ABSTRACT
Common bean (Phaseolus vulgaris) is one of the main Brazilian agricultural crop. It is affected by numerous diseases during its life cycle; one of these includes the white mold caused by the fungus Sclerotinia sclerotiorum. The objective of this study was to verify the control of white mold in bean plants by homeopathic medicines. Two tests were performed: In the first test, the medicine Calcarea carbonica was used, while phosphorus was used in the second test. Both medicines were administered at dynamizations of 6, 12, 24, 36, and 48 CH (Hahnemanniana Centesimal). In the control treatment, 30% hydroalcoholic solution was used. The study was conducted using a completely randomized design, analyzing the following variables: Area under the disease progress curve and percentage of dead plants. The results were submitted to Tukey’s test and regression analysis (p > 0.05). Calcarea carbonica at 6 CH and Phosphorus at 6 CH, 12 CH, 24 CH, 36 CH, and 48 CH reduced the intensity of white mold in bean plants. With the exception of Calcarea carbonica at 12 CH and 24 CH, no other medicine reduced the percentage of plant death due to white mold disease.
Key words: Homeopathy, alternative control, Sclerotinia sclerotiorum, Phaseolus vulgaris.
The common bean plant (Phaseolus vulgaris L.) is one of the most important edible legumes, and is distributed from the tropics to the temperate zones across the five continents. It is also one of the most important components of the Brazilian diet and represents the main source of income for a considerable number of farmers (Borém and Carneiro, 2008).
In Brazil, one of the main causes for the low yield of bean plants is diseases, they lower the physiological, sanitary, nutritional, and commercial quality of the product, thus limiting its production (Carneiro et al., 2015). Among these diseases is white mold. It affects large areas of bean production in Brazil, causing losses in excess of 50% (Meyer and Campos, 2009).
The fungus,
Sclerotinia sclerotiorum that causes white mold. It is a polyphagous pathogen with over 300 host species belonging to approximately 200 botanical genera (Fancelli and
Neto, 2007). An adequate combination of methods must be used, whenever possible, to control it, adapting the available strategies to keep the pathogen population below the threshold of economic damage and, at the same time, minimizing negative effects on the environment (
Zambolin and Paula, 2008).
In this regard, alternative methods, such as homeopathy, may emerge as an innovative and effective practice to control diseases in plants. According to homeopathic science, the causes of illness in living systems are the suppressive procedures that act contrary to the vital principle, and once suppressive forces are internalized, symptoms are caused by the purging of everything that affects the vital equilibrium (Lisboa et al., 2005). This disequilibrium in the vital energy, when somatized, causes diseases or physiological disturbance in plants, which may lead to the death of plants or reduction in yield. However, when homeopathic medicine is applied, it leads to minimization of the harmful effects caused to the vital energy through biotic and abiotic factors (Bonato and Silva, 2003) and the restoration of equilibrium through the stimulation of the plant’s defense systems. Therefore, plants can resist diseases and pests, combat viruses, fungi, bacteria, and other types of agents with their own means (Lisboa et al., 2005). At present, the activity of homeopathic substances has already been verified in many types of biological systems and for many variables; biochemical, morphological, or physiological (Castro, 2013).
Thus, homeopathy emerges as an alternative for production systems, with a view toward preserving the ecological equilibrium of the cultivated plants (Casali, 2004). Hence, the goal of this study was to verify the control of white mold in bean plants by the use of the homeopathic medicines Phosphorus and Calcarea carbonica.
In Brazil, when the author has junior or grandson in name, if often use both last names, as is the case of Dourado Neto.
Two tests, using a completely randomized design, were conducted in a greenhouse. C. carbonica was used as the treatment in the first test and Phosphorus in the second. In both cases, the homeopathic medicines were in dynamizations of 6, 12, 24, 36, and 48 CH. As a control, distilled water and 30% hydroalcoholic solution was used, totaling seven treatments with five repetitions per test.
The homeopathic medicines were selected by means of repertorization using the program HomeoPro. Based on past studies (
Modolon et al., 2009 ; Toledo et al., 2009; Modolon et al., 2012), dynamizations of 0, 6, 12, 24, 36, and 48 CH were selected for this study.
The medicines were obtained from a homeopathic pharmacy in a dynamization of 6 CH and manipulated to 12, 24, 36, and 48 CH in accordance with the Brazilian Homeopathic Pharmacopoeia (Farmacopéia Homeopática Brasileira 1997), diluting 1:100 and succussing 100 times. Next, the pluralist dilution proposed by Hahnemann was followed, where a flask was used for each dilution, and suction was applied in unidirectional, sequential, and vertical movements using a mechanical stirrer.
Bean seeds of the cultivar IPR Tuiuiu were disinfested in 70% alcohol for 1 min, in a sodium hypochlorite solution (3:1) for 2 min, and washed in running distilled water. Next, the seeds were placed equidistant inside Gerbox plates on three sheets of germitest paper moistened with autoclaved distilled water in the proportion of 2.5:1 (1 mL of water for every 2.5 g of germitest paper).
The bean seeds were kept in
biochemical oxygen demand (
BOD) incubators and removed for permanent planting after three days. Seedlings free of disease with the potential to develop into normal plants and with a complete and proportional radicle were selected for planting. Three-liter pots were used for planting, which contained a mixture of soil, sand, and organic material in the proportion of 2:1:1, which had been autoclaved at 120°C for 60 min to sterilize.
When the plants grew their first fully expanded trifoliate leaf (stage V2), a 7-mm diameter disk replete with
S. sclerotiorum mycelia were inoculated at the base of each plant. It was later covered with soil and straw to provide the fungus the required
conditions for development: Constant humidity and absence of light. The treatments were administered in the soil three days before inoculation, on the day of inoculation, and
3, 10, and 17 days after inoculation. They were applied to the soil at a concentration of
0.1%, based on other works (Damin et al., 2014; Sukul et al., 2006).
Daily measurements of the lesions caused by S. sclerotiorum were taken using a caliper as soon as the first symptoms appeared at the base of the plants, and ceased when the control plants were dead. The values for the area under the disease progress curve (AUDPC) for each treatment were obtained through the equation:
Where: AUDPC = area under the disease progress curve (adimensional); Yi and Yi+1 = size of lesion of the disease observed in two consecutive evaluations (cm); I = interval between two consecutive evaluations (days). The evaluation of plant mortality from white mold disease took place after the tests were completed when the number of dead plants per treatment was counted. The percentage of dead plants (PDP) was obtained through the equation:
Where: PDP = Percentage of dead plants for the treatment in question; NDP = Number of dead plants in the treatment; TNP = Total number of plants in the treatment.
The original data of PDP was transformed into √ ( x + 0.5). To analyze the data, an analysis of variance (ANOVA) was performed and, when relevant, regression analysis and the test of averages at a 5% probability of error were conducted using the statistical program GENES (Cruz, 2006).
In terms of the resistance-inducing activity of the homeopathic medicine C. carbonica, both the variables studied showed statistical significance (Table 1). For the AUDPC, only the 12 CH dynamization differed from the control treatment and was able to reduce the progression of the disease by 67%. Similarly, the dynamizations of 12 CH and 24 CH reduced plant mortality by 61%.
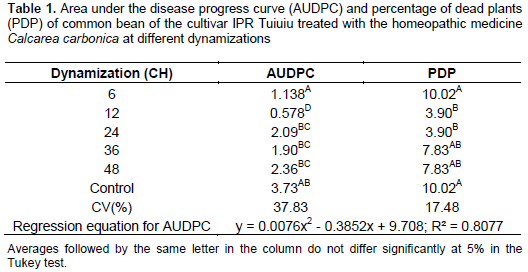
White mold is difficult to control in bean plants, furthermore, there are only
two registered and approved products with no restrictions for use in cultivation: Cantus®, Rovral®, Spot®, Trichodermax® e Unix® (SEAB, 2016). This complicates and at times even hinders the rotation of active ingredients; consequently, it elevates the resistance of the pathogen to them. Thus,
C. carbonica at 12 CH emerges as a promising alternative to control and reduce the severity of white mold in bean plants, as well as a tool for rural farmers to manage crops in an integrated and sustainable manner.
Furthermore, healing through homeopathy is based primarily on the
Law of Similars and o vitalism (Boericke, 2003). Thus, the cure from a disease, from the homeopathic point of view, occurs when the vital force that distinguishes living beings (both animals and plants) from inanimate beings is reestablished (Bonato, 2007). However, this healing goes beyond this. It is not enough for the symptoms of the disease to disappear. The manner in which producers manage their crops must change in order for bean plants to maintain their vital force and become less susceptible to the development of the disease (Boff, 2009).
We observed that the replicates of the control treatment showed 100% plant mortality, whereas those treated with C. carbonica 12 CH exhibited continuous resistance to the progression of the disease, emphasizing its role in resistance induction. Barros and Leonel (2013) observed a similar finding when using homeopathic preparations to control coffee rust (Hemileia vastatrix). They noted that after three months without applying any product, coffee rust on the crops was controlled, demonstrating that the residual effect of homeopathy is a result of the stimulus provided to plant metabolism.
On the other hand, plants treated with the same medicine with a dynamization of 6 CH exhibited grater disease severity than plants in the control treatment, and showed 100% plant death before the end of the evaluations. These results stress the importance of using various dynamizations in experiments, because the responses may vary according to the dynamization of the medicine under study (Bonato and Silva, 2003). In addition, because it acts on the vital energy, which represents a dynamic, immaterial principle, distinct from the body and integrating the totality of the organism, organizing all the physiological phenomena, the same medicine may apply to various organisms and distinct situations (Boff, 2009).
Fonseca et al. (2006), when evaluating the effect of a single application of C. carbonia on Porophyllum ruderale plants, observed an increase in the concentration of polyphenols in the plant leaves, demonstrating the resistance-inducing character of the homeopathic medicine. The C. carbonica preparation is also mentioned for its effects in inhibiting the production of ethylene in tomatoes, reducing the proportion of fruits for sauce, and increasing the proportion of colorful salad fruits.
In terms of area under the disease process curve, all the dynamizations of Phosphorus (
Table 2) differed statistically from the control treatment. Thus, there was a significant reduction in the severity of the disease, with a reduction of up to 78% in the progression of white mold. Regarding the percentage of dead plants, the analysis did not show a difference between the treatment and control.
In the tests performed, it was generally observed that the plants did not respond to the dynamizations tested in a linear manner. The same medicine that was capable of increasing the values of AUDPC and PDP also showed a suppressive effective with different dynamizations, such that the same medicine that proved to be innocuous for one variable was efficient for the same variable under a different dynamization.
The potential of a certain medicine and dynamization to activate plant defense mechanisms in only a few cases may be explained by the absence of similarities between the vital energy of the homeopathic solution and the organism, leading to disorder in the plant’s metabolic system and resulting in deterioration of plant development and growth. Thus, the lack of significance for some treatments in the control of white mold in bean plants may have occurred because of the dissimilarity between the vital energy of the medicine and/or the dynamization of the plant treated. Thus, as Bonato (2007) stated, taking into consideration that each substance has a different dynamic, it is important to emphasize that when conducting an experiment with plants, various dynamizations of the homeopathic medicine should be used. Otherwise, there is the risk of not achieving results, or even erroneously concluding that the homeopathic medicine is inefficient.
This study confirms that C. carbonica is effective in controlling the moth in common beans. C. carbonica at 6 CH and Phosphorus at 6 CH, 12 CH, 24 CH, 36 CH, and 48 CH reduced the intensity of white mold in bean plants. However, no other medicine reduced the percentage of plant death due to white mold disease with the exception of C. carbonica at 12 CH and 24 CH. However, this findings needs to be evaluated further for efficacy at higher doses and combinations of doses.
The authors have not declared any conflict of interests.
REFERENCES
|
Barros BHR, Leonel AH (2013). Use of homeopathic reparations to control coffee leaf rust (Hemileia vastratrix) in the region of Alta Mogiana. Resumos do VIII Congresso Brasileiro de Agroecologia. Cadernos Agroecol. 8:2.
View
|
|
|
|
Boericke WO (2003). Manual de Materia Medica Homeopatica [Compendium of homeopathic materia medica]. São Paulo: Ed. Robe. P 638.
|
|
|
|
|
Boff P (2009). Saúde Vegetal e a Contribuição da Homeopatia na Transição Ecológica da Agricultura. Rev. Bras. Agroecologia 4(2):3963-3966.
|
|
|
|
|
Bonato CM (2007). Homeopatia em modelos vegetais. Cultura Homeopática 2:24-28.
|
|
|
|
|
Bonato CM, Silva EP (2003). Effect of the homeopathic solution Sulphur on the growth and productivity of radish. Acta Sci. Agron. 25(2):259-263.
|
|
|
|
|
Borém A, Carneiro JES (2008). A cultura. In: Vieria C, Júnior TJ de P, Borém A. Feijão. Viçosa: Ed. UFV. P 600.
|
|
|
|
|
Carneiro JES, Paula Júnior TJ de, Borém A (2015). Feijão: do plantio à colheita. Viçosa: Ed. UFV. P 384.
|
|
|
|
|
Casali VWD (2004). Utilização da homeopatia em vegetais. In: Seminário Brasileiro Sobre Homeopatia Na Agropecuária Orgânica, 5., Toledo. Anais. Toledo. pp. 89-11.
|
|
|
|
|
Castro DM (2013). Homeopathy: principles and applications. In: International Conference on Homeopathy in Agriculture P 2.
|
|
|
|
|
Cruz CD (2006). Programa Genes: Biometria. Viçosa: UFV. P. 382.
|
|
|
|
|
Damin S, Alves LFA, Alexandre TM, Bonini AK, Bonato CM (2014). Homeopathic preparations on the activity of the entomopathogenic Beauveria bassiana (Bals.) Vuill. (Ascomycota: Cordycipitaceae). Rev. Bras. Agroecologia 9(3):41-53.
|
|
|
|
|
Fancelli AL, Dourado ND (2007). Produção de Feijão. 2 ed. Piracicaba: Os Autores. P 386.
|
|
|
|
|
Farmacopéia Homeopática Brasileira (1997). 2 ed, parte 1. São Paulo, SP: Atheneu Editora São Paulo.
|
|
|
|
|
Fonseca MCM, Casali VWD, Cecon PR (2006). Efeito de aplicação única dos preparados homeopáticos Calcarea carbonica, Kalium phosphoricum, Magnesium carbonicum, Natricum muriaticum e Silicea terra no teor de tânico em Porophyllum ruderale (Jacq.) Cassini. Rev. Cultura Homeopática 4(6):6-8.
|
|
|
|
|
Lisboa SP, Cupertino MC, Arruda VM, Casali VWD (2005). Nova visão dos organismos vivos e o equilíbrio pela homeopatia. Viçosa, MG. P 103.
|
|
|
|
|
Meyer MC, Campos HD (2009). Guerra ao mofo. Cultivar Grandes Culturas 120(11):16-18.
|
|
|
|
|
Modolon TA, Boff P, Rosa JM, Sousa PMR, Miquelluti DJ (2012). Qualidade pós-colheita de frutos de tomateiro submetidos a preparados em altas diluições. Hortic. Bras. 30(1):58-63.
Crossref
|
|
|
|
|
Modolon TA, Boff P, Boff MIC, Borghezan SF (2009). Preparados homeopáticos na produção de tomate em sistemas orgânicos. Rev. Bras. Agroecologia 4(2):702-705.
|
|
|
|
|
Secretaria da Agricultura e do Abastecimento do Paraná (SEAB) (2016). Agrotóxicos registrados para o controle de mofo branco em feijoeiro. Disponível em
View
|
|
|
|
|
Sukul NC, Ghosh S, Sukul A, Sinhababu P (2006). Amelioration of root-knot disease of lady's finger plants by potentizes Cina and Santonin. Homeopathy 95:144-147.
Crossref
|
|
|
|
|
Toledo MV, Stangarlin JR, Bonato CM (2009). Uso dos medicamentos homeopáticos Sulphur e Ferrum sulphuricum no controle da doença pinta preta em tomateiro. Rev. Bras. Agroecol. 4(2):475-478.
|
|
|
|
|
Zambolin L, Paula TJ de (2008). Doenças. In: Vieria C, Júnior TJ de P, Borém. Feijão. 2 ed. Viçosa: Ed. UFV. P 600.
|
|