ABSTRACT
Changes on soil microbial activity may be triggered by different management approaches and the study of the effects of such changes on xenobiotics, of non-target populations, may represent a valuable strategy to evaluate their environmental risk potential. The objective of the present study was to evaluate the effect of Phytosanitary control measures over microbial activity and genetic variability of bacteria in soil cultivated under the forage cactus Opuntia ficus-indica. The experiment was performed at Caetés region in Pernambuco, Brazil. Three days after the application of the xenobiotics (water (control); detergent + sodium hypochlorite; Neem oil; Methomyl and Thiamethoxam + Lambda-cyhalotrin), soil samples (0-20 cm) were collected and transported to laboratory. Respirometry, density of bacterial population and morphologic and genetic variability of bacteria were evaluated with molecular techniques, using BOX-PCR in a completely randomized statistical design. Regarding to respirometry, the amount of CO2 released from the soil samples was greater within the plots where the insecticide Thiamethoxam + Lambda-cyhalotrin was applied, when compared with control. Soil treatments with only water and water + sodium hypochlorite showed the highest population densities (0.96 and 0.94 × 102 CFU.g-1, respectively). Concerning to morphological characteristics (color), there was prevalence of white color colonies, with a little visual phenotypic variability. However, the use of molecular techniques revealed high genetic variability among the white colored colonies evaluated, demonstrating the importance of more detailed studies on the effects of xenobiotics on soil microbiota prior to its use of recommendation.
Key words: Respirometry, microbiological indicators, BOX-PCR, ecotoxicology, pesticides.
Barbary fig, Opuntia ficus-indica, is a cactus species native to Mexico introduced in Brazil at the end of the 19th century. Under the edaphoclimatic conditions of the semi-arid region in Brazil, this cactus assumes a relevant role within livestock farming, due to its high resistance against drought and high temperatures, allied to its adaptability to low fertility soils. Its high efficiency in the use of water contributes to increase economic feasibility of intermediate and small farm producers of low incomes within the Brazilian semi-arid region (Santos et al., 2013; Ramos et al., 2014).
Brazil has the largest Barbary fig planted area in the world, however, over the last few years, the incidence of the cochineal insect (Dactylopius opuntiae Cockerell) has threated the viability of the main variety of Barbary fig within, where this insect has become the leading pest of this cactus plant (Santos et al., 2013). Farmers have accomplished control of the insect with the use of xenobiotics, registered or not for this pest. The compounds used for this purpose are constituted by a great number of molecules with different modes of action and toxicity, and its impact over non-target organisms within the agricultural ecosystem of the Barbary fig was not yet been evaluated.
According to Ros et al. (2006), the ideal xenobiotic must be toxic to aim only target organisms, totally biodegradable and able to not leaving any intermediary compounds in the environment or being lixiviated to underground waters. In a general way, the main problems resulting from the use of such compounds in agriculture are their toxicity to non-target organisms, their persistence in the soil, the development of resistant species and their influence over soil microorganism dynamics. Thus, the study of pesticide’s effects on non-target populations represents an acceptable strategy to evaluate their potential environmental risk (Ferreira et al., 2009). Soil microorganisms promote organic matter breakdown, formation and stabilization of soil aggregates, bio-geo-chemical nutrient cycling within the soil, pathogen suppression, production of phytohormones, breakdown of xenobiotic compounds, among others (Reis et al., 2009; Pôrto et al., 2009).
Little changes in the activity of soil microorganism may be associated with distresses caused by management (Reis et al., 2008). Biochemical and microbiological criteria are the most responsive, in short time, due to the higher sensibility to distresses from the improper management (Chaer and Tótola, 2007). Amongst the most remarkable soil quality microbiological indicators, respiratory rate, microbial quantification and bacterial diversity studies are fundamental parameters to understand the ecosystem functioning.
According to Zilli et al. (2003), the analysis of genetic variability is a soil quality indicator. Nowadays, the structure of microbial diversity it’s being studied trough methods based on the research of parts of DNA sequences, with emphasis onthe 16S rDNA gene, through the BOX-PCR technique. The bacterium are primarily in combination with different species, forming the bacterial community, occupies all terrestrial niches and colonize environments such as soil, water, air, plants and animals (Andreote et al., 2009).
Based on these considerations, the objective of the present study was to evaluate the effect of phytosanitary control measures on microbial activity and the genetic variability of bacteria in soils planted with Barbary fig.
The experiment was performed in a private farm at the county of Caetés in the State of Pernambuco, Brazil, where the xenobiotics were applied on plants naturally infected with the carmine cochineal, D. opuntiae. A completely randomized design with five treatments (xenobiotics) and three replicates was used. Treatments consisted in: Only water (control); Detergent (3%) + sodium hypochlorite (1.5%); Commercial Neem oil (0.66%); Methomyl (0.3%) and Thiamethoxam + Lambda-cyhalotrin (0.01%), that were sprayed with the aid of a 20 L capacity back sprayer, directly over the plant’s cladodes, until the initial signs of runoff. Sprayings were performed to avoid interference between treatments, with the use of parcels of 20 × 15 m (width x length).
Soils were collected three days after application of the xenobiotics, in order to quantify CO2 released by the microorganisms and for bacterial evaluation. Soil samples were collected near Barbary fig plants, at a 0 to 20 cm depth, collecting three simple random samples within each parcel to form a final compound sample. Sterile centrifuge tubes were filled with 50 g soil and conditioned inside Styrofoam boxes containing ice and water soaked paper to refrigerate samples. These samples were used for bacterial isolation. With the purpose of quantify CO2, approximately 1.000 g of soil samples were conditioned into clean plastic bags. Soil samples were transported to the Laboratory of Microbial Genetics and Biotechnology from the Garanhuns Academic Unity (UFRPE/UAG) and to the Applied Entomology Laboratory (UFRPE/UAG), respectively, both in the city of Garanhuns - Brazil, to proceed with the sample processing.
To quantify respiration produced by microbes present in the soil, samples were dried for a period of 24 h in the dark, before determining their field capacity, using 500 g of soil for 500 mL of water. During the assays, 100 g of dried soil were incubated in 600 mL capacity glass containers, moistened with distilled water at 70% field capacity. Plastic vases (40 mL) containing 10 mL NaOH (0,91 mol L-1) were placed inside the glass containers. Containers were closed hermetically and preserved at room temperature (± 26ºC) for a period of 30 days. Vase without soil, containing NaOH to capture CO2 from the environment were used as control.
Quantification of CO2 production was performed 3, 6, 9, 13, 16, 20 and 30 days after soil incubation. For each incubation period the plastic pots were removed from the glass containers. Then, the sodium hydroxide (NaOH) of each sample, 5 mL of BaCl2 (1 mol.L-1) and 3 drops of phenolphthalein 1% were placed in separated Beaker. Later, this solution was titrated with hydrochloric acid (HCl 0.45 mol.L-1), and the volume of HCl was registered. After removing NaOH, new solutions were changed for the subsequent incubation periods. The same procedure was performedfor pots without the addition of soil (control).
The quantity of CO2 released, in mg.kg-1 of C-CO2 in the soil, was calculated according Stotzky (1965): C-CO2 mg = (B-V) *M*E. Where B = volume of HCl in mL, used for titration of NaOH from control; V = volume of HCl in mL, used for titration of NaOH from the sample; M = molar concentration of the acid used (HCl 0.45 mol/L); E = carbon equivalent weight.
For each of the five products applied on the field three replicates were used, consisting in two vase each (duplicate) for each incubation period, in a factorial 5 × 7 (xenobiotics x incubation period) design. Data underwent a variance analysis and means were compared by the Tukey test at 5% probability, using SISVAR® software.
The procedures for bacterial isolation followed the methodology described by Araújo et al. (2010), with modifications. From each soil sample collected within the parcel, 5 g were transferred to Erlenmayer flasks (125 mL) containing 50 mL of PBS (Phosphate Buffered Saline: NaCl 8.0 g L-1; KCl 0.20 g L-1; Na2HPO4 1.44 g L-1; KH2 PO4 0.24 g L-1; pH 7.4) per liter and about 5 g of glass beads (0.1 cm diameter). Then, these flasks were located in a shaker table during 30 min at 100 rpm. After agitation, serial dilution (10-4 and 10-5) were executed in PBS buffer and aliquots of 100 µL were inoculated in Petri dishes containing TSA 10% (Triptone Soy Agar: Triptone 1.5 g L-1; Peptone 0.5 g L-1; NaCl 1.5 g L-1; Agar 15 g; pH 7.3) culture medium per liter, plus the fungicide Cercobin 700 (50 µg mL-1). Then, flasks were incubated at 28ºC and evaluated after 24, 48 and 72 h. Population density was evaluated by counting colonies and expressed in grams of fresh soil (CFU g-1 of soil). In addition, time of bacterial growth and morphology (color) were also evaluated.
The experiment was performed under completely random design with five treatments (applied products) and three replicates. Data underwent variance analysis and the means were compared by the Scott-Knott test at 5% probability, using SISVAR® software.
To evaluate genetic variability, 19 bacterial colonies of white color were selected, isolated from culture dishes and individually identified with a UAGtx nomenclature. With the aid of sterile wood-needles, each isolated colony was re-inoculated in a tube containing 4 mL TSA 10% liquid medium and preserved in shaker table (100 rpm) during 24 h. After the bacterial growth period, the culture was transferred to microtubules of 2 mL and centrifuged for 5 min at 12.000 G to precipitate bacterial cells, discharging supernatant. The resulting pellet was re-suspended in 300 uL of TE (10 mMTris-HCl; 1 mM EDTA; pH 8,0), and used as a DNA source for molecular analysis. Samples were preserved at a temperature of -20ºC.
The evaluation of genetic diversity of the bacterial isolates was performed by means of the BOX-PCR technique, using the primer BOX 1AR (‘5 – CTACGGCAAGGCGACGCTGAC G-3’). The polymerase chain reactions (PCR) were prepared to a final volume of 25 µL, containing the sequence: Ultra-pure water; 1 × Taq Buffer; 3.5 mM MgCl2; 1 mM of each dNTP; 0.4 µM of the primer BOX 1AR; 1 × DMSO (dimethyl sulfoxide) and 0.08U/µL of enzyme Taq DNA Polymerase. After mix preparation, micro tubes were placed in a thermal cycler programed to perform initial denaturation at 95°C during 2 min, followed by 35 cycles for denaturation at 94ºC during 2 s, 92ºC during 30 s, 50ºC during 1 min, 65ºC during 8 min, 65ºC during 10 min and 4ºC during 59 min. After amplification PCR reactions were evaluated in agarose gel (1.5%) electrophoresesat 1 × TAE (40 mMTris-Aacetate; 1 mM ETDA) buffer with addition of 10 µL of each reaction and 2 µL of Blue Green Loading Dye (LGC Bio), ending with observation under UV light and photo documentation.
Lanes obtained by amplification were transformed in binary data (lane presence or absence) and used to obtain a similarity dendrogram calculated by Jaccard’s coefficient and clustered using the algorithm UPGMA (Unweighted Pair-Group Method with Arithmetical Average), using PAST® 1.90 software.
The evaluation of CO2 quantity captured from soils in parcels where the plants were treated with different xenobiotics, showed effects of the applied products on the microbiota. In accordance with a similar study conducted by Sebiomo et al. (2011) evaluating effect of herbicides on microbial population, in the present study, the synthetic organic insecticides (Methomyl (0.3%) and Thiamethoxam + Lambda-cyhalotrin) promoted significant microbial activity. A higher respiration rate of the soil, with 48.33 and 55.33 mg of CO2 gathered at the end of the evaluation period, was found compared to the other treatments. Neem oil and detergent + sodium hypochlorite, did not differed from the control treatment when regarding to the quantity of CO2 released (Table 1).
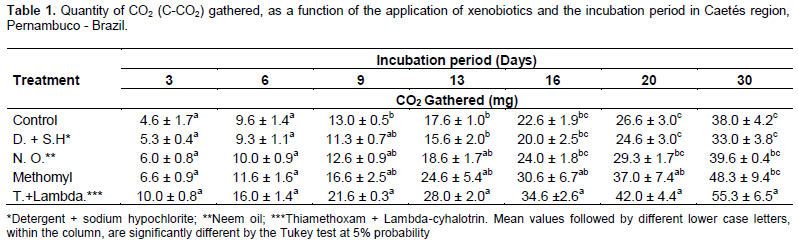
All incubation periods showed increments regarding CO2 quantification. According Sá et al. (2000), a greater microbial activity is revealed by a higher respiratory rate, causing the breakdown of organic matter of the soil and consequently, allowing nutrient availability for the plants. On the other hand, Islam and Weil (2000) quoted that higher respiratory rates may be related to the high level of productivity of the soil ecosystem or to ecological disturbance (that may be caused by the use of agrotoxics).
During the initial incubation periods (3 and 6 days), no difference between xenobiotics was noticed concerning microbial respiration, demonstrating that the effect of the products on the soil microbiota, does not happen immediately. Starting from 9 days of incubation becomes apparent that released CO2 quantities within soil samples was higher in parcels with the use of the insecticide Thiamethoxam + Lambda-cyhalotrin, if compared to control, but not differing from the other treatments. From 16 days of incubation until the last record (30 days), the same tendency was observed for the insecticides Thiamethoxam + Lambda-cyhalotrin and Methomyl, regarding to the accumulated CO2, being higher when compared with the other treatments (control and detergent + sodium hypochlorite).
The lowest quantity of C-CO2, in the last reading from parcels with use of water and detergent + sodium hypochlorite, may be an evidence of the higher efficiency in the use of soil resources by the microorganisms. The microbial biomass uses death cells as source of C and energy. In that manner, according Reis et al. (2008), it is reasonable, at least in part, to observe an increase in C-CO2 production. However, Castro et al. (2006), while studying the effect of xenobiotics on soil microbiota, verified that some compounds are easier metabolized and used as energy and nutrient sources, thus increasing activity due to a higher CO2 release, probably due to promote higher stimuli and consequently, increasing the soil microbiota.
Moreno et al. (2007), while evaluating high dosages of the herbicide Atrazine in semi-arid soil, verified a tendency to an increasing microbial respiration with the incubation period. According to the authors, this may be explained by the ability of a small fraction of the microbial population to completely reduce Atrazine to produce CO2 and H2O. Tironi et al. (2009), while studding the effect of herbicides on soil microbial activity, verified higher accumulated C-CO2 progression when using two times the reference dosages (10 mg dm-3 of soil) of the herbicide Ametryn, with the lowest evolution ratio of C-CO2 registered in soils without application of herbicide (control). The authors observed that in treatments using a mixture of Trifloxysulfuron-sodium + Ametryn, C-CO2 progression were higher when using two, four and eight times the reference dosages, differing form the one time dosage and the control, without herbicide.
With dosages 10 times higher to the field recommendation, Zabaloy et al. (2008) verified that in general, the herbicides 2.4-D, Metsulfuron-methyl and Glyphosate had little effect over soil microbial communities. Araújo et al. (2003) verified that soils with application of Glyphosate exhibited higher microbial respiration during the beginning of incubation, showing that microorganisms are the main responsible by the biodegradation of the herbicide in the soil. However, Reis et al. (2009) did not detected any alteration in respiratory rates in soils treated with Fomesafen + Fuazifop-p-butyl and Glyphosate and with or without application of the mixture of insecticide (Endosulfan) + fungicide (Tebuconaloze). We highlight that scarce studies are available concerning the effect of insecticides on soil microbiology.
The products used for carmine cochineal control substantially affected bacterial community. Soils treated with only water (control) and detergent + sodium hypochlorite showed the highest quantities of colony forming units (CFU), with 0.96 and 0.94 × 102 UFC/g of soil, respectively, but not differing among them self. In the remaining treatments a reduction in the quantity of CFU’s was observed. Neem oil, though being a natural product, affected the bacterial community in a similar way, when compared to synthetic insecticides (Figure 1).
Oliveira (2004), while evaluating microbial diversity in different agricultural systems in the semi-arid region, verified population densities of 18, 21 and 127 CFU x 10 g/soil, for bacteria, fungi and actinomycetes respectively, in an area planted with Barbary fig (O. ficus-indica). In the present study, a mean value of 0.93 × 102 UFC.g-1 bacteria were registered, thus showing a low CFU rate.
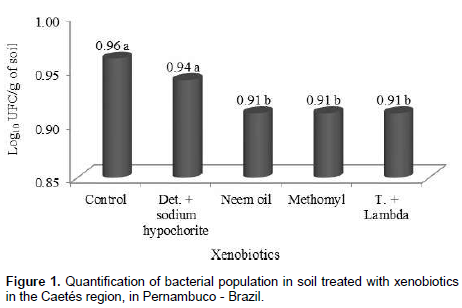
This difference must be related to the content of organic matter, temperature and humidity, once the abundance and activity of microorganisms are susceptible to seasonal variations (Zilli et al., 2003). Absence of rain fall during the period of the experiments may have been another factor that influenced the lower densities of CFU in this soil. According to Costa and Melo (2012), a considerable number of bacterial species, particularly those associated with plant’s rhizosphere, exert beneficial effects for plant growth.
Kuklinsky-Sobral et al. (2004), while evaluating the total density of bacterial community in soybean cultivars growth in areas with and without pre-planting application of the herbicide Glyphosate, observed densities of about 104 to 106 CFU/g and 108 to 1010 CFU/g in endophytic and epiphytic bacterial communities, respectively. The author evidences the occurrence of interactions between densities and other factors analyzed, as cultivar, vegetal tissue, seasonal variation and developmental stage of the host.
Soils with application of only water (control) and detergent + sodium hypochlorite, showed a slower growth of bacteria, within the three evaluation periods (24, 48 and 72 h). It is evident that for the other treatments (Neem oil, Methomyl and Thiamethoxam + Lambda-cyhalotrin), bacterial growth increased after 24 h, with a decrease starting at 48 h of incubation. At 72 h there was an absence of new colonies formation in the treatments with insecticides Methomyl and Thiamethoxam + Lambda-cyhalotrin (Figure 2).
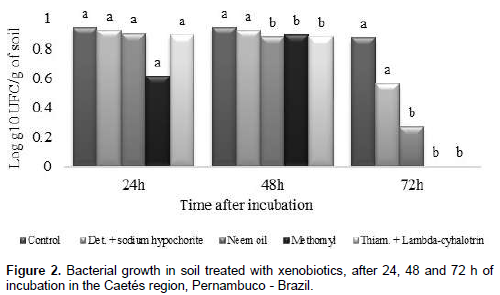
Studying growth of the diazotrophic bacteria Herbaspirillum seropedicae, Fernandes et al. (2012), verified that insecticides Imidacloprid, Fipronil and Thiamethoxam had no effect in bacterial growth when used at a concentration corresponding to the commercial dosage. However, when using dosages two times higher they verified that insecticides Endosulfan and Carbofuran resulted in growth reduction of H. seropedicae. According to this author, the use of insecticide molecules and less aggressive formulations must be pursued for all those using such technologies to increase food and energy production, without compromising sustainability of the agricultural ecosystems. Different results were found by Shamsuddeen and Inuwa (2013) which reported slow growth of bacteria Pseudomonas aeruginosa up to four hours after application of Cipermetrine and after this period there was rapid growth, showing that this bacteria used the Cipermetrine as carbon source up to certain limits, which thereby stimulating their growth. This way, demostrating that can serve as a tool for environmental mitigation.
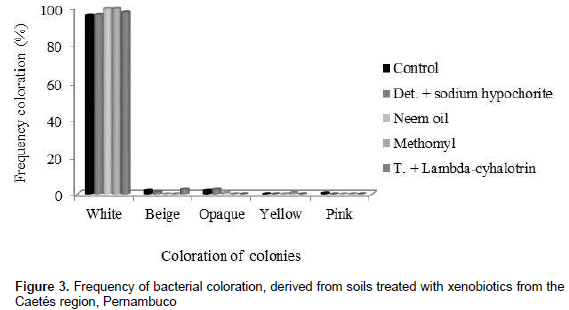
Castro et al. (2006), when evaluating the number of bacterial colonies with 1 and 13 days of incubation, verified a variation in the control treatment, pure Glyphosate (95%) and commercial Glyphosate (43%), respectively from 1.78; 1.33; 1.11 × 105 CFU g soil, in the first day of incubation, to 2.63; 2.89; 2.45 × 105 UFC g soil after 30 days of incubation. By means of morphological characterization (color) it was possible to verify that bacterial colonies showed white, beige, pale pink, yellowish color and dull appearance. White colonies were predominant, independently of xenobiotic application (Figure 3).
Although this feature points morphological similarity, visually assuming low genetic variability among colonies, the BOX-PCR technique allowed to observe a high genetic variability among colonies, demonstrating that such morphological variable is not always efficient to show genetic variability, becoming indispensable the use of different and complementary methods, as for example molecular techniques (PCR).
Analysis of genetic variability through the BOX-PCR technique was completed by amplification of bacterial genomic DNA repetitive sequences, using the primer BOX A1R. A similarity dendrogram, with absence and presence of bands was constructed based on the analysis of the band’s profiles. Similarity index of Jaccard showed high genetic diversity among bacterial colonies. Only colonies UAGtx 6 and UAGtx 15 showed a higher similarity rate (60%), all the other colonies evaluated showed similarity under 60%, confirming high genetic diversity (Figure 4).
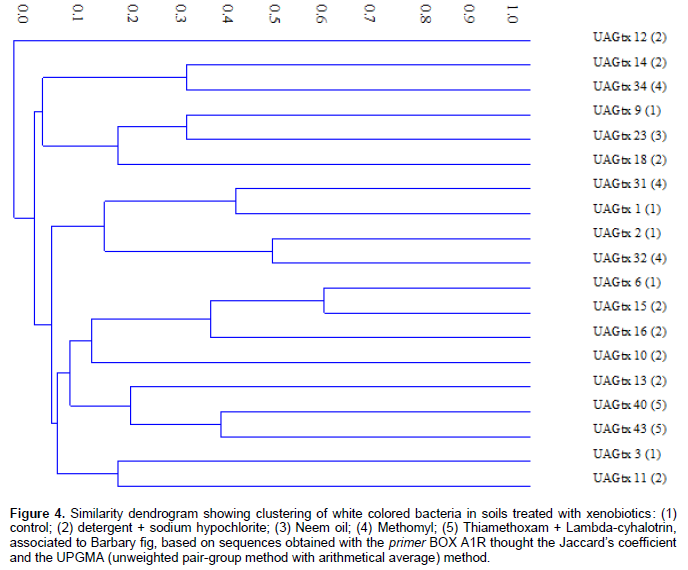
Colonies UAGtx 6 and UAGtx 15 were isolated from soils with the application of different xenobiotics. The first colony is original from an area with application of water (control), while the second is original form an area with application of detergent + sodium hypochlorite. A clear effect of the xenobiotics applied was not showed by the dendrogram obtained. Given the importance of the Barbary fig for the semi-arid region it is of the foremost importance to advance with more detailed studies regarding the effects of xenobiotics on the microbiota and the associated insects. In association, using a slow liberation of xenobiotics in soils in order to prolong its action could promote the environmental protection. For example, the microencapsulation could be used to reach this propose (Barbat et al., 2013). In this study, we demonstrate the insecticides Methomyl and Thiamethoxam + Lambda-cyhalotrin, applied to the soil, increased CO2 release by the soil microbiota, the higher bacterial densities occurred in soils treated with water (control) and with detergent + sodium hypochlorite, when compared with the rest of xenobiotics that reduced bacterial community, soils with application of water (control) and detergent + sodium hypochlorite, showed a slower growth of bacteria, while the other treatments showed an accelerated growth with 24 h incubation, to reduce growth from 48 h until the end of the incubation periods; concerning the morphological characteristic (color) of the colonies, a higher frequency of white colored colonies was observed, independently of the xenobiotic applied in the treatment and the BOX-PCR molecular technique, revealed high genetic variability amongst the white colored bacteria analyzed.
In this study, it was found that the amount of CO2 released from the soil samples was greater within the plots where synthetics insecticides were used. However, we found lowest bacteria population densities in those same plots. More detailed studies on the effects of xenobiotics on soil microbiota in forage cactus agroecosytem must be done prior to recommend its use in the fields.
The authors have not declared any conflict of interests.
The Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE) for granting the study scholarship to the first author.
REFERENCES
|
Andreote FD, Azevedo JL, Araújo W (2009). Assessing the diversity of bacterial communities associated with plants. Braz. J. Microb. 40:417-432.
Crossref
|
|
|
|
Araújo ASF, Monteiro RTR, Abakerli RB, Souza LSD (2003). Biodegradação de glifosato em dois solos brasileiros. Pesticidas 13:157-164.
|
|
|
|
|
Araújo WL, Lacava PT, Marcon J, Lima AOS, Sobral JK, Pizzirani-Kleiner AA, Azevedo JL (2010). Guia prático: Isolamento e caracterização de microrganismos endofíticos. Cop. Luiz de Queiroz, Piracicaba 167 p.
|
|
|
|
|
Barbat C, Rodino S, Petrache P, Butu M, Butnariu M (2013). Microencapsulation of the allelochemical compounds and study of their release from different products. Digest J. Nanomater. Biostruct. 8:945-953.
|
|
|
|
|
Castro JJV, Selbach PA, Záchiaayub MA (2006). Avaliação do efeito do herbicida glifosato na microbiota do solo. Pest.: Rev. de Ecotox. e Meio Amb. 16:16-30.
|
|
|
|
|
Chaer MC, Tótola MR (2007). Impacto do manejo de resíduos orgânicos durante a reforma de plantios de eucalipto sobre indicadores de qualidade do solo. Rev. Bras. Ciênc. Solo 31:1381-1396.
Crossref
|
|
|
|
|
Costa FEC, Melo IS (2012). Endophytic and rhizospheric bactéria from Opuntia ficus-indica milland their ability to promote plant growth in cowpea, Vigna unguiculata (L.) Walp. Afr. J. Microbiol. Res. 6(6):1345-1353.
|
|
|
|
|
Fernandes MF, Procópio SO, Teles DA, Sena FJG, Cargnelutti FA, Machado TNM (2012). Toxicidade de inseticidas utilizados na cultura da cana-de-açúcar para a bactéria diazotrófica Herbaspirillum seropedicae. Rev. Ciênc. Agrár. 55:318-326.
Crossref
|
|
|
|
|
Ferreira EPB, Dusi AN, Costa JR, Xavier GR, Rumjanek NG (2009). Assessing insecticide and fungicide effects on the culturable soil bacterial community by analyses of variance of their DGGE fingerprinting data. Europ. J. Soil Biol. 45:466-472.
Crossref
|
|
|
|
|
Islam KR, Weil RR (2000). Land use effects on soil quality in a tropical forest ecosystem of Bangladesh. Agricult. Ecosyst. Sand Environ. 79:9-16.
Crossref
|
|
|
|
|
Kuklinsky–Sobral J, Araújo WL, Mendes R, Geraldi IO, Pizzirani-Kleiner AA, Azevedo JL (2004). Isolation and characterization of soybean associated bacteria and their potential for plant growth promotion. Environ. Microbiol. 12:1244-1251.
Crossref
|
|
|
|
|
Moreno JL (2007). Effects of atrazine on microbial activity in semiarid soil. Appl. Soil Ecol. 35:120-127.
Crossref
|
|
|
|
|
Oliveira VC (2004). Atividade enzimática, população e análise de DNA da biodiversidade microbiana do solo em agroecossistemas do Semi-Árido. 2004. 127 f. Dissertação (Mestrado em Desenvolvimento e Meio Ambiente: Área de Concentração em Desenvolvimento Regional) - Universidade Federal de Sergipe, São Cristóvão.
|
|
|
|
|
Pôrto ML, Alves JC, Diniz AA, Souza AP, Santos D (2009). Indicadores biológicos de qualidade do solo em diferentes sistemas de uso no brejo paraibano. Ciênc. Agrotec. 33:1011-1017.
Crossref
|
|
|
|
|
Ramos JPF, Santos EM, Freitas FF, Candido EP, Lima Junior AC, Leite MLV, Oliveira Jr S (2014). Caracterização técnica dos sistemas de produção de palma forrageira em Soledade, PB. Agropec. Téc. 35:23-30.
|
|
|
|
|
Reis MR, Silva AA, Costa MD, Guimarães AA, Ferreira EA, Santos JB, Cecon PR (2008). Atividade microbiana em solo cultivado com cana-de-açúcar após aplicação de herbicidas. Plan. Danin. 26:323-331.
Crossref
|
|
|
|
|
Reis MR, Silva AA, Freitas MAM, Pereira JL, Costa MD, Picanço MC, Ferreira EA, Belo AF, Coelho ATCP, Silva GR (2009). Impacto do glyphosate associado a inseticida e fungicida na atividade microbiana e no potencial de solubilização de fosfato em solo cultivado com soja Roundup Ready®. Plan. Danin. 27:729-737.
Crossref
|
|
|
|
|
Ros M, Goberna M, Moreno JL, Hernandez T, García C, Insam H, Pascual JA (2006). Molecular and physiological bacterial diversity of a semi-arid soil contaminated with different levels of formulated atrazine. Appl. Soil Ecol. 34:93-102.
Crossref
|
|
|
|
|
Sá TCLL, Marques M, Vasconcelos CA, Pereira Filho I, França GE, Cruz JC (2000). Envolvimento de dióxido de carbono e mineralização de nitrogênio em Latossolo vermelho-escuro com diferentes manejos. Pesq. Agrop. Bras. 35:581-589.
|
|
|
|
|
Santos DC, Silva MC, Dubeux Júnior JCB, Lira MA, Silva RM (2013). Estratégias para Uso de Cactáceas em Zonas semiáridas: Novas Cultivares e Uso Sustentável das Espécies Nativas. Rev. Cienc. Prod. Anim. 15:111-121.
Crossref
|
|
|
|
|
Sebiomo A, Ogundero VW, Bankole SA (2011). Effect of four herbicides on microbial population, soil organic matter and dehydrogenase activity. Afr. J. Biotechnol. 10:770-778.
|
|
|
|
|
Shamsuddeen U, Inuwa AB (2013). Utilization of cypermethrin by bacteria isolated from irrigated soils. Bay. J. Pure Appl. Sci. 6:19-22.
Crossref
|
|
|
|
|
Stotzky G (1965). Microbial respiration. In: BLACK CA (Ed.). Methods of soil analysis. Madison: Am. Soc. Agron. 2:1551-1572.
|
|
|
|
|
Tironi SP, Belo AF, Fialho CMT, Galon L, Ferreira EA, Silva AA, Costa MD, Barbosa MHP (2009). Efeito de herbicidas na atividade microbiana do solo. Planta Daninha 27:995-1004.
Crossref
|
|
|
|
|
Zabaloy MC, Garland LJ, Gomez MA (2008). Na integrated approach to evaluate the impacts of the herbicides glyphosate, 2,4-D and metsulfuron-methyl on soil microbial communities in the Pampas region, Argent. Appl. Soil Ecol. 40:1-12.
Crossref
|
|
|
|
|
Zilli JE, Rumjanek NG, Xavier GR, CoutinhO HLC, Neves MCP (2003). Diversidade microbiana como indicador de qualidade do solo. Cad. Ciênc. Tecnol. 20:391-411.
|
|