ABSTRACT
Late season crops in planossoil are prone to waterlogging associated with high temperatures that are characteristic of the season, during brief periods of time in early summer. The aim of the present study was to evaluate growth, assimilate partitioning and seed vigor of bean plants subjected to periods of waterlogging and high temperatures during late season. Bean plants of the IPR Tuiuiú genotype were submitted to conditions of soil field capacity and to waterlogging for 8, 16 and 24 h. In order to obtain growth data, plants were collected in regular intervals of seven days until the end of the crop cycle, starting after sowing, dry matter content and leaf area were determined and used to estimate dry matter production, relative growth and net assimilation rates, leaf area index, solar energy conversion efficiency and organs’ dry matter partitioning. Seeds were collected at the end of the developmental cycle and used for seedling emergence test and evaluation of initial growth. The level of stress imposed by waterlogging and high temperatures is time dependent, paralyzing dry matter allocation in bean plants and reducing the conversion efficiency of solar energy. Seeds produced by plants under this stress present low vigor and reduced initial growth.
Key words: Phaseolus vulgaris L., stress, dry matter, physiological performance of seed.
Brazil, the world biggest common bean consumer, has Rio Grande do Sul as one of its states with highest per capita consumption. Common beans (Phaseolus vulgaris L.), belong to family Fabaceae, presenting an average lifecycle of 90 days (Ctsbf, 2010), at the Rio Grande do Sul State, the cultivated area of more than 3.3 million ha with an average productivity of 1026 kg ha-1 in the 2013/14 crop year (Conab, 2013). However, this state presents major climatic fluctuations, especially in relation with the quantity and distribution of pluvial precipitation. In such cases, sowing date, set according to the crops’ agro-ecological zoning, is anticipated or delayed by thefarmers (Ribeiro et al., 2008; Conab, 2013).
The stress imposed by soil water saturation causes morpho-anatomical and physiological alterations in plants. In those conditions, maize plants tend to reduce the number of leaves, dry matter accumulation (Coelho et al., 2013) and leaf expansion, and to induce leaf abscission (Bailey-Serres and Voesenek, 2008). Yet, in common bean plants there is an imbalance on the production and distribution of photoassimilates, due to an decrease in the conversion efficiency of solar energy (Didonet and Silva, 2004). In common beans, the excess of water during flowering may produce losses up to 60% in seed production (Silva et al., 2006).
In flooded soil regions, gas diffusion is hampered (Jackson and Colmer, 2005) reducing oxygen levels (Fries et al., 2007). Those conditions cause the diversion from the aerobic to anaerobic metabolism which reflects on low energy yields (Kolb and Loly, 2009); this metabolic modification induces the increase in the synthesis of enzymes that utilize pyruvate as the substrate to produce lactate and ethanol (Shingaki-Wells et al., 2011) leading to prejudice on root growth and metabolism (Amarante et al., 2007) and reducing nutrient absorption by roots (Pires et al., 2002), negatively affecting morphology and growth of various species.
High air temperatures also represent a limiting factor for commercial exploitation of beans (Junior et al., 2007). Consecutive periods under high temperatures may lead to damage on crop development (Barbano et al., 2001), chiefly on flowering and fructification, being a decisive factor on production due to its influence on flower abortion and pod formation (Didonet, 2002).
Late-season crops, in January and February, have shown growth trend on small farms, seeking for higher incomes. However, cultivations performed during this period are prone to soil waterlogging during brief periods of time, water saturation due to high pluvial precipitation, primarily on regions of planossoil with a B textural horizon (Streck et al., 2008). Furthermore, high temperatures, common of the season, may negatively affect the crop and the performance of vegetal growth, which is a major consideration for high-quality seed production.
Given the aforementioned, the work intents to analyze growth, assimilate partitioning and vigor of seeds from common bean plants subjected to periods of soil waterlogging in late-season cultivation.
The experiment was performed in a chapel greenhouse, with a north-south orientation, in an experimental area of the Phytotechny Department at Universidade Federal de Pelotas – Capão do Leão, RS. The region presents temperate climate with well distributed rainfall and hot summer, Cfa type by Köppen’s climate classification. Bean seeds cv. IPR Tuiuiú were placed to germinate in black polyethylene pots with 12 L capacity containing substrate soil collected from an A1 horizon characterized as a Haplic Eutrophic Solodic Planos soil, previously amended according to soil analysis and based on the “Fertilization and liming manual for the states of Rio Grande do Sul and Santa Catarina” (Cqfs RS/SC, 2004).
The plants were cultivated from January to March, 2014, which is the late-season for this crop. According to the climate normal for the period of 1971 to 2000, the mean values of maximum air temperature are 28.2, 27.9 and 26.9°C for January, February and March, respectively (latest data for the region). During the course of the experiment, the mean maximum temperatures were 30.5, 29 and 26.2°C for the respective aforementioned months. Yet, temperature data obtained by a thermometer located inside the greenhouse indicate mean maximum temperature values of 39°C during the period of the evaluations.
In the V4 growth stage (Ctsbf, 2010) the periods of soil waterlogging were applied, corresponding to eight, 16 and 24 h of waterlogging. An additional treatment consisted of the maintenance of soil field capacity, which was determined using tension table equipment (Embrapa, 1997). The waterlogging was performed providing a water level of 20 mm aboveground by fitting each pot inside a second pot with no perforations, preventing soil aeration and gas exchange. For drainage, these external pots were later removed allowing drainage until the field capacity.
In order to obtain primary growth data of leaf area and dry matter content, successive harvests were performed according to a seven day regular intervals alongside the whole crop cycle. In every collection, plants were cut at ground level, split into plant parts (leaf, shoot, roots and pods, if present) and accommodated separately kraft paper envelops.
Dry matter was obtained using four repetitions during each collection time and treatment; the samples were later transferred to a force ventilation oven, at the temperature of 70+2°C for 72 h.
The leaf area (AL) was determined using the equipment Licor model LI-3100 and leaf area index (LAI) calculated by the formula: LAI=AL/St, where St corresponds to the superficial area occupied by the plant. Primary data of total dry matter accumulated (Wt) were adjusted by the simple logistic equation, Wt=Wm/(1+Ae–Bt), where Wm stands for maximum growth asymptotic estimate, A and B represent the constants, e is the base of the Naperian logarithm and t the time in days after transplant. Leaf area primary data were adjusted using orthogonal polynomials (Richards, 1969). The instantaneous value for the rate of dry matter production (Ct) was obtained from the derivative of the fitted equation of total dry matter (Radford, 1967). In order to obtain the instantaneous values for relative growth (Rw) and net assimilatory (Ea) rates, the equations Rw=1/Wt.dW/dt and Ea=1/Af.dW/dt were employed, accordingly to Radford (1967).
Conversion efficiency of solar energy (ξ) was determined from the equation ξ=(100.Ct.δ)/Ra, where Ra is the mean value of incident radiation (cal m-² dia-1) fourteen days before the corresponding Ct, registered using a pyrometer and δ is the calorific value of 3800 cal g-1 cited by Cuesta et al. (1995). Assimilate partitioning between different plant organs (root, shoot, leaves and pods) along plant development were determined, separately, through measurement of the mass allocated in each plant structure followed by data transformation to percentage.
At the end of the crop cycle, seed harvest was performed for use in seedling emergence test which was accomplished using eight subsamples of 50 seeds each treatment. Every repetition was disposed to germinate in black polyethylene trays filled with the soil previously detailed and held at soil field capacity at the greenhouse. The following variables were assessed:
Seedling emergence at 21 days from sowing date
Emergence speed index, obtained by daily count of emerged
Seedlings, accordingly to Nakagawa (1994).
At the end of the seedling emergence test, leaf area and dry matter of leaf, shoot and root were evaluated by measurement of 10 seedlings each sample. The experimental design was completely randomized with three repetitions, factorial scheme 4 x 10 (waterlogging period and harvest times), performing weekly randomization. Data concerning seedling emergence, emergence speed index, leaf area and organ’s dry matter were subjected for analysis of variance and, when F-statistic was significant, Tukey-test was applied at 5% probability level. Primary data of total dry matter, leaf area and dry matter of leaves, roots, shoot and pods were subjected to analysis of variance, wherein data referring to growth analysis, were submitted to the simple logistic regression (Radford, 1967).
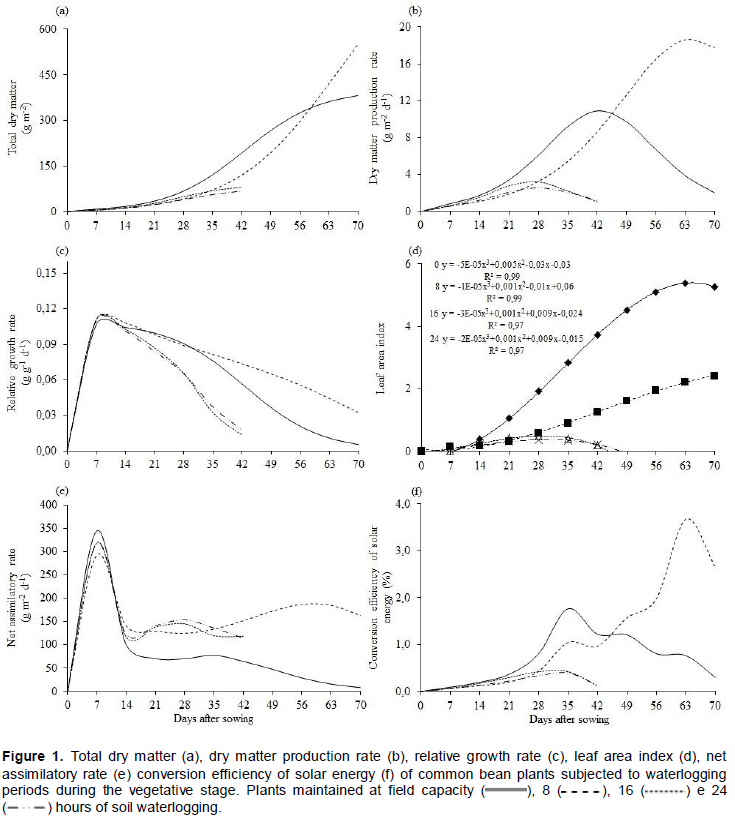
Total dry matter production (Wt) of common bean plants at different waterlogging periods fitted the logistic tendency with a coefficient of determination higher than 80% (Figure 1a). The growth was slow until 21 days after sowing (DAS) and presented maximum growth at the end of the crop cycle (70 DAS).
Plants cultivated under field capacity conditions or 8 h of waterlogging showed increasing growth up to the 70 DAS. However, maximum dry matter accumulation occurred at the 42 DAS for plants subjected to 16 and 24 h of soil waterlogging, presenting loss of vitality after this period. The difference in the allocation of Wt on plants exposed to periods of 16 and 24 h comparatively to other periods can be related to the high temperatures that occurred during the crop cycle, leading to stoppage of growth and premature plant death (Figure 1a). Temperature values above 27°C are harmful to plants under the effect of soil waterlogging (Lizaso et al., 2001).
Maximum dry matter production rate (Ct) was achieved at 42, 63, 27 and 28 DAS in plants maintained at field capacity and under waterlogging for 8, 16 and 24 h, respectively (Figure 1b). Plants subjected 8 h of waterlogging presented a higher Ct, corroborating with the higher Wt (Figure 1c) and demonstrating that plants under waterlogging reduce the metabolism during and after the stress, extending crop cycle.
Relative growth rate (Rw) presented maximum values at early development (seven DAS) with later systematic decrease until the end of common bean crop cycle (Figure 1c). The high initial Rw can be attributed to a high photosynthetic capacity of young leaves and later decrease, due to auto-shadowing, caused by the development of new leaves (Lopes et al., 1986).
Throughout the cycle, greater values of Rw were found in plants subjected to 8 h of waterlogging, followed by plants kept at field capacity (Figure 1c), possibly due to the occurrence of soil waterlogging causing a greater stagnation of dry matter increment relative to the preexisting. However, in plants under longer periods of soil waterlogging (16 and 14 h), Rw values decreased quickly and prematurely. Even though different genotypes present different responses regarding Rw, it is worth mentioning that under temporary waterlogging, three varieties and four accessions of Panicum maximum Jacq. Presented lower relative growth rates (Silva et al., 2009).
Leaf area index (LAI) presented high coefficient of determination (R2 ≥ 0.97) and, at 63 and 70 DAS, the maximum value was reached in plants maintained at soil field capacity and under the effect of 8 h of soil waterlogging, respectively (Figure 1d). Nevertheless, plants that had 16 or 24 h of soil waterlogging, the maximum LAI was verified at 28 DAS (Figure 1d). The decrease, after the peak, is the result of an increase on leaf senescence and alterations of hormonal balance (Moura et al., 2008).
The maximum values of net assimilatory rate (Ea) were obtained at early vegetative growth (7 DAS) with secondary peaks at 35 and 56 DAS for plants maintained at field capacity, and under the effect of 8 h of waterlogging, respectively (Figure 1e). At the early development, Ea tends to be higher (Gondim et al., 2008), the later decrease is associated with auto-shadowing and the second peak, results from the initiation of the reproductive stage (Lopes et al., 1986).
The reduction of photosynthetic rate, stomatal conductance and water absorption by roots (Folzer et al., 2006) can result in low photoassimilate production at the leaves and consequent low translocation to other plant parts (Parent et al., 2008). Such aspects may have been affected by the combination of soil waterlogging and high temperature, leading to stoppage of carbon fixation and growth, followed by premature death of common bean plants.
The curves for conversion efficiency of solar energy (ξ) presented different trends according to the treatment (Figure 2f). The maximum values of ξ were 1.75% for plants at field capacity, 0.43 and 0.40 % for plants subjected to 16 and 24 h of soil waterlogging, at the 35 DAS. However, for plants subjected to eight hours of soil waterlogging, the maximum ξ was 3.70% at the 63 DAS.
Up to 42 DAS the maximum values were obtained at zero and 8 h of soil waterlogging, respectively. The ξ increased along with the maximum obtained for Ct (Figure 1d) and solar radiation (Figure 1b). Similar results were reported for soybean plants (Marenco and Lopes, 1998) and for common bean plants subjected to different nitrogen sources (Cuesta et al., 1995).
Dry matter partitioning was modified along the developmental stages of common bean plants (Figure 2). At field capacity, a greater dry matter allocation was observed on leaves up to the 42 DAS, followed by shoot and roots (Figure 2a). The accumulation of dry matter atpods started at 49 DAS, modifying preferential metabolic sink which reduced the allocation on leaves and roots. However, the period of 8 h of waterlogging extended the period of dry matter accumulation up to 49 DAS. After this period, accumulation on pods took place, resulting in diminution of accumulation on leaves and roots (Figure 2b).
The responses of dry matter partitioning were similar on plants under 16 and 24 h of soil waterlogging (Figures 2c and 2d) ceasing the accumulation of dry matter at the 42 DAS, and without forming pods. Thus, the results obtained in this work evidence that extending the period of waterlogging associated with high temperatures leads to stoppage of growth and premature death on bean plants.
The differences observed on dry matter allocation among plant organs, at the periods of zero and 8 h of waterlogging, can be associated with the fact that waterlogging reduces the production and translocation of photoassimilates from leaves to roots (Yordanova et al., 2004). Nevertheless, the absence of oxygen required to reduce sugars by the glycolytic pathway, may have been a limiting factor for survival when periods of 16 and 24 h of waterlogging were used (Parent et al., 2008a).
The increase in the period of soil waterlogging tends to reduce dry matter accumulation of shoot and roots in plants of the Poaceae family, and the magnitude of the reduction is dependent of the genotype (Silva et al., 2009). Cultivation conditions under high air temperatures reduce the number of pods per plant, seeds per pod and seed mass, which negatively affects the yield (Aidar et al., 2002).
The results of seedling emergence (E), emergence speed index (ESI), leaf area (AL), dry matter of leaves (WL), shoot (WS) and roots (WR) are presented on Table 1. Plants subjected to 16 and 24 h of waterlogging did not developed pods. Thus, the seedling emergence test, represented by the variables emergence and emergence speed index were performed solely using seeds originated from plants subjected to field capacity or 8 h of soil waterlogging.
Seedling emergence was affected by soil waterlogging, in which the plant was maintained, resulting in seeds with a lower percentage of emerged seedlings and emergence speed index. The same was observed to leaf area and for dry matter of leaves (WL), shoot (WS) and roots (WR).
Lowers values for emergence and emergence speed index demonstrate the decrease on vigor of common bean seeds collected in mother plants subjected to soil waterlogging. Since seed vigor is related to stresses occurred directly or indirectly on the seed, any stress during vegetative or reproductive stage affects seed quality. Therefore, vigor decrease is related to the environmental stress imposed, affecting the biosynthesis of storage compounds, the formation of cellular membranes and probably, enzymatic mechanisms involved in the hydrolysis and translocation of reserves from the cotyledons to the embryo, during the resumption of growth (Peske et al., 2012). This sense, the decrease of seed vigor, imposed by soil waterlogging, reflected in poor development of seedlings, reducing dry matter of shoot, roots and leaves and additionally, the leaf area.
A reduced leaf area imposes a smaller area for capturing radiant energy, which can affect the photosynthetic process due to reduction of absorbed radiation (Aumonde et al., 2011). Under conditions of soil hypoxia, the low energy production caused by photosynthetic reduction (Alaoui-Sossé et al., 2005) leads to the depletion of carbohydrate reserves and total protein synthesis (Parent et al., 2008).
The analysis of variance of primary data of growth is presented on Table 2. Analyzing the mean squares for leaf area and organs’ dry matter, high level of significance (0.1%) between waterlogging conditions and harvest times was noticeable. Different aspects related to management, the use of water and soil have been studied with a view to maximizing the use of agricultural agro-ecosystems (Valipour, 2013), as irrigation efficiency (Valipour, 2014a; Yannopoulos et al., 2015) and drainage systems (Valipour, 2014b). Thus, soil waterlogging causes low levels of oxygen in the roots, leading to fermentation with low energy production. In this work, it was found lower allocation of dry matter and reduced vigor of bean seeds produced under soil waterlogging.
Thereby, it was observed that growth and seed quality of common beans can be negatively affected according to environmental conditions. Thus, the selection of more propitious areas and proper management regarding cultivation conditions are paramount.
The increase of soil waterlogging periods on late-season provokes alterations on growth and assimilates partitioning of common bean plants, delaying the attainment of the second peak of net assimilation rate. Soil waterlogging and high temperatures cause decrease in conversion efficiency of solar energy and seed vigor of the aforementioned specie.
The authors have not declared any conflict of interests.
REFERENCES
|
Aidar H, Silvia SC, Kluthcouski J, Thung M (2002). Sistema de produção do feijoeiro comum em várzeas tropicais: época de plantio. Santo Antonio de Goiás, Embrapa. 4p. (Circular Técnica, 55).
|
|
|
|
Alaoui-Sossé B, Gérard B, Binet P, Toussaint ML, Badot PM (2005). Influence of flooding on growth, nitrogen availability in soil, and nitrate reduction of young oak seedlings (Quercus robur L.). Ann. For. Sci. 62(6):593-600.
Crossref
|
|
|
|
|
Amarante L, Colares DS, Oliveira ML, Zenzen IL, Badinelli PG, Bernardi E (2007). Teores de clorofilas em soja associada simbioticamente com diferentes estirpes de Bradyrhizobium sob alagamento. Rev. Bras. Biocienc. 5:906-908.
|
|
|
|
|
Aumonde TZ, Lopes NF, Moraes DM, Peil RMN, Pedó T (2011). Análise de crescimento do híbrido de mini melancia Smile® enxertada e não enxertada. Interciencia 36(9):677-681.
|
|
|
|
|
Bailey-Serres J, Voesenek LACJ (2008). Waterlogging stress: acclimations and genetic diversity. Annu. Rev. Plant. Biol. 59:313-339.
Crossref
|
|
|
|
|
Barbano MT, Brunini O, Wutke EB, Castro JL, Gallo PB, Kanthack RAD (2001). Comparação entre valores observados e estimados de duração dos diferentes subperíodos de desenvolvimento da cultura do feijoeiro. Rev. Bras. Agrometeorologia, Santa Maria 9(1):103-110.
|
|
|
|
|
Coelho CCR, Neves MG, Oliveira LM, Conceição AGC, Okumura RS, Oliveira Neto CF (2013). Biometria em plantas de milho submetidas ao alagamento. Rev. Agroecossistemas 5(1):32-38.
|
|
|
|
|
Conab (2013). Companhia Nacional de Abastecimento. Acompanhamento de safra brasileira de grãos, 1(2).
|
|
|
|
|
Cqfs (2004). Manual de adubação e calagem para os Estados do Rio Grande do Sul e Santa Catarina. Sociedade Brasileira de Ciência do Solo. Comissão de Química e Fertilidade do Solo. 10 ed. Porto Alegre. P 400.
|
|
|
|
|
Ctsbf (2010). Comissão Técnica Sul-Brasileira de Feijão. Informações técnicas para o cultivo do feijão na Região Sul brasileira 2009. Epagri, Florianópolis. P 164.
|
|
|
|
|
Cuesta RR, Lopes NF, Oliva MA, Franco AA (1995). Crescimento e conversão da energia solar em Phaseolus vulgares L. em função da fonte de nitrogênio. Rev. Ceres. 42(242):405-422.
|
|
|
|
|
Didonet AD (2002). Caracterização das respostas da cultivar de feijão comum BRS Valente ao choque térmico com altas temperaturas. Comunicado Técnico Embrapa Arroz e Feijão. P 4.
|
|
|
|
|
Didonet AD, Silva SC (2004). Elementos climáticos e produtividade do feijão. Info. Agropecu. 25(223):13-19.
|
|
|
|
|
Embrapa (1997). Empresa Brasileira de Pesquisa Agropecuária, Centro Nacional de Pesquisa de Solos. Manual de métodos de análise de solo. 2 ed. ver. atual. Rio de Janeiro: Embrapa/Cnps. P 212.
|
|
|
|
|
Folzer H, Dat J, Capelli N, Rieffel D, Badot PM (2006). Response to waterlogging of sessile oak: An integrative study. Tree Physiol. 26(6):759-766.
Crossref
|
|
|
|
|
Fries DD, Alves JD, Filho ND, Magalhães PC, Goulart PFP, Magalhães MM (2007). Crescimento de plântulas do milho saracura e atividade da α-amilase e invertases associadas ao aumento da tolerância ao alagamento exercido pelo cálcio exógeno. Bragantia 66(1):1-9.
Crossref
|
|
|
|
|
Gondim ARO, Puiatti M, Ventrella MC, Cecon PR (2008). Plasticidade anatômica da folha de taro cultivado sob diferentes condições de sombreamento. Bragantia 67(4):1037-1045.
Crossref
|
|
|
|
|
Jackson MB, Colmer TD (2005). Response and adaptation by plants to waterlogging stress. Ann. Bot. 96(4):501-505.
Crossref
|
|
|
|
|
Junior LH, Ribeiro ND, Da Rosa SS, Jost E, Poersch NL, Medeiros SLP (2007). Resposta de cultivares de feijão à alta temperatura do ar no período reprodutivo. Cienc. Rural 37(6):1543-1548.
Crossref
|
|
|
|
|
Kolb RM, Joly CA (2009). Waterlogging tolerance of Tabebuia cassinoides: Metabolic, morphological and growth responses. Flora 204:528-535.
Crossref
|
|
|
|
|
Lizaso JI, Melendez LM, Ramirez R (2001). Early waterlogging of two cultivars of tropical maize. I. Shoot and root growth. J. Plant. Nutr. 24(7):979-995.
Crossref
|
|
|
|
|
Lopes NF, Oliva MA, Cardoso MJ, Gomes MMS, Souza VF (1986). Crescimento e conversão da energia solar em Phaseolus vulgares L. submetido a três densidades de fluxo radiante e dois regimes hídricos. Rev. Ceres 33(183):142-164.
|
|
|
|
|
Marenco RA, Lopes NF (1998). Solar radiation conversion efficiency and growth of soybean plants treated with herbicides. Rev. Ceres 45(259):265-275.
|
|
|
|
|
Moura EG de, Albuquerque JM, Aguiar ACF (2008). Growth and productivity of corn as affected by mulching and tillage in alley cropping systems. Sci. Agric. 65(2):204-208.
Crossref
|
|
|
|
|
Nakagawa J (1994). Testes de vigor baseados na avaliação das plântulas. In: Vieira RD, Carvalho NM (Eds.). Testes de vigor em sementes. Jaboticabal: FUNEP. pp. 49-85.
|
|
|
|
|
Parent C, Berger A, Folzer H, Dat J, Crèvecoeur M, Badot PM, Capelli N (2008a). A novel nonsymbiotic hemoglobin from oak: cellular and tissue specificity of gene expression. New Phytol. 177(1):142-154.
|
|
|
|
|
Parent C, Capelli N, Berger A, Crèvecoeur M, Dat JF (2008). An overview of plant responses to soil waterlogging. Plant Stress 2(1):20-27.
|
|
|
|
|
Peske ST, Villela FA, Meneguello GE (2012). Sementes: Fundamentos Científicos e Tecnológicos, 3 ed. P. 573.
|
|
|
|
|
Pires JLF, Soprano E, Cassol B (2002). Adaptações morfofisiológicas da soja em solo inundado. Pesqui. Agropecu. Bras. 37(1):41-50.
Crossref
|
|
|
|
|
Radford PJ (1967). Growth analysis formulae: their use and abuse. Crop Sci. 7:171-175.
Crossref
|
|
|
|
|
Ribeiro ND, Antunes IF, Souza JF de, Poersch NL (2008). Adaptação e estabilidade de produção de cultivares e linhagens-elite de feijão no Estado do Rio Grande do Sul. Cienc. Rural 38:2434-2440.
Crossref
|
|
|
|
|
Richards FJ (1969). The quantitative analysis of growth. In: Stewward FC (Ed). Plant Physiology. A treatise. New York: Academic press. pp. 3-76.
Crossref
|
|
|
|
|
Shingaki-Wells RN, Huang S, Taylor NL, Carroll AJ, Zhou W, Millar AH (2011). Differential molecular responses of rice and wheat coleoptiles to anoxia reveal novel metabolic adaptations in amino acid metabolism for tissue tolerance. Plant Physiol. 156(4):1706-1724.
Crossref
|
|
|
|
|
Silva AS, Laura VA, Jank L (2009). Soil flood tolerance of seven genotypes of Panicum maximum Jacq. Braz. Arch. Biol. Technol. 52(6):1341-1348.
Crossref
|
|
|
|
|
Silva VR, Reichert JM, Reinert DJ (2006). Variação na temperatura do solo em três sistemas de manejo na cultura do feijão. Rev. Bras. Cienc. Solo 30(3):391-399.
Crossref
|
|
|
|
|
Streck EV, Kämpf N, Dalmolin RSD, Klamt E, Nascimento PC, Schneider P, Giasson E, Pinto LFS (2008). Solos do Rio Grande do Sul. 2 ed. Porto Alegre: Emater/RS; UFRGS. P 222.
|
|
|
|
|
Valipour M (2013). Comparison of different drainage systems usable for solution of environmental crises in soil. In: The 1st International Conference on Environmental Crises and its Solutions, Kish Island, Iran.
|
|
|
|
|
Valipour M (2014a). Pressure on renewable water resources by irrigation to 2060. Acta Adv. Agric. Sci. 2(8):23-42.
|
|
|
|
|
Valipour M (2014b). Handbook of drainage engineering problems. Published by OMICS Group eBooks, Foster City. P. 126.
|
|
|
|
|
Yannopoulos SI, Lyberatos G, Theodossiou N, Li W, Valipour M, Tamburrino A, Angelakis NA (2015). Evolution of water lifting devices (Pumps) over the centuries worldwide. Water 7:5031-5060.
Crossref
|
|
|
|
|
Yordanova R, Christov K, Popova L (2004). Antioxidative enzymes in barley plants subjected to soil waterlogging. Environ. Exp. Bot. 51(2):93-101.
Crossref
|
|