ABSTRACT
Various species and varieties of the genus Solanum (Solanaceae) are among the most important market crops produced worldwide. They may be considered as multipurpose crops since leaves and/or fruits are eaten fresh or cooked in various dishes. The increase of garden surfaces and the permanence of gardening have induced frequent outbreaks and diversification of pests and cryptogrammic diseases. In order to improve farmer’s capacities in controlling these constraints, the present study assessed diversity, abundance and incidence of fruit pests on Solanum spp. Data collection was conducted in two agroecological zones of southern Cameroon: Okola (Center Region) and Koutaba (Western Region), on three plant varieties from the genus Solanum: Solanum aethiopicum (African scarlet eggplant) with two varieties (jakatu and zong) and Solanum melongena (brinjal eggplant) var. inerme. The study aimed to (1) characterise the community of fruit pests associated with these plant varieties, (2) assess damage due to the main pests and (3) determine their impact on fruit yield. To achieve this, systematic sampling were done by visual observations in experimental gardens set up at the above cited sites. Harvests and incubations of infested fruits allowed identifying 15 insect species, belonging to three orders and 12 families: Lepidopterans with Leucinodes orbonalis, Cryptophlebia leucotreta, Helicoverpa armigera, Chrysodeixis chalcites and Hypolycaena phylippus), Dipterans with Batrocera (Batrocera) dorsalis, Ceratitis (Ceratitis) capitata, Ceratitis (Pterandrus) anonae, Neosilba sp., Atherigona sp. three unidentified species belonging to undetermined genera and Coleopterans with Diplognatha gagates and Formicomus sp.. Among them, H. phyllipus and C. chalcites were absent from Koutaba’s samples. Without site considerations, L. orbonalis was the most abundant with higher incidence on fruit production. At Okola, fruit losses on S. aethiopicum var. jakatu, on S. aethiopicum var. zong and on S. melongena var. inerme were 53.8-76.97, 43.65-61.51 and 29.42-46.61% respectively while at Koutaba, they were 49.51-68.57, 33.41-60.23 and 11.68-30.44%. The present study provided baseline data for integrated pest management strategies of Solanum in Cameroon.
Key words: Solanum, yield, fruit pests, L. orbonalis, damages.
The increase of market crop trade in national and sub-regional markets has led to intensification of gardening in various production basins in Cameroon. Among crops involved are various species of the genus Solanum (Solanaceae) that includes species of high nutritional and sociocultural values. Fruits and vegetables production in Cameroon provides 154 billion of CFA francs (representing about 3% of the country's GDP 1997/1998). Theses incomes constitute a great component in the fight against poverty (Temple, 2001). The gardening faces important threats among which the presence of insect pests that include mainly dipteran and lepidopteran fruit feeders. Eggs are laid on various parts of the plant for lepidopterans or under the fruit cuticle for dipterans. After hatching, larvae develop in the fruit pulp from which they leave at the pre-nymph stage to pupate in the soil. Because larvae feed and live inside the fruit, they cannot be effectively controlled by contact insecticides (Djiéto-Lordon and Aléné, 2002, 2006), while systemic insecticides are not appropriates for vegetables. In these conditions efficient control strategies may associates appropriates use of pesticides with other control technics such as biological control, mass trapping, or physical and biotechnology control technics in integrated pest management strategies. Implementation of these strategies needs a good knowledge of insects diversity in the ecosystem and the ecology of the pests. In this framework, the present study aimed to gather baseline data necessary for improvement of pest control strategies, the present study aimed to characterize fruit pests on three plant varieties belonging to two species of the genus Solanum, commonly grown in the Central and West African sub-regions: (1) The highly economic potential crop S. aethiopicum with two varieties, the var. zong (Figure 1a to c) and the var. jakatu (Figure 1 d to f). The first variety is among the most important native varieties cultivated in the forest region of Central Africa. Despite its weak commercial value, it is a great component of various unsalted meals e.g. ‘Sanga’, ‘Kpwem’ (Franqueville, 1972). The second variety, also called sweet Solanum constitutes a significant source of income for farmers and retailers in urban and peri-urban zones and can be encountered in almost all the markets in West and Central Africa, where it is one of the five most important vegetables (Adeyeye and Adanlawo, 2011); its fruits are rich in vitamins and minerals and are consumed fresh, dried or transformed into association with other vegetables (salads), cooked in soup or boiled (Messiaen, 1989; Lester and Seck, 2004; Shippers 2004). (2) The third variety is Solanum melongena var. inerme (Figure 1 g to i). It is less known than the two others, but is an important component of traditional and highly valuable meals of the western highlands of Cameroon, e.g. ‘Yellow soup’, ‘Nah poh’ and ‘Nkui soup’ (Noumi, 1984; Tchiégang and Mbougueng, 2005).
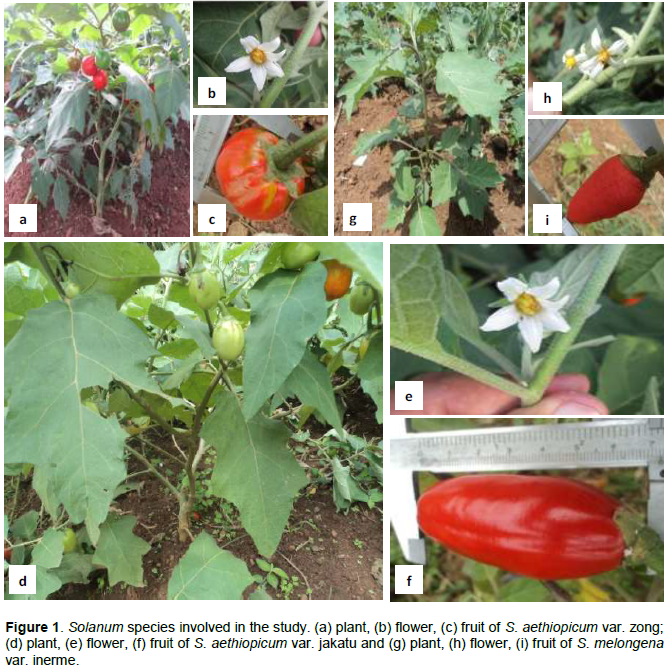
The species S. aethiopicum is a plant of sunny area distributed throughout the southern Cameroon and other countries in Africa (Sekara et al., 2007). Its phenology is closely correlated with the local climatic conditions. During the rainy season, the plant has a greenish appearance with several leaves and maturing fruits while in the dry season, it has a jagged appearance (Schippers, 2004). The var. zong of S. aethiopicum and inerme of S. melongena grow well in full sunny woodland on relatively well drained deep soils with a pH of 5.5 to 6.8 and temperatures ranging from 25 to 35°C during diurnally and 20 to 27°C nightly. The var. jakatu of S. aethiopicum grows in warmer climates (45°C during the day) but may tolerate relatively dry conditions with sometimes less than 20% moisture (when irrigated) (Grubben and Denton, 2004; Schippers, 2004).
Despite highly nutritional, cultural and commercial values, few studies have focused on the diversity and geographic distribution of fruit pests of Solanum spp. in southern Cameroon (Djiéto-Lordon and Aléné, 2006; Djieto-Lordon et al., 2007). The aim of the present study, conducted in two agroecological zones of southern Cameroon, Central plateau and Western highlands were: (i) To identify the insect pests associated with the fruit of three Solanum varieties; (ii) To assess host specificity of the pests on the three plant varieties, and finally, (iii) To evaluate the incidence of each specific fruit pests on the yield of each variety.
Study site
Field study was carried out simultaneously in two localities from two agro-ecological zones of southern Cameroon: Okola (04°01’39.0’’N, 011°23’00.1’’E, asl: 604 m) in the Southern plateau, and Koutaba (05°38’47.9’’N, 010°48’22.2’’E, asl: 1186 m) in the Bamoun Plateau. The latter is situated in one of the main market crop basin of Cameroon (Westphal et al., 1981). Both agroecological zones differed in their topographic and climatic characteristics. At Okola, the study area undergoes an equatorial climate with bimodal rainfall regime marked by a succession of four seasons while Koutaba is under a humid tropical climate characterized by two seasons with unimodal rainfall regime (Suchel, 1988). For landscapes, more description and climatic conditions of the study sites, is found in Mokam et al. (2014) and Heumou et al. (2015). The study period extended from June 2010 to October 2011 at Okola and from August 2010 to November 2011 at Koutaba.
Biological material
The biological material in this study was composed of healthy and infested harvested fruits from three varieties Solanum spp. (Solanaceae): S. aethiopicum var. zong with less sweet fruits (Figure 1a to c), S. aethiopicum var. jakatu sweet (Figure 1d to f) and Solanum melongena var. inerme with bitter fruits (Figure 1g to i).
Experimental design
For data collection, experimental gardens were set up at each site. At Okola, the experimental garden consisted in 06 ridges of 10 m × 1 m separated each other by furrow of 0.5 m wide. Each ridge supported 24 plants of the same variety (two ridges per variety), placed on two lines, and separated by 0.80 m. At Koutaba, they were made of 9 ridges (three ridges per variety). For the two localities, seedlings were obtained from the same nursery set up at the Laboratory of Zoology of the University of Yaoundé I (03°51’35.3’’N, 011°30’00.6’’E, asl: 770 m).
Sampling method
Biological diversity and abundance of fruit pest insects
Data were collected during 184 sampling days unequally distributed among the two study sites; 112 at Okola and 72 at Koutaba. From the Blooming stage of the earliest variety up to the end of the fruiting period of the latest, plots were visited weekly at Okola and Koutaba in order to assess by count, the number of fruits produced, the number of fruits affected by insects and/or diseases and the number of mature fruits. All mature fruits and those affected were systematically
harvested, individually isolated in labelled bags and brought to the laboratory for further observations. Once in the Laboratory, fruits were weighed and categorised as followed: (i) Fruits attacked by fruit feeding insects, characterised by the presence of entrance/exit holes, (ii) quasi-healthy fruits, with no sign of insect attack, (iii) fruits with other affection symptoms. Fruits with insect attack signs were individually incubated in plastic boxes of appropriate size. Each box was previously provided with wet sand, then covered with a fine gauze and followed up to the emergence of adult insects (Djiéto-Lordon and Aléné, 2006, Djieto Lordon et al. 2007; Mokam et al., 2014; Heumou et al., 2015).
Fruits of the two other categories were dissected in order to detect any cryptic insect attack (in case of cryptic attacks, they were incubated as above). This process allowed us discriminating healthy fruits, fruits lost by insects’ attacks, and fruits lost by other biotic factors. To improve sample collection for the biodiversity study, sampling was extended to peasants’ gardens in the neighboring areas. In this particular case, only infested fruits were collected and incubated separately. After the emergence, adult insects from each incubating box were separated on the base of morphological features, counted and preserved dry (Lepidoptera) or in vial tubes provided with 70% alcohol (other taxa) for future identifications.
Assessment of yield loss due to specific fruit pests
The number of healthy and infected fruits per plant variety was recorded at each harvest to calculate the incidence of specifics fruits pests on the yield. For this purpose, the ratio of the number of fruits of each cultivated plant which have been attacked over the total number of fruits harvested was used (Heumou et al., 2015). The total number of fruits and the number of infested fruits were recorded from all varieties weekly during the period from June 2010 to October 2011 at Okola and from August 2010 to November 2011 at Koutaba. Damage index from each plant variety was calculated and expressed in percentage using the following formula:
ID (%) = (Nif / Nthf)*100
Where: ID (%) = Damage index; Nif = Number of infested fruits by specific pest; Nthf = Number of total harvested fruits
Identification method
Insect determinations were done at the Laboratory of Zoology of the University of Yaoundé I, and confirmed by the taxonomists of CIRAD/CBGP at Montpellier (France). These determinations were based on various keys, including Delvare and Aberlenc (1989) for insect families in general, White (2006) and White and Elson-Harris (2004) for fruit flies, Wharton and Gilstrap (1983) and Wharton et al. (1992) for parasitoïds. The monographs of Bordat and Arvanitakis (2004) and Bordat and Daly (1995) were also used. Voucher specimens were deposited in the collections of the two above cited institutions.
Statistical analysis
After transformation, data were submitted to variance analysis test (ANOVA) using the Statistica software version 6.0. Then, a multiple comparison of means was performed using the Tukey HSD test. These statistics treatments aimed to compare the diversity, the abundance and the incidence of fruit pest insects between species/varieties of Solanum form one study site to another and from a season to another. All probabilities were appreciated at the 5% threshold.
Biological diversity of the fruit feeding insects and abundance
A total of 15 insect species belonging to 12 families and 3 orders were recorded from incubated fruits of the three species/varieties of Solanum in the two study sites. Orders represented were (i) Lepidoptera with 5 species, (ii) Diptera with 8 species and (iii) Coleoptera with 2 species (Table 1). At the site level, there were weak variations as all the 15 species were recorded at Okola while two species Hypolycaena phylippus and Chrysodeixis chalcites were absent from Koutaba. It is important to notice that the two species absent from Koutaba are highly polyphagous and weakly represented at Okola. The butterfly Leucinodes orbonalis (Lepidoptera: Pyralidae) and the fly Neosilba sp. (Diptera: Lonchaeidae) were the most important pests at both the two sites (Table 1). Their relative abundances were higher than those of other fruit pests at both Okola and Koutaba without plant species/variety considerations. At Okola, the relative abundance of the pests did not varied significantly in relation to plants species/varieties. For S. aethiopicum var. zong, S. aethiopicum var. jakatu and S. melongena var. inerme, the relative abundances were: 75.1, 68 and 70.3% for L. Orbonalis, 15.5, 17.9 and 18.2% for Neosilba sp. respectively while at Koutaba, they were 65.9, 68.84 and 67.6% for L. orbonalis, and 19.44, 16.47 and 20.98% for Neosilba sp. respectively. Apart from these two main species, other pests were weakly abundant on Solanum (≤ 10 and 2%) (Table 1).
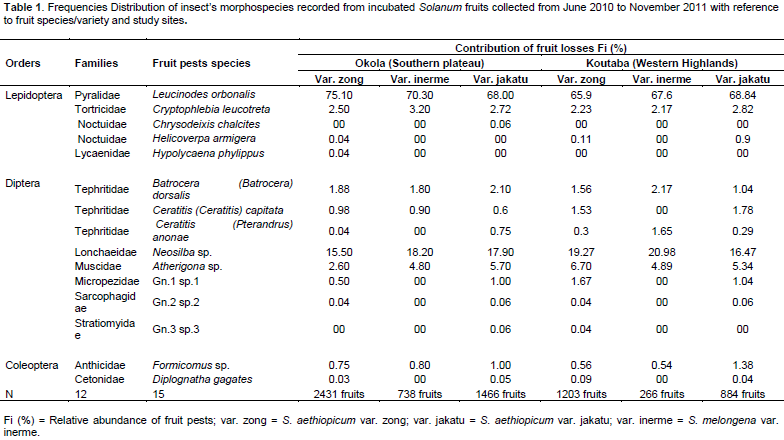
Insect orders and families associated with species/varieties of Solanum
The ANOVA test for the three orders of fruit pests showed non-significant variation of the relative abundance between the two study sites with Lepidoptera (F = 0.001, DF=1, P = 0.96); Diptera (F = 0.02, DF = 1, P = 0.87) and Coleoptera (F = 0.001, DF = 1, P = 0.97). Lepidopterans were the most represented group, with a relative abundance of 73.98% at Okola and 70.19% at Koutaba, followed by dipterans (25.11% at Okola, 28.95% at Koutaba) and coleopterans (0.9% at Okola and 0.86% at Koutaba) (Figure 2).
At the family level, Pyralidae (Lepidoptera) were the most represented with relative abundances of 71.13% at Okola and 67.44% at Koutaba, followed by Lonchaeidae (Diptera) which contributed for 17.2% at Okola and 18.96% at Koutaba. These two families were followed by Muscidae (Diptera) (4.03% at Okola, 5.64% at Koutaba), Tephritidae (Diptera) (3.4% at Okola, 3.43% at Koutaba) and Tortricidae (Lepidoptera) (2.8% at Okola, 2.4% at Koutaba). The other families showed the lowest abundances throughout the study (< 1%) in the both agroecological sites. Pyralidae and Tortricidae were more abundant at Okola than at Koutaba while all other families were more abundant at Koutaba than at Okola (Figure 3).
Mean number of individuals per fruit on Solanum spp.
In the both study sites, larvae of L. orbonalis were present in field during the period of pre-flowering, flowering (on shoot and flowers) and fructification (on fruits) and those of Neosilba sp. during fruit maturation only. The means number of pest individual per fruit varied significantly in relation to
fruit species/varieties and pest species.
At Okola, the variation of the mean number of L. orbonalis individuals per fruit was highly significant between the three species/varieties of Solanum (F = 18.06, DF = 2, P = 0.0001), with 1.51 ± 0.77 individuals per fruit on S. aethiopicum var. zong (N = 1891 fruits), 1.61 ± 0.82 individuals per fruit on S. aethiopicum var. jakatu (N = 1069 fruits) and 1.37 ± 0.61 individuals per fruit on S. melongena var. inerme (N = 545 fruits). Similar trend was observed between the mean number of other fruit pest individuals per fruit (F = 3.9, DF = 2, P = 0.02), with 1.89 ± 1.54 individuals per fruit on S. aethiopicum var. zong (N = 151 fruits), 2.21 ± 2.30 individuals per fruit for S. aethiopicum var. jakatu (N = 115 fruits) and 1.38 ± 0.97 individuals per fruit for S. melongena var. inerme (N = 52 fruits). Contrarily, with Neosilba sp., the mean number of individuals per fruit did not vary significantly with respect to Solanum varieties (F = 197, DF = 2, P = 0.13) (with 4.37 ± 3.7 individuals per fruit for S. aethiopicum var. zong, (N = 389 fruits), 4.74 ± 4.20 individuals per fruit for S. aethiopicum var. jakatu (N = 282 fruits) and 3.97 ± 3.27 individuals per fruit for S. melongena var. inerme (N = 141 fruits). Globally, the highest number of fruit pests per fruit was recorded on S. aethiopicum var. jakatu, followed by the one on S.aethiopicum var. zong and finally the one on S. melongena var. inerme (Figure 4).
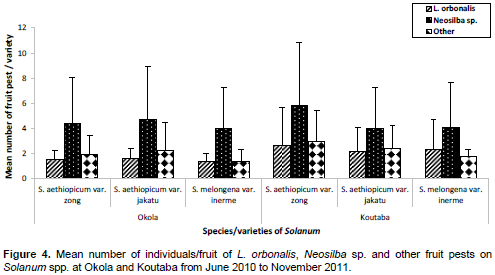
At Koutaba, the mean number of L. orbonalis individuals per fruit also varied significantly on Solanum species/varieties (F = 6.24, DF = 2, P = 0.01), with 2.62 ± 3.03 individuals per fruit on S. aethiopicum var. zong (N = 896 fruits), 2.16 ± 1.87 individuals per fruit on S. aethiopicum var. jakatu (N = 674 fruits) and 2.35 ± 2.38 individuals per fruit on S. melongena var. inerme (N = 184 fruits). Also, the mean number of individuals per fruit of other pests varied significantly (F = 3.8; DF = 2; P = 0.02), with 2.98 ± 2.48 individuals per fruit on S. aethiopicum var. zong (N = 131 fruits), 2.40 ± 1.86 individuals per fruit on S. aethiopicum var. jakatu (N = 99 fruits) and 1.76 ± 0.53 individuals per fruit on S. melongena var. inerme (N = 21 fruits). Also, the mean number of Neosilba sp. individuals per fruit presented a significant variation (F = 7.7, DF = 2, P = 0.005), with 5.84 ± 5.04 individuals per fruit on S. aethiopicum var. zong (N = 176 fruits); 4.03 ± 3.61 individuals per fruit on S. melongena var. inerme (N = 57 fruits) and 4.0 ± 3.22 individuals per fruit on S. aethiopicum var. jakatu (N = 111 fruits).
So, the mean numbers of L. orbonalis and Neosilba sp. individuals per fruit were high on S. aethiopicum var. zong followed by those on S. melongena var. inerme and the mean number of other fruit pests was high on S. aethiopicum var. zong followed by the one on S.aethiopicum var. jakatu (Figure 4).
With the ANOVA, a significant variation was revealed on the mean abundance per fruit of L. orbonalis (F = 360.3, DF= 1, P = 0.00), Neosilba sp. (F = 3.9, DF = 1, P = 0.04) and other fruit pests (F = 15.27, DF = 1, P = 0.0005) in the two sites:
(i) For L. orbonalis, the mean abundance was higher at Koutaba with 2.42 ± 2.58 individuals per fruit (Min = 2.34, Max = 2.49, N = 1754 fruits) than at Okola with 1.52 ± 0.77 individuals per fruit (Min = 1.46, Max = 1.57, N =
3505 fruits);
(ii) For Neosilba sp., the mean abundance was higher at Koutaba with 4.94 ± 4.38 individuals per fruit (Min = 4.52, Max = 5.37, N = 344 fruits) than at Okola with 4.43 ± 3.82 individuals per fruit (Min = 4.16, Max = 4.71, N = 812 fruits);
(iii) For the other species, the mean abundance was higher at Koutaba than at Okola. These data allowed demonstrating that the mean number of L. orbonalis, Neosilba sp. and other pests were higher at Koutaba than at Okola (Figure 5).
Damage due to specific fruit pests on Solanum spp.
The losses of young fruits of Solanum spp. in the present study were mainly caused either by the borer L. orbonalis or the black fruit fly Neosilba sp. and sometimes the both. Then, the two insect species could be regarded as the main causes of fruit losses on the three varieties. The presence of other pest species was so far not neglected despite the fact that their injuriousness in different sites remained weak. It could however cause significant damage in other agroecological zones. So the pattern of damage caused by each species was as follow:
1. Leucinodes orbonalis: At Okola, the mean damage index per month due to L. orbonalis showed a significant variation on Solanum species/varieties (F = 9.12, DF = 2, P = 0.005) with 52.58 ± 15.46% (Min = 43.65%, Max = 61.51%, N = 14 months) on S. aethiopicum var. zong, 65.39 ± 20.06% (Min = 53.8%, Max = 76.97%, N = 14 months) on S. aethiopicum var. jakatu and with 38.01 ± 14.88% (Min = 29.42%, Max = 46.61%, N = 14 months) on S. melongena var. inerme.
At Koutaba, the mean damage index per month due to L. orbonalis showed a significant variation on the Solanum species/varieties (F = 16.73, DF = 2, P = 0.0028) with 46.82 ± 17.44% (Min = 33.41%, Max = 60.23%, N = 9 months), 59.04 ± 12.39% (Min = 49.51%, Max = 68.57%, N = 9 months) and 21.06 ± 12.19% (Min = 11.68 %, Max = 30.44%, N = 9 months) respectively on S. aethiopicum var. zong, S. aethiopicum var. jakatu and S. melongena var. inerme (Figure 6).
By comparing this value from the two sites using the Student test, there was no significant difference (t = 1.42, P = 0.15) (Table 2).
2. Neosilba sp.: At Okola, mean damage index due to this pest showed a significant variation on the Solanum species/varieties (DF= 2, F = 10.02, P = 0.003) with 11.19 ± 5.10% (Min = 8.24%, Max = 14.17%, N = 14 months) on S. aethiopicum var. zong, 17.42 ± 7.55% (Min = 13.06%, Max = 21.78%, N = 14 months) on the S.
aethiopicum var. jakatu and 7.81 ± 4.05% (Min = 5.47%, Max = 10.15%, N = 14 months) on S. melongena var. inerme.
At Koutaba, the mean damage index due to Neosilba sp. showed a significant difference on the Solanum species/varieties (F = 4.10, DF = 2, P = 0.029) with 13.27 ± 7.71% (Min = 7.34%, Max = 19.20%, N = 9 months), 23.72 ± 12.84% (Min = 13.85%, Max = 33.59 %, N = 9 months) and 11.01 ± 8.82% (Min = 4.23%, Max = 17.79%, N = 9 months) on S. aethiopicum var. zong, S. aethiopicum var. jakatu and S. melongena var. inerme respectively (Figure 6).
The comparison of this value from the two sites using the Student test showed no significant difference (t = -1.77, P = 0.08) (Table 2).
3. Other fruit pests: The mean damage index due to other fruit pests showed a significant variation on Solanum species/varieties at Okola and Koutaba (F = 5.16, DF = 2, P = 0.01 and F = 7.73, DF = 2, P = 0.002 respectively). Damage were higher on S. aethiopicum var. jakatu in both sites with 11.63 ± 6.75% (Min = 7.73%, Max = 15.53%, N = 14 months at Okola and 16.97 ± 7.32% (Min = 11.34%, Max = 22.60%, N = 9 months at Koutaba) (Figure 6).
The mean indexes of damage due to other pests in the two study sites were not significantly different on the two study sites (t = -1.72, P = 0.09) (Table 2).
According to these results, L. orbonalis was the main pest for which the highest damage index was recorded on the three Solanum species/varieties in the two sites. This butter fly appeared to be one of the most injurious pests on Solanum spp. fruits in southern Cameroon, followed somewhat by Neosilba sp. This study also reveals that fruits of S. aethiopicum var. jakatu were the mostly attacked by the pests at the two sites, followed by fruits of S. aethiopicum var. zong. Solanum melongena var. inerme was slightly less susceptible than the two others.
Biodiversity of fruit pests on species/varieties of Solanum
The inventory of fruit pests on the three Solanum species/varieties recorded the main pest species and revealed their economic importance. Although some species fed on leaves and stems, they were usually controlled by various chemicals. However, the task was highly difficult for fruit pests since they were hidden inside the carpels of young fruits. Thus, the borer L. orbonalis, which was present on all the studied species/varieties in the two study sites, appeared to be the most injurious pest on fruits of Solanum species. This borer has been reported in Ghana, Uganda and Nigeria by Schippers(2004) on the same species/varieties. Its young larvae (13 mm) bore into the fruit and develop living holes which persist after their emergence. These holes induce fruit rot, making them uneatable and then unmarketable. Furthermore, Neosilba sp. is known to be the most injurious pest on fruits of Jatropha curcas in Brazil by Dias et al. (2012). Apart from these two species, we also found some other fruit flies such as Atherigona sp., Batrocera (Batrocera) dorsalis, Ceratitis (Ceratitis) capitata and Ceratitis (Pterandrus) anonae, some other caterpillars such as C. leucotreta, H. armigera and C. chalcites which could be regarded as minor fruit pests. These fruit pests have never been recorded as pest on the plants involved in this study but as main fruit pests on other fruits species. The members of the genus Ceratitis, widespread on the African continent were found on guajava fruits in the region of Yaounde by Ndzana Abanda et al. (2007). These authors and Bwomushana et al. (2008) also found on the same fruit species members of the genus Batrocera.
Abundance of fruit pest’s insects on Solanum spp.
From the present study, L. orbonalis and Neosilba sp. are recorded as the most abundant species among all other fruit pests associated with species/varieties of Solanum at Okola and Koutaba. In both sites, S. aethiopicum var. jakatu supported the highest abundance of L. orbonalis, Neosilba sp. and other pests per fruit followed by S. aethiopicum var. zong and finally by S. melongena var. inerme. The jakatu and zong are among the most common Solanum varieties in Africa and therefore more attractive to various fruit pests (Schippers, 2004). The species L. orbonalis was the most abundant pest on the three studied varieties. The variety S. melongena inerme presented a relatively low abundance compared to the other two varieties. This low abundance correlated with low attack rate. Mannan et al. (2003) showed that the eggplant variety ‘Jumki’ was resistant to L. orbonalis so that infestation due to this pest on its fruits was low. These authors also noticed that the attacks due to fruit pests were low for this variety because the fruit is smaller than other varieties. Then the variety inerme is therefore unable to contain a large population of fruit pests and due to its bitterness, only a few species of fruit pests able to support the bitter taste was feeding on this variety. Moreover, the mean number of L. orbonalis per fruit in both sites is lower than those of Neosilba sp. This can be linked to the late colonization of larvae of Neosilba sp. after fruits maturation. Some larvae of L. orbonalis end their development cycle before fruit maturation while other fruit pests colonized fruits up to maturation. In addition, larvae of some flies secondary colonized fruits and emerged in large number during incubations (e.g. Neosilba sp. and other fruit flies such as Atherigona sp. which prefered very ripe fruits or fruits at the end of maturation) (Dias et al., 2012). The lower number of L. orbonalis individuals could also be explained by the presence of a high number of parasites associated with larvae during incubations and field predators that would have reduced their populations.
Damage due to fruit pest insects on species/varieties of Solanum
S. aethiopicum var. jakatu presented the highest damage among the three varieties followed by S. aethiopicum var. zong and finally by S. aethiopicum var. inerme. This may be due to the difference in tastes of the studied varieties (S. aethiopicum var. jakatu is sweet, S. aethiopicum var. zong is moderately sweet and S. melongena var. inerme is bitter). Resistance and susceptibility of Solanum spp. to fruit feeder insects’ attacks could then be due to some biochemical characteristics such as chlorophylls, Phenols and Sugars in fruits (Mannan et al., 2003). Elanchezhyan et al. (2008) reported that the sweet Solanum varieties with high rate of chlorophyll and low rate of phenol were infested. This could explain why the S. aethiopicum var. jakatu presented the highest damage index. These biochemical parameters can play a great role in the behaviour of Solanum spp. vis-à-vis fruit pests attacks (Mofazzel et al., 2002). In S. melongena var. inerme, damage were the lowest compared to S. aethiopicum var. zong and S. aethiopicum var. jakatu. This variety is stronger than S. aethiopicum var. zong and S. aethiopicum var. jakatu which in turn would be respectively tolerant and susceptible to fruit pests’ attacks. Indeed, the study of Mannan et al. (2003) showed that some local varieties of Solanum such as the Jumki-1 and Jumki-2 were strongly resistant to L. orbonalis whose attack rates ranged from 1 to 10 %, whereas other varieties were weakly resistant (Islampuri-3, BL-34 and Muktakeshi) with infestation rate ranging from 11 to 20 % rate, tolerant (with infestation rates ranging from 21-30 %) and susceptible (with infestation rates ranging from 31 to 40 %). In this way, S. melongena var. inerme was resistant to the attacks of L. orbonalis with a lower attack rate.
In this study, L. orbonalis caused fruits losses on S. aethiopicum var. jakatu, var. zong, and S. melongena var. inerme in the two study sites. These losses were almost constant from June 2010 to November 2011 and varied on the three species/varieties of Solanum. In India, attacks due to L. orbonalis were studied on 25 varieties of eggplants and these varieties showed different sensitivities to this fruit pest (Elanchezhyan et al., 2008). It is known that the estimated damage caused by L. orbonalis may considerably vary (Djiéto-Lordon et al., 2014). On S. melongena for example, Patnaik (2000) reported field damage due to L. orbonalis ranged from 47.6 to 85.8%. Mehto et al. (1983) observed a reduction in yield ranged from 50 to 60% and Mall et al. (1992) an average field loss of 13% due to L. orbonalis. Neosilba sp. caused also damage on Solanum spp. fruits at Okola (12.14%) and Koutaba (16.04%). Infestation due to this pest on Jatropha fruit was reported by Dias et al. (2012) in Brazil with 53 pupae/kg of fruit and 0.6 pupae/fruit. According to this author, Neosilba sp. was regarded as secondary invader of fruit. Helicoverpa armigera obtained on Solanum during the study were also observed by Etienne and Delvare (1987) on the fruits of S. aethiopicum var. jakatu in Casamance (Senegal). These authors noticed that H. armigera did not cause serious damage. Merely, it perforated fruits and was known to cause similar damage on tomato (Appert and Deuse, 1982, 1988).In the present study, attacks due to L. orbonalis and somewhat Neosilba sp. were always observed during the study period, and more than half of the harvested fruits of S. aethiopicum var. jakatu, var. zong, and S. melongena var. inerme were affected in the two sites.
At Okola and Koutaba, 15 and 13 species belonging to three orders were inventoried. Leucinodes orbonalis and Neosilba sp. were the most abundant and the damages they caused on species/varieties of Solanum in both sites were noticeable. The borer L. orbonalis was the most injurious. Fruits of S. aethiopicum var. jakatu were the most susceptible in the two study sites. Infestation from this pest was due to its larvae which develops in carpel of the young fruits.
The authors have not declared any conflict of interests.
The present study was carried out with the financial support of the French Ministry of Foreign Affairs via the program “Corus 2” (Project CORUS 6080). The authors are especially grateful to Mrs. Nji Moussa, Ndaga, Nga Tchanga and other farmers who allowed them to work in their farms and gave them land for setting up trap gardens. Their hospitality during their stay in the various study sites is quite appreciable.
REFERENCES
|
Adeyeye EI, Adanlawo IG (2011). Amino acid composition of the ripe fruits of Solanum aethiopicum and Solanum macrocarpon. Int. J. Pharm. BioSci. 2(2):39-51.
|
|
|
|
Appert J, Deuse J (1982). Les ravageurs des cultures vivrières et maraîchères sous les tropiques. Maisonneuve et Larose, Paris, France.
|
|
|
|
|
Appert J, Deuse J (1988). Insectes nuisibles aux cultures vivrières maraîchères. Maisonneuve et Larose, Paris, France.
|
|
|
|
|
Bordat D, Arvanitakis (2004). Catalogues des Arthropodes des cultures légumières d'Afrique de l'Ouest, Centrale, Mayotte et Réunion. CIRAD, Montpellier. France 291 p.
|
|
|
|
|
Bordat D, Daly P (1995). Catalogue des principaux Arthropodes présents sur les cultures légumières de Nouvelle-Calédonie. CIRAD/Mandat de gestion Nouvelle-Calédonie., Montpellier. France 95 p.
|
|
|
|
|
Bwomushana I, Ekesi S, Gordon I, Ogol GKPO (2008). Host Plants and Host Plant Preference Studies for Batrocera invadens (Diptera: Tephritidae) in Kenya, a new Invasive Fruit Fly Species in Africa. Ann. Entomol. Soc. Am. 101(2):331-340.
Crossref
|
|
|
|
|
Delvare G, Aberlenc HP (1989). Les insectes d'Afrique et d'Amérique tropicale. Clés pour la reconnaissance des familles. Prifas/cirad/Gerdat. Montpellier, France. 302 p.
|
|
|
|
|
Dias NS, Broglio SMF, Santos DS, dos Santos JM, Strikis PC (2012). First record of Neosilba (Diptera: Lonchaeidae) on Jatropha curcus L. in Brasil. Scientific communication. Arq. Inst. Biol. 79(3):423-424.
Crossref
|
|
|
|
|
Djiéto-Lordon C, Aléné DC (2002). Inventaire des insectes ravageurs et auxiliaires des cultures maraîchères dans la région de Yaoundé. Rapport – CIRAD Yaoundé P 35.
|
|
|
|
|
Djiéto-Lordon C, Aléné DC (2006). Inventaire diagnostique des insectes de quelques cultures dans les exploitations maraîchères périurbaines dans la région de Yaoundé – Cameroun. In «Pôle de compétence en Partenariat (PCP) Grand Sud Cameroun. Actes atelier de présentation des résultats de recherche participative». IRAD/IRAD. Yaoundé, Cameroun pp. 7-17.
|
|
|
|
|
Djiéto-Lordon C, Heumou CR, Elono Azang PS, Aléné DC, Ngueng AC, Ngassam P (2014). Assessment of pest insects of Capsicum annuum L.1753 (Solanaceae) in a cultivation cycle in Yaoundé. Int. J. Biol. Chem. Sci. 8(2):621-632.
Crossref
|
|
|
|
|
Elanchezhyan K, Murali Baskaran RK, Rajavel DS (2008). Fiel screening of brinjal varieties on major pests and their natural enemies. J. Biopest 1(2):113-120.
|
|
|
|
|
Etienne J, Delvare G (1987). Les insectes associés au fruit du diakhatu (Solanum aethiopicum) en Casamance (Sénégal) : composante de l'entomofaune et phénologie des principaux ravageurs. L'AGRONOMIE TROPICALE, 42-3.
|
|
|
|
|
Franqueville A (1972). Les relations ville-campagne sur la route au nord de Yaoundé. Cah. O.R.S.T.O.M., sér. Sci. HI. 9(3):337-387.
|
|
|
|
|
Heumou CR, Djiéto-Lordon C, Aléné DC, Elono Azang PS (2015). Diversity and agronomic status of tomato and pepper fruit pests in two agroecological zones of Southern Cameroun: Western Highland and the Southern Plateau of Cameroun. Afr. J. Agric. Res. 10(11):1224-1232.
|
|
|
|
|
Lester RN, Seck A (2004). Solanum aethiopicum L., pp. 530-536. In: Grubben G.J.H. and Denton O.A. (Eds.). Ressources végétales de l'Afrique Tropicale 2: Légumes. Fondation PROTA / Backhuys Publishers / CTA, Wageningen-Pays Bas.
|
|
|
|
|
Mall NP, Pandey RS, Singh SV, Singh SK (1992). Seasonal incidence of insect-pests and estimation of the losses caused by shoot and fruit borer on brinjal. Indian J. Ent. 54(3):241-247.
|
|
|
|
|
Mannan MA, Begum A, Rahman MM, Hossain MM (2003). Screening of Local and Exotic Brinjal Varieties/Cultivars for Resistance to Brinjal Shoot and Fruit Borer, Leucinodes orbonalis Guen. Pak. J. Biol. Sci. 6 (5):488-492.
Crossref
|
|
|
|
|
Mehto DN, Singh KM, Singh RN, Prasad D (1983). Biology of brinjal fruit and shoot borer, Leucinodes orbonalis Guen. Bull. Entomol. 24(2):112-115.
|
|
|
|
|
Messiaen CM (1989). Le potager tropical. 2nd ed. ACCT/CILF. PUF. Paris, France 580 p.
|
|
|
|
|
Mofazzel HM, Shahjahan M, Mosaddeqque HM, Bari MN (2002). Chlorophyll Contents of Brinjal Plants Influencing the Resistance and Susceptibility to Brinjal Shoot and Fruit borer, Leucinodes orbonalis Guenne. Pak. J. Biol. Sci. 5(8):825-829.
Crossref
|
|
|
|
|
Mokam DG, Djiéto-Lordon C, Bilong Bilong CF (2014). Patterns of species richness and diversity of insects associated with Cucurbit fruits in the Southern part of Cameroun. J. Insect Sci. 14(248):1-9.
|
|
|
|
|
Ndzana Abanda FX, Quilici S, Vayssières JF, Kouodiekong L, Woin N (2007). Inventaire des espèces de mouches des fruits sur goyave dans la région de Yaoundé au Cameroun. Fruits 63(1):19-26.
Crossref
|
|
|
|
|
Noumi E (1984). Les plantes à épices, à condiments et à arômes du Cameroun: Thèse de Doctorat 3ième cycle en sciences biologiques. Faculté des Sciences, Université de Yaoundé, Cameroun 165 p.
|
|
|
|
|
Patnaik HP (2000). Flower and fruit infestation by brinjal shoot and fruit borer Leucinodes orbonalis Guenée. Damage potential vs. weather. Veg. Sci. 27(1):82-83.
|
|
|
|
|
Schippers RR (2004). Légumes africaines indigènes : Présentation des espèces cultivées. Margraf Publishers. CTA, Wegeningen, Pays-Bas 482 p.
|
|
|
|
|
Sekara A, Cebula S, Kunicki E (2007). Cultivated eggplants – origin, breeding objectives and genetic resources, a review. Folia Hortic. Ann. 19/1:97-114.
|
|
|
|
|
Suchel FG (1988). Les régions climatiques du Cameroun. Les climats du Cameroun. Thèse de Doctorat d'Etat. Université de St. Etienne, France 1188 p.
|
|
|
|
|
Tchiégang C, Mbougueng FD (2005). Composition chimique des épices utilisées dans la préparation du Nah poh et du Nkui de l'ouest Cameroun. Tropicultura 23(4):193-200.
|
|
|
|
|
Temple L (2001). Quantification des productions et des échanges de fruits et légumes au Cameroun. Cahiers Agric. 10(2):87-94
|
|
|
|
|
Westphal E, Embrechts J, Mbouemboue PMB, Westphal-Stevels JMC (1981). Agriculture autochtone au Cameroun. Veenman & B.V Wageningen, Pays-Bas 175 p.
|
|
|
|
|
Wharton RA, Gilstrap FE (1983). Key to and status of opiine braconid (Hymenoptera) parasitoids used in biological control of Ceratitis and Dacus s. l. (Diptera: Tephritidae). Annals of the Entomological SociR. Aety Am. 76:721-742.
Crossref
|
|
|
|
|
Wharton RA, Shaw SR, Sharkey NJ, Wahl DB, Woolley JB, Marsh PM, Johnson W (1992). Phylogeny of the subfamilies of the family Braconidae (Hymenoptera: Ichneumonoïdae). A reassessment. Cladistics 8:199-235.
Crossref
|
|
|
|
|
White IM (2006). Taxonomy of the Dacina (Diptera: Tephritidae) of Africa and the Middle East. Afr. Entomol. Memoir 2:1-156.
|
|
|
|
|
White IM, Elson-Harris MM (2004). Fruit flies of economic significance: their identification and bionomics, London, CAB/ACIAR.
|
|