’s Nematology Laboratory of the Semiarid Region located in Petrolina, State of Pernambuco, Brazil, respectively. The climate of Petrolina is BSwh' of Koppen classification agreement, therefore, semi-arid with a rainy season of high annual average temperatures of 26.03ºC order and average rainfall of 522.3 mm.
Seedling plants were cultivated in expanded polystyrene seedling trays with 128 cells. The substrate used was a commercial compost based on expanded vermiculite and vegetable organic matter. Fourteen days after sowing, the plantlets were transplanted into the soil contained in plastic pots. The soil used was alfissol dystrophic.
To obtain the inoculum, roots infected with the plant nematode in areas of guava tree production (Psidium guajava L. cultivar Paluma) were collected in Petrolina, and by means of α-esterase revelation of phenotype, according to the vertical electrophoresis technique in polyacrylamide gel (Alfenas et al., 2006), the presence of M. enterelobii species was determined in the sample.
The eggs for preparation of the inoculum were extracted ten days after sowing, according to the method proposed by Coolen and D’herde (1972). Aliquots of 1 mL were used, in which the count of eggs and the calibration of suspension for inoculation of the plants in Petri dishes were performed using a microscope.
Inoculation was made the fourth day after transplanting by adding 1.5 mL of the suspension containing 2200 eggs per parcel into two holes separated at a distance of five centimeters from the plant’s base and approximately two centimeters deep. The “Santa Cruz Kada Gigante” cultivar of tomato (Lycopersicon esculentum Mill.) was used as control to confirm the inoculum viability.
Seventeen days after transplanting, the plants were tied to stakes with plastic strings. The branches were kept vertically until completion of the experiment and the tiers were adjusted every week.
The first experiment was conducted by the 52nd day after inoculation with average temperature of 28.5°C. Harvesting was performed by cutting the plants base five centimeters above the ground. Each plant was stored in separate plastic bag and taken to the laboratory for evaluation.
To measure the genotypes resistance degree, the gall index (GI) and egg mass index (EMI), egg mass number (EMN), egg number (EN) per plant and the reproduction factor 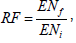 as obtained by the ratio between the final population (ENf) and the initial population (ENi) was determined.
as obtained by the ratio between the final population (ENf) and the initial population (ENi) was determined.
The gall index and egg mass were determined by examining the root system of each infected plant under a stereoscopic microscope. To estimate the indices, the score rating of the International Meloidogyne Project - IMP, cited by Taylor and Sasser (1978), was used with susceptibility reaction determined when the mean of the gall indices or the egg mass indices was equal to or over three.
For the reproduction factor, plants with RF < 1 were considered resistant and the plants with RF ≥ 1 as susceptible, as proposed by Oostembrink (1966). Complementarily, the fresh mass of the root system was measured to determine the correlation between the infection severity and the root system mass of each of the accessions evaluated.
The first experiment was performed under a completely randomized design, with 20 treatments corresponding to accessions, and ten replications, each parcel consisting of a plastic pot with three liters of medium textured soil, autoclaved, containing one plant.
For the statistical analysis of data, a variance-stabilizing transformation was applied in 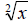 for the fresh mass of the root system and egg mass number (EMN); in
for the fresh mass of the root system and egg mass number (EMN); in 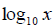 for the egg number (EN) and
for the egg number (EN) and  as indicated by Asmus et al. (2005) for the reproduction factor (RF). Analysis of variance was processed for all sources of variation. The post-ANOVA procedures used were the correlation study using Pearson’s correlation coefficient method for the RM, EMN and EN quantitative variables, and application of the Scott-Knott’s means comparison procedure at a 5% probability level for the RF.
as indicated by Asmus et al. (2005) for the reproduction factor (RF). Analysis of variance was processed for all sources of variation. The post-ANOVA procedures used were the correlation study using Pearson’s correlation coefficient method for the RM, EMN and EN quantitative variables, and application of the Scott-Knott’s means comparison procedure at a 5% probability level for the RF.
Analysis of the parcels reaction in the treatments was made by comparing the RF mean value of each parcel with a constant value (k), which corresponded to the parcel with the lowest reproduction factor within each accession. Pair comparisons were made usingthe t-test for orthogonal contrasts  , with
, with 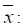 parcel mean; K: constant value relating to the lowest reproduction factor within each accession and
parcel mean; K: constant value relating to the lowest reproduction factor within each accession and 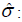 standard deviation of the parcels around the treatment mean.
standard deviation of the parcels around the treatment mean.
The second experiment comprised accessions 02, 07, 09 and 10 and the Crimson Sweet commercial variety. Accessions 02, 07, 09 and 10 were selected not for presenting the lowest mean RF but because they presented at least one parcel with RF < 1, that is, rated as resistant and with suppressed expression by using the RF arithmetical mean of treatments for rating. The accession 14 was used as a control for the species’ susceptibility and the Crimson Sweet variety as a commercial standard genotype.
All planning, installation, conduction and evaluation procedures used in the second experiment were identical to those used in the first experiment.
The only variable determined in the experiment for measurement of the genotypes resistance was the reproduction factor (RF).
For statistical analysis, experiments were subjected to the analysis of variance and the unfolding of the genotypes × environments (G x E) interaction was performed.
RESULTS AND DISCUSSION
With regard to the GI and EMI variables, the 20 accessions examined showed susceptibility to the parasite according to the criteria described by Taylor and Sasser (1978). Severity of the root systems infection, which was found in all accessions assessed, suggests determination of other variables to characterize the germplasm. Taking into account that the infection occurred in a generalized form, it is suggested that for the development of a breeding program for resistance to M. enterolobii, variables relating to the reproduction of this parasite be considered, in order to allow identification of genotypes that are potentially capable of suppressing the parasite reproduction after infection. In this context, accessions with reduction character in the pathogen population densities below the economic injury level is configured as a viable alternative for watermelon breeding. Likewise, when Anwar and Mckenry (2010) evaluated seeds from 16 localities in Pakistan, susceptibility reaction of all watermelon genotypes evaluated was found using these criteria. In general, in this study, all accessions were on average susceptible. However, there exists possibility of obtaining resistant genotypes with the completion of new collections in municipalities of Rio Grande do Norte, Brazil.
In this study, the choice of RF was an alternative to quantitative character GI and EMI. The reproduction inhibitor character was suggested as more robust, considering the refinement of the technique of determining the number of eggs per root.
In contrast with the present work, Pontes (2009) observed resistance of four accessions of C. lanatus var. citroides and one C. lanatus var. lanatus according to the gall index, using 2,000 and 5,000 eggs as inoculum, room temperature of 32 ± 5°C, and conducted evaluation 45 days after inoculation. Divergence between the results is suggested by the longer period of exposure of the accessions to the parasite in this work, associated with relatively mild temperatures of 27±0.9ºC, which may have favored the M. enterolobii reproductive cycle, which, according to Agrios (2005), is optimum at 27ºC.
Thies and Levi (2007), studying the reaction of accessions of C. lanatus var. lanatus and Citrullus colocynthis to M. incognita race 3 and M. arenaria race 2, observed resistance of the first species as to the gall index, corroborating the results found by Pontes (2009) for the nematode genus and, for this reason, in disagreement with the present work.
Lima (2008), evaluating the reaction of 4 cultivars and 15 accessions of C. lanatus var. lanatus to M. javanica, observed susceptibility to infection in all accessions and, therefore, corroborate the results found in this work. However, the author found a resistance reaction of all genotypes to the nematode oviposition.
The analysis of variance showed significance in the mean squares of RM, EMN, EN and RF, but the intensity of association between the variables was nearly null (Table 1).
High correlation values would allow the selection of genotypes with mitigation potential, or restriction, of M. enterolobii reproduction using only one descriptor simple determination (EN;RF), however, not observed in this study. To evaluate the resistance of a host plant, it is necessary to determine the ability of restricting the nematode multiplication in the plant (Mckenry and Anwar, 2006), thus indicating the egg number or the reproduction factor as the most important variables to be recorded.
Regarding the RF variable, it was found that the extreme values presented by some parcels within each accession had a direct influence on the mean RF in each treatment. Thus, the RF mean values seemed to be overestimated (RF > 1), making ineffective the Scott and Knott’s test, like any other procedure of multiple means comparisons, to explain the resistance reaction based on relative contrasts to the RF value (Table 2).
The variability observed within each accession, that is, of each representative subsample of a given population was mostly due to the seeds management by the family farmers. The selection of seeds for the next planting, besides the variability that exists in the samples introduced by the African continent (Romão, 2000) by different routes (Correa, 2010), showed to be a very important tool for the maintenance of the variability found in the germplasm existing in traditional farming.
From the analysis of the reaction of each parcel in each treatment assessed, it was possible to identify plants with resistance reaction in the accessions 02, 07, 09 and 10, because they are plants with reproduction factors lower than one (RF < 1) (Table 3).
The accessions 09 and 10 had the lowest mean reproduction factors in the first experiment, 1.94 and 1.31, respectively, but in the second experiment, all plants indicated higher reproduction factors. The accessions 02 and 07 showed different plant reactions, sometimes lower in the first accession and higher in the second and vice versa. By analyzing the RF magnitude in each accession, it was found that the accession 02 showed one parcel with RF = 0.45 in the first experiment and standard deviation (σ) around the average of 5.85, indicating resistance reaction to M. enterolobii. In the second experiment, the accession 02 showed susceptibility reaction to M. enterolobii in all parcels. The accession 07 behaved similarly to accession 02.
The accessions 09 and 10 presented a differentiated behavior, with respectively 40 and 30% of their parcels with RF < 1 in the first experiment. The interval of 0.57 ≤ RF ≤ 1.94 comprised 80% of the parcels of the accession 09 with a standard deviation of 1.93. In the second experiment, there was only one plant with RF < 1 (RF = 0.82); however, it reaffirmed its potential as a source of resistance genes for use in breeding programs.
Seventy percent of the parcels showed resistance reaction in the genotype 10 in the first experiment, which synthetized the interval 0.80 ≤ RF ≤ 1.44. In the second trial, no parcel exhibited RF < 1, suggesting that the frequency of individuals with this characteristic, in this seeds subsample, is low, lower than that found in accession 09.
This difference in behavior is likely related to different genus contents in the plants; as even in slightly contrasting conditions, they enabled or mitigated the nematode development. Damasceno (2013), in a study of parents and F1s of watermelon Citrullus spp., showed that the general and specific resistance combination ability to the nematode were highly significant, indicating a genetic control of the character. In general, segregation for the RF character can be observed in the plants of all accessions.
Regarding the Crimson Sweet commercial variety evaluated in the second trial, there was variability in the expression of resistance within the cultivar, with a minimum and mean RF of 1.39 and 29.24, respectively.
Commercial lines with high ability of nematode reduction viability can be promising sources of watermelon resistance to the nematode species, either for breeding programs per se or crossings.
Similar result was found by Lima (2008), who evaluated six cultivars and 15 accessions of C. lanatus var. lanatus species, showing resistance reaction of all treatments to M. javanica based on the RF.
On the other hand, Pontes (2009) found RF < 1 in two accessions of the C. lanatus var. citroides species. Also, Damasceno (2013) corroborated the results found by Lima (2008) and Pontes (2009) in accessions of Citrullus spp., showing the germplasm potential of traditional farming as a source of tolerance alleles for the characteristics, which was also confirmed by the present study on C. lanatus var. lanatus accessions.
CONCLUSIONS
Variation was found in the response among and within the accessions regarding the ability to inhibit reproduction of M. enterolobii species in the root system; as the accessions 9 and 10 showed potential for implementation of breeding programs aiming to develop tolerant genotypes to the reproduction of M. enterolobii. However, in future research, the data collection area will be extended to all municipalities in the State of Rio Grande do Norte, Brazil, for characterization and conservation of genetic variability present in germoplasm of traditional agriculture.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Agrios GN (2005). Plant pathology. Burlington: Elsevier Academic Press P 922. |
|
|
Alfenas AC, Lfenas AC, Brune W (2006). Eletroforese em gel de poliacrilamida. In: Alfenas, A.C. Eletroforese e marcadores bioquímicos em plantas e microrganismos. Viçosa: UFV pp. 151-182. |
|
|
Anwar SA, Mckenry CMV (2010). Incidence and reproduction of Meloidogyne incognita on vegetable crop genotypes. Pakistan Journal of Zoology 42(2):135-141. |
|
|
Asmus GL, Inomoto MM, Sazaki CSS, Ferraz MA (2005). Reação de algumas culturas de cobertura utilizadas no sistema de plantio direto a Meloidogyne incognita. Nematologia Brasileira 29(1):47-52. |
|
|
Coolen WA, D'herde CJ (1972). Method for the quantitative extraction of nematode plant tissue. Ghent State Agriculture Research Center P. 777. |
|
|
Correa SMS (2010). Africanidades na paisagem brasileira. Revista Internacional Interdiscinar Interthesis 7(1):96-116. |
|
|
Damasceno LS (2013). Reação de genótipos de melancia a Meloidogyne enterolobii, BA. 2013. P. 100. Dissertação (Mestrado) – Universidade do Estado da Bahia, Juazeiro. |
|
|
Instituto Brasileiro de Geografia e Estatística Estados (IBGE) (2014). Instituto Brasileiro de Geografia e Estatística. Estados: lavoura temporária 2014. |
|
|
Lima GKL (2008). Reação de cultivares e acessos de melancia ao parasitismo de Rotylenchulus reniformis e Meloidogyne javanica, RN. 2008. P.60 Dissertação (Mestrado) - Universidade Federal Rural do Semi-Árido, Mossoró. Universidade Federal do Rio Grande do Norte. |
|
|
Mckenry MV, Anwar SA (2006). Nematode and grape rootstock intereactions including an improved understanding of tolerance. Jounal of Nematology 38(3):312-318. |
|
|
Moura RM, Pedrosa EMR, Guimarães LMP (2002). Nematoses de alta importância econômica da cultura do melão no estado do Rio Grande do Norte. Nematologia Brasileira 27:225. |
|
|
Moura RM, Moura AM (1989). Meloidoginose da Goiabeira: doença de alta severidade no estado de Pernambuco, Brasil. Nematologia Brasileira 13(1):13-19. |
|
|
Oostenbrink M (1966). Major characteristics of the relation between nematode and plants. Meded. Landbouwhogeschool 66:3-46. |
|
|
Pontes MFC (2009). Resistência de melancieira a Meloidogyne mayaguensis e avaliação dos mecanismos envolvidos P 69. Dissertação (Mestrado)–Universidade Federal Rural do Pernambuco, Recife. Universidade Federal de Pernambuco. |
|
|
Romão RL (2000). Northeast Brazil: A secondary center of diversity for watermelon (Citrullus lanatus). Genetic Resource and Crop Evolution 47:207-213. |
|
|
Terao D, Castro JMC, Lima MF, Batista DC, Barbosa MAG, Reis A, Dias RCS (2015). Doenças causadas por nematoides. In: Mendes MAS, Silva AF, Oliveira AR, Faria CMB, Silva DJ, Teixeira FA, Souza FF, Resende, Barbosa GS, Alencar JA, Anjos JB, Alves JCSF, Damaceno LS, Queiroz MA, Calgaro M, Braga MA, Lima MAC, Costa ND, Correia RC, Souza RNC, Cunha TJF. Sistemas de produção de melancia. 2015. |
|
|
Taylor AL, Sasser JN (1978). Biology, identification and control of root-knot nematodes, Meloidogyne spp. Raleigh: North Carolina State University Graphics P 111. |
|
|
Thies JA, Levi A (2007). Characterization of Watermelon (Citrullus lanatus var. citroides) Germplasm for Resistance to Root-knot Nematodes 42(7):1530-1533. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0