ABSTRACT
Management techniques that can assist in treating only parasite infested animals can reduce anthelmintic resistance arousing from frequent dosing of all animals. This study aimed to investigate the application of FAMACHA© with no formal training of resource-poor farmers to identify anaemic or helminthes infested animals. Two sites (KwaMthethwa (KM) village and Owen Sitole College of Agriculture (OSCA) farm) with 40 animals each of mixed sex were used in this study. The animals grazed on natural pasture during the day and housed in Kraal at night. FAMACHA© chart scored animals on eye colour; 1 (Red, non-anaemic and acceptable), 2 (red-pink, non-anaemic and border line), 3 (pink, mildly anaemic and dangerous), 4 (pink-white, anaemic and fatal) or 5 (porcelain white, severely)). Eye scoring was over four seasons (autumn, winter, spring and summer) alongside faecal egg count (FEC) as a positive control to FAMACHA© diagnosis method. Anaemic animals varied (P<0.05) between the sites, 76.72, 40.34 and 49.37% for KM, OSCA and KM+OSCA, respectively. Comparison of FAMACHA© with FEC showed that only 61.53, 33.5 and 40.12% of the animals were anaemic at KM, OSCA and KM+OSCA, respectively, due to false positive results. Spring, autumn and summer were identified as seasons for frequent monitoring due to higher (P<0.05) gastrointestinal parasite especially Trichostrongylus axei and Moniezia expansa. Approximately 80% of all anaemic animals were identified by no skilled resource-poor farmers using FAMACHA©. This reinforces the economic (cheap) importance and reliability of FAMACHA© chart in parasite resistance management but emphasized on formal training as 20% false negative anemic animals is a lot for a resource-poor farmers.
Key words: FAMACHA©, Anthelmintic, resistance, faecal egg count, Nguni goat.
The world’s goat population is estimated to be 876 million with 276 million coming from Africa where about 6.2 million is produced by South Africa according to the Food and Agriculture Organization of the United Nations Statistical database (
FAOSTAT, 2011). However, the goat population in South Africa has been declining (from 2008 (6.5 million), 2009 (6.4 million), 2010 (6.3 million) to 2011 (6.2 million)) which is a major concern especially to the rural communities where it serves as a major source of income or protein supply in diets (FAOSTAT, 2011). Therefore, it is possible to postulate that by 2025 the goat population will be less than 5 million in South Africa. These are significant and alarming statistics especially with the growing demand for animal protein (Mmbengwa et al., 2008) by the ever increasing human population (48.6 (2008) to 51.8 (2011) million people) in South Africa (STATSA, 2011). No specific reason has been associated to the decrease in goat production though different researchers have advanced the following reasons; low research on goats as their contribution towards formal national economy is assumed to be less, lack of etiology of diseases (internal and external) prevalence, inaccessibility of conventional knowledge and information among small-scale farmers, minimal resources (land and capital), poor food security and informal labour. Goat production is bound to decline without any intervention especially among the small scale farmers in South Africa who owns about 50% of the total goats population (Shabalala and Mosima, 2002). KwaZulu-Natal has the second largest human population (10.3 million) and the third largest goat population in South Africa, with the indigenous Nguni breed as majority (Slippers et al., 2006). This breed is preferred by most resource-poor farmers because of its resistance to some diseases and parasites, high conception rates, lower mortality rate, hardiness, adaptability and good mothering ability (Barry and Godke, 2001). Though the Nguni breed is superior over the other goat species, health issues such as worms, diarrhea, poor condition, external parasites, fertility, abortion, eyes problems are still a major task to eradicate.
Infection of the gastrointestinal tract by helminthes (nematodes) has been coined as a major limiting factor in goat production particularly among resource-poor farms (Hoste et al., 2002). Anthelmintic resistance on both commercial and some resource-poor small ruminant farmers has been reported in South Africa (Spickett et al., 2012; Van Wyk and Bath, 2002; Vatta et al., 2002). However, anthelmintic resistance prevalence has been reported to be higher in goats than in sheep (Hoste et al., 2002). Therefore alternative methods for worm management in other to reduce worm resistance are vital as goat production is no longer cost effective (millions of Rands are spent on drugs that parasite population has
already developed resistance). The treatment of only sick animals has been suggested as it favours unselected worms coming from untreated animals (Wyk and Bath, 2002). FAMACHA© system (grading animal according to their anaemic level) and body condition scores has been used by trained participants to identify sick (infested with Haemonchus contortus) animals (Spickett et al., 2012; Van Wyk and Bath, 2002). The results demonstrated benefits such as reduction in treatments, lower selection pressure on helminthes for anthelmintic resistance and discriminate animals with variable ability to cope with infection but demanded more work on the application of FAMACHA© chart on goats (Leask et al., 2012). Though some of the resource-poor farmers are gradually getting access to anthelmintic drugs (subsidized) due to government intervention, wastage is still a major problem as all animals are often treated at once instead of selecting for sick animals. Management methods for sick animal identification such as FAMACHA© has shown 93% success after training (Reynecke et al., 2011) but can it work for resource-poor farmers without any formal training? This study aimed to use the FAMACHA© chart as a tool to control parasite infestation and to manage anthelmintic resistance in goats of resource-poor farmers at KwaMthethwa (KM) village and Owen Sitole College of Agriculture (OSCA) of the province of KwaZulu-Natal in South Africa. Fecal egg loads were counted as a positive control for the FAMACHA© diagnostic method (Quijada et al., 2012). It was hypothesized that no skill resource-poor farmers will not be able to manage sick animals on their farm with the FAMACHA© chart.
Experimental sites, animals, nutrition and sampling
The study sites were KwaMthethwa (KM) village and Owen Sitole College of Agriculture (OSCA), located at the north coast of KwaZulu-Natal Province, South Africa. KM is located at 28°31’S Latitude and 31°51’E Longitude while OSCA bearings are 28°45’S Latitude and 31°53’E Longitude both of which have an annual rainfall of 900 mm and an average temperature of 26°C. Forty Nguni goats in each area KM (belonging to four resource-poor farmers) and OSCA (owned by government for student research) were monitored (Mitchell, 1982). In both areas the animals were allowed to graze during the day on communal or natural pastures (under the supervision of a shepherd) and housed in kraal at night. Animal sampling was carried out monthly in four seasons; summer (October-January), autumn (February-April), winter (may-July), and spring (August-September) from 2012-2013. Sex (male and female), ages (between 1-4 years calculated from number of incisors (Mitchell, 1982) and Alba-Hurtado et al. (2010) ) and weight of goats were some of the independent variables considered. All animals were ethically treated as per the University of Zululand Ethical Committee research guide manual (S590904).
Depicting anaemia level by FAMACHA© chart
FAMACHA© chart is an eye colour based stratification method, with five colour categories of the conjunctival mucous membrane from bright red to pale as an indicator of anaemia (Malan and Van Wyk, 1992; Van Wyk and Bath, 2002). The lower eyelid mucous membrane of each goat were examined and compared to a laminated colour chart picture of eye with five different levels of anaemia and assigned a score of either 1 (Red, non-anaemic and acceptable), 2 (red-pink, non-anaemic and border line), 3 (pink, mildly anaemic and dangerous), 4 (pink-white, anaemic and fatal) or 5 (porcelain white, severely). Animals with scores of 3-5 were advised for treatment. FAMACHA© chart level rating at KM was done by the farmers while at OSCA it was done by the Shepard. All the farmers received equal advised from researcher on anaemia rating using FAMACHA© chart.
Determination of faecal egg counts per gram of faeces
Faecal egg count (FEC) was carried out to investigate any correlations with FAMACHA rating of animal’s health by resource-poor farmers. One gram of faeces collected from the rectum was dissolved in 40 ml of sugar solution and allowed to stand for five minutes. Faecal egg count was counted using a modified McMaster Technique because it estimates egg count per gram (EPG) of faeces (Hansen and Perry, 1994). The number of nematode eggs in both wells of the McMaster chamber was multiply by 133 ([40 ml ÷ (0.15 ml x 2) = 133]) to get EPG. Generally, 500 EPG has been suggested for treatment by many authors in order to decrease pasture contamination and prevent the development of subclinical diseases (Stephen et al., 2003). However, subclinical symptoms development would depends on a lot of factors such as animal’s genetic resistance, age, state of nutrition and specific worm species (Stephen et al., 2003). Although FAMACHA© chart is often linked to the wireworm (Haemonchus contortus) infection, these study considered total egg counts including wireworm when relating faecal egg counts to FAMACHA scores. Because pathogenicity count is greatly influenced by species type and number, total egg counts greater than 2000 were considered as potential anaemic counts.
Trichostrongylus axei and Moniezia expansa were monitored throughout the season by observing the diagnostic features of their eggs under the microscope as described by Thienpont et al. (1979). M. expansa identification was much easier due to its diagnostic features peculiarity whereas T. axei was a bit difficult due to its similarities with Strongyles spp under the microscope. Means of FEC and number of anaemic animals estimates were subjected to analysis of variance (ANOVA) using the general linear model of SAS (2002). Student Newman-Keuls’ test was used to compare means.
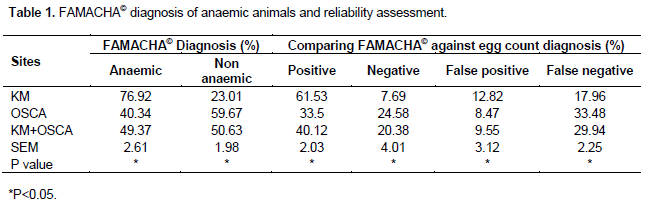
FAMACHA© scores showed that the number of anaemic animals were higher (P<0.05) in KM than at OSCA or KM+OSCA (Table 1). Non anaemic animals were lower (P<0.05) in KM than at OSCA and KM +OSCA. The results obtained from comparing clinical FAMACHA© scores to faecal egg counts showed that the percentage of anaemic animals were still higher in KM than at OSCA or combined sites. False negative results were prevalent (P<0.05) at OSCA than KM while false positive results were prevalent at KM when compared to OSCA.
Faecal egg count varied (P<0.05) between seasons with the highest counts recorded in spring and autumn, followed by summer and least in winter (Table 2). Animal sex had an effect on FEC as a higher number of eggs (P<0.05) were observed in males than in females (Table 2). The number of FEC was also influenced by age as the number of eggs increased (P<0.001) with animal age. The least number of FEC were observed in Nguni goats of 1 year and below while the highest were observed in goats of 5 years and beyond. Generally, FEC increased with increased in FAMACHA© scores but for the FAMACHA© score of 5 that was the same with 4.
The highest (P<0.05) number of T. axei and M. expansa eggs in Nguni goats were recorded in spring and summer for both KM and OSCA (Table 3). T. axei and M. expansa egg counts in Nguni goats were lower in winter than autumn for both KM and OSCA.
Most studies in the literature utilize people with formal or partial training on FAMACHA© chart (Leask et al., 2012; Papadopoulos et al., 2012) while this study used resource-poor farmers with no form of training, which is equivalent to buying off the counter. The reason behind this trial was based on the fact that formal training by Non-Governmental Organizations or local government programmes rarely reaches these farmers in the remote areas. Application of FAMACHA© chart by resource-poor farmers with no formal training was able to identify anaemic (sick) animals in this study which is similar to the results obtained by Ribeiro et al. (2012). The animals at KM were heavily infested with helminthes as shown by the higher number of anaemic animals compared to OSCA. The lower percentage of anaemic animals at OSCA was associated to frequent dosing when compared to KM. Animal identification results by FAMACHA© chart were similar to those obtained by other researchers but with either partial or formal trained staff (Van Wyk and Bath, 2002; Vatta et al., 2011). The correlation between FAMACHA© and FEC diagnosis was quite high with an R2 value of 0.91. When FEC was used as a positive control to FAMACHA© chart, it was found that 17.96, 33.48 and 29.94% of anaemic animals at KM, OSCA and KM+OSCA, respectively, were false negative. Though the numbers of false positive animals were lower compared to false negative, false negative is still worth improving as one dead animal is a lot to resource-poor farmers. The false negative and positive results were associated to no formal or very little training of the resource-poor farmers rather than reliability of the FAMACHA© chart. Even when formal training on FAMACHA© system has been used in trials, only 93% of anaemic animal identification has been achieved (Vatta et al., 2011). Therefore the result obtained in this study is important but can be improved by formal training of rural farmers. If only two people are properly trained in each community, they can pass on the technology easily rather than sending a few professionals who will not be able to reach the needy (Vatta et al., 2011). A high false positive result implies that animals that were not sick will be treated which comes at a price. Firstly, anthelmintic drug wastage on treating animals that are not sick and secondly anthelmintic resistance will be prevalent because of frequent dosing (Hoste et al. 2002). Under-dosing which promotes anthelmintic resistance may also be problematic in false negative animals because of incomplete treatment of parasite especially when a general deworming programme is being followed. Incomplete treatment implies exposure of parasites to low doses of anthelmintic drugs hence the development of parasite resistance (Degen et al., 1995; Jabbar et al., 2013).
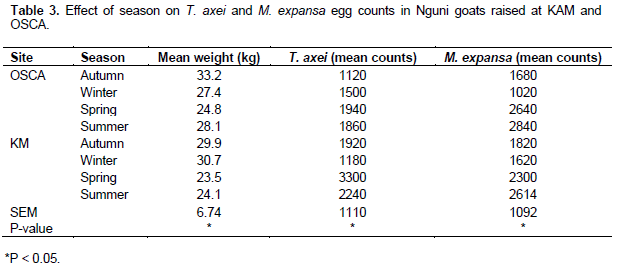
The results from FEC shows that autumn, spring and summer are the periods to constantly observe the animals for parasite infestation than winter. Similar studies emphasizing on frequent observation and treatment of sick animals during spring and autumn has also been reported as breeding conditions are suitable (Githigia et al., 2001; Reynecke et al., 2011). The incidence of helminthes in male and female goats were similar to those obtained by Asanji and Williams (1984). An explanation of male prevalence to anthelmintic over female goats where not clear but has been associated to the following factors; sex of the host, genetic composition, environmental factors, feeding food habits of the host or the host parasite interaction. It has also been observed that females are more susceptible to infection during pregnancy and peri-parturient period because of weaker immune system and stress (Dagnachew et al., 2011; Khan et al., 2010). FAMACHA© chart seasonal egg counts and T. axei and M. expansa egg counts per gram of sample also demonstrated that gastrointestinal parasitic infestation was prevalent in spring and summer than winter. Therefore animals should be frequently checked and treated for any helminthes infection during those periods. It is also worth mentioning that parasite infestation varied with age of animal with the least egg counts observed in the youngest animals. This was contrary to the results obtained in most literature where younger animals are more infested with helminthes due to weaker immune response (Dagnachew et al., 2011; Khan et al., 2010). The hypothesis was therefore rejected as anaemic animals were identified by farmers with no formal training.
It was concluded that FAMACHA© chart is a good and an economical tool to manage anthelmintic resistance by resource-poor farmers but can be a better management tool if strategies on formal training are properly outlined. Spring, autumn and summer were confirmed as the seasons for frequent checks on the conjunctival mucous membrane (helminthes infestation) to reduce pathogenicity of egg counts. Future studies on expanded projects with formal training of field workers that can educate more resource-poor farmers in the remote areas can assist in decreasing the number of false negative anaemic animals identified in this study.
The authors declare that they have no conflict of interest.
Authors would like to acknowledge the National Research Founder and University of Zululand for sponsoring the project and the farmers at KM village and OSCA for their contribution in data collection.
REFERENCES
Alba-Hurtado F, Romero-Escobedo E, Munoz-Guzman MA, Torres-Hernandez G, Becerril-Perez CM (2010). Comparison of parasitological and productive traits of Criollo lambs native to the central Mexican Plateau and Suffolk lambs experimentally infected with Haemonchus contortus. Vet. Parasitol. 172(3-4):277-282.
Crossref |
|
|
Asanji MF, Williams MO (1984). The effect of sex on seasonal variation in single and double infection of cattle in Sierra Leone by Dicrocoelium hospes and Fasciola gigantica. Vet. Parasitol. 15(3-4):247-55.
Crossref |
|
|
|
Barry DM, Godke RA (2001). The Boer Goat: The Potential for Cross Breeding. Louisiana State University, Baton Rouge, USA dnafrica@worldonlinecoza. |
|
|
Dagnachew S, Amamute A, Temesgen W (2011). Epidemiology of gastrointestinal helminthiasis of small ruminants in selected sites of North Gondar zone, Northwest Ethiopia. Ethiop. Vet. J. 15(2):57-68.
Crossref |
|
|
Degen AA, Becker K, Makkar HPS, Borowy N (1995). Acacia saligna as a fodder tree for desert livestock and the interaction of its tannins with fibre fractions. J Sci Food Agric. 68(1):65-71.
Crossref |
|
|
|
FAOSTAT (2011). Food and Agriculture Organisation of the United Nations Statistical database |
|
|
Githigia SM, Thamsborg SM, Munyua WK, Maingi N (2001). Impact of gastrointestinal helminths on production in goats in Kenya. Small Rumin Res. 42(1):21-29.
Crossref |
|
|
|
Hansen J, Perry B (1994).The epidemiology, diagnosis and control of helminth parasites of ruminants. (Handbook), International Laboratory for Research on Animal Diseases. ILRAD, Nairobi. |
|
|
|
Hoste H, Chartier C, Frileux YL (2002). Control of gastrointestinal parasitism with nematodes in dairy goats by treating the host category at risk. Vet Res. 33(5):531-545. |
|
|
Jabbar A, Campbell A, Charles J, Gasser R (2013). First report of anthelmintic resistance in Haemonchus contortus in alpacas in Australia. Parasit. Vectors 6(1):243.
Crossref |
|
|
Khan M, Sajid M, Khan M, Iqbal Z, Hussain A (2010). Gastrointestinal helminthiasis: prevalence and associated determinants in domestic ruminants of district Toba Tek Singh, Punjab, Pak. Parasitol. Res. 107(4):787-794.
Crossref |
|
|
Leask R, van Wyk JA, Thompson PN, Bath GF (2012). The effect of application of the FAMACHA (c) system on selected production parameters in sheep. Small Rumin. Res. 110(1):1-8.
Crossref |
|
|
|
Malan FS, Van Wyk JA (1992). The packed cell volume and colour of the conjunctivae as aids for monitoring Haemonchus contortus infestations in sheep. Biennial national veterinary congress. 1:139. |
|
|
|
Mitchell T (1982). How to tell the age of goats. Agfact A7. 2:Department of Agriculture, New South Walles, Australia. |
|
|
|
Mmbengwa V, Gundidza JRM, Fair M, du Toit J, Greyling J (2008). South African indigenous goat milk: a potential alternative source of macro-nutrients for poverty-stricken rural areas. Livest. Res. Rural. Dev. 20:121. |
|
|
Papadopoulos E, Gallidis E, Ptochos S, Fthenakis GC (2012). Evaluation of the FAMACHA (c) system for targeted selective anthelmintic treatments for potential use in small ruminants in Greece. Small Rumin. Res. 110(2-3):124-127.
Crossref |
|
|
|
Quijada J, Bethencourt A, Sulbaran D, Salcedo P, Aguirre A, Vivas I, Lopez E, Perez A (2012). Digestive Strongylids in Caprines: Fecal Egg Counts and FAMACHA (R) Score Values in a Naturally Infected Herd. Rev Cient-Fac Cien V. 22(5):418-425. |
|
|
Reynecke DP, van Wyk JA, Gummow B, Dorny P, Boomker J (2011). Validation of the FAMACHA© eye colour chart using sensitivity/specificity analysis on two South African sheep farms. Vet. Parasitol. 177(3–4):203-211.
Crossref |
|
|
Ribeiro VVL, Feitosa TF, Linhares EF, Rodrigues Athayde AC, Molento MB, Azevedo SS (2012). FAMACHA (c) method as an auxiliary strategy in the control of gastrointestinal helminthiasis of dairy goats under semiarid conditions of Northeastern Brazil. Vet. Parasitol. 190(1-2):281-284.
Crossref |
|
|
|
SAS (2002). Statiscal Analysis System user's guide (version 8). SAS Institude Inc., SAS CampusDrive, Cary, N.C., USA. |
|
|
|
Shabalala N, Mosima B (2002). Report on the survey of large and small scale agriculture. Statistics SA Library Cataloguing-in-Publication Pretoria: Statistics South Africa |
|
|
|
Slippers S, Letty B, De Villiers J (2006). Prediction of the body weight of Nguni goats. S Afr. J. An. Sci. 30(4):127-128. |
|
|
Spickett A, de Villiers JF, Boomker J, Githiori JB, Medley GF, Stenson MO, Waller PJ, Calitz FJ, Vatta AF (2012). Tactical treatment with copper oxide wire particles and symptomatic levamisole treatment using the FAMACHA© system in indigenous goats in South Africa. Vet. Parasitol. 184(1):48-58.
Crossref |
|
|
|
STATSA (2011). Statistics South Africa. |
|
|
|
Stephen L, Gareth H, Elizabeth M (2003). WormTest for livestock and guide to egg counts. |
|
|
|
Thienpont D, Rochette F, Vanparijs O (1979). Diagnosing helminthiasis through coprological examination. Janssen Res. Found. P. 187. |
|
|
Van Wyk JA, Bath GF (2002). The FAMACHA system for managing haemonchosis in sheep and goats by clinically identifying individual animals for treatment. Vet. Res. 33(5):509-529.
Crossref |
|
|
Vatta AF, de Villiers JF, Harrison LJS, Krecek RC, Pearson RA, Rijkenberg FHJ, Spickett A, Worth SH (2011). A framework for the transfer of animal health knowledge to rural goat owners. Small Rumin. Res. 98(1):26-30.
Crossref |
|
|
Vatta AF, Krecek RC, Letty BA, van der Linde MJ, Grimbeek RJ, de Villiers JF, Motswatswe PW, Molebiemang GS, Boshoff HM, Hansen JW (2002). Incidence of Haemonchus spp. and effect on haematocrit and eye colour in goats farmed under resource-poor conditions in South Africa. Vet. Parasitol. 103(1–2):119-131.
Crossref |
|
|
Wyk JAV, Bath GF (2002). The FAMACHA system for managing haemonchosis in sheep and goats by clinically identifying individual animals for treatment. Vet. Res. 33(5):509-529.
Crossref |