ABSTRACT
The experiment was conducted with three replication in randomized complete block design in field laboratory of Department of Agronomy, Bangaladesh Agricultural University, Mymensingh. Extracts of garlic (Allium sativum) and neem (Azadirachta indica), BAU-Biofungicide (Trichoderma based preparation) and Proud (Propiconazole) and Bavistin (Bavistin DF) were evaluated under field condition in controlling brown spot disease Bipolaris oryzae (Breda de Haan) Shoem of rice cv BRRI dhan28. Foliar spray of BAU-Biofungicide (2%) and Proud (0.1%) showed profound effect in marked reduction of disease severity. BAU-Biofungicide (2%) resulted grain yield 5785 kg/ha in the field and reduction in cost of production was found with benefit-cost ratio (BCR). BCR 2.78:1 was achieved in BAU-Biofungicide (2%) which is close to 2.87:1 in Proud (0.1%). Highest (98.00%) germination percentage was observed with BAU-Biofungicide (3%) as well as maximum (35.64%) increase of vigour index was found with Proud (0.1%) over control. BAU-Biofungicide and Proud were found to have in inhibiting seed borne fungi when they were applied as foliar spray. Trichoderma harzianum was associated with B. oryzae infected seeds and when kept for few days for incubation it overgrew B. oryzae.
Key words: BAU-Biofungicide, Bipolaris oryzae, benefit-cost ratio (BCR), disease severity, seed borne fungi, vigour index.
Rice is the staple food crop in Bangladesh. The country is occupying 4th position as a rice producer in the world (USDA, 2015). About 76% of the total cultivated land covers about 11.37 million hectares under rice cultivation (BBS, 2014). At present, the total amount of rice production is approximately 34.36 million tons (BBS, 2014). The average yield of rice in our country is 3.03 t/ha but the world average yield of rice is 4.42 t/ha (USDA, 2014). Disease plays an important role for low yield of rice in Bangladesh. Out of 32 diseases reported to occur on rice, 10 diseases have the potentiality to cause economic damage to the crop in Bangladesh (Haq et al., 2008) but rice is suffering from brown spot to a great extent and caused “Bengal Famine” in 1943 (Padamanabhan, 1973). Brown spot disease of rice caused by Bipolaris oryza impairs grain quality and results in about loss of 4 to 52% yield (Barnwal et al., 2013). Kamal and Mia (2009) reported that 18.75 to 22.50% yield loss was found due to brown spot disease of rice in Banngladesh. Application of fungicides for the control of brown spot is the most effective management option, but under high disease pressure effective control is not achieved (Lore et al., 2007).
Fakir (2004) stated that roughly 10% production loss of rice may be incurred annually due to seed borne diseases in Bangladesh and according to this estimate, 2.5 million tons of rice worth TK 30,000 millions is lost annually in Bangladesh. Quality seed for planting is an important input for successful crop production. Good quality seed possess major characteristics such as high yielding potentiality, viability, purity, free from varietals mixtures and being healthy, that is, free from infection by pathogens or having maximum acceptable tolerance limit of infection by a given pathogen in a given seed lot (Fakir and Mia, 2004). In Bangladesh, 27 of the 43 diseases known to occur on rice are seed-borne (Fakir, 2004). Seed borne diseases caused by fungi such as brown spot (B. oryzae), blast (Pyricularia grisea), sheath rot (Sarocladium oryzae), seed rot and seedling blight (B. oryzae, Sclerotium rolfsii and Fusarium spp.) and grain spots (B. oryzae, Curvularia lunata, Nigrospora oryzae, Phoma glumarum and Cladosporium sp.); by bacteria such as Bacterial leaf blight (Xanthomonas oryzae pv. oryzae) and bacterial leaf streak (Xanthomonas oryzae pv. oryzicola); and by nematode like white tip (Aphelenochoides besseyi) are harmful to rice seed health for inflicting diseases in seed bed as well as in the field (Fakir, 2004). Rice disease management strategies mainly aim at prevention of outbreak or epidemics through the use of host plant resistance and chemical pesticides. Although some plants have been with antifungal properties (Mia et al., 1990), but recommended dose of plant extracts has not yet been completely formulated. Biocontrol assumes special significance being an eco-friendly and cost effective strategy which can be used in integration with other strategies for a greater level of protection. An antagonist is an organism that exerts a damaging effect on another organism by producing lytic enzymes and antibiotics or by competition. Trichoderma spp. elicits biocontrol mainly by being mycoparasites and by being aggressive competitor of the pathogens (Cumagun, 2012). The present study has been designed to control brown spot disease by using plant extracts and biocontrol agent as an alternate means avoiding environmental pollution.
The experiment was conducted during two Boro seasons of 2012 and 2013 in the field Laboratory of the Department of Agronomy, Bangladesh Agricultural University (BAU), Mymensingh. The field was fertilized as per recommendation of Bangladesh Rice Research Institute, Gazipur (BRRI, 2004). The experiment was carried out in Randomized Complete Block Design having three replications. The individual plot size was 5.0 m × 2.0 m (10 m2). Block to block and plot to plot distances were 1.5 m and 1.5 m, respectively. Thirty five days old seedlings was uprooted from the seed bed and three seedlings per hill were transplanted in the field on January 21 in two successive 2012 and 2013. Hill to hill and row to row distances were 15 and 25 cm, respectively. The spray schedule was started just after commencement of disease symptom and two sprays were maintained at 15 days interval. Disease severity of each plot was assessed following the procedure of Standard Evaluation System for Rice (IRRI, 1996).
Healthy leaves of neem and garlic cloves were collected and the samples were washed thoroughly under running tap water followed by sterile distilled water (SDW). The extracts were prepared by homogenizing 5 g of plant sample in 50 ml of sterile distilled water (SDW) using a blender and the extracts were then prepared at 1 and 2% concentration by dilution with water and kept in conical flasks separately before use (Hossain, 2012). BAU-Biofungicide was used at 2 and 3% in this experiment. BAU-Biofungicide is a Trichoderma based preparation (Hossain, 2011). Bavistin DF (Carbendazim) and Proud 250 EC (Propiconazole) were also used at 0.1 and 0.05% concentration.
70 g seeds were taken randomly from each harvested sample for dry inspection. The seed samples were categorized visually for the presence of any distinct disease symptom or any other physiological abnormalities and were grouped into (a) apparently healthy seeds, (b) spotted and discoloured seeds and (c) chaffy grain. Each category was recorded and expressed in percentage (Chowdhury, 2012).
The experiment (Tray method) was conducted in the nethouse of the Seed Pathology Centre, Bangladesh Agricultural University Mymensingh. Sand was collected from Brahmaputra River, Mymensingh. The collected sand was sterilized with formalin (40%) at the rate of 5 ml formalin Diluted with 20 ml of water for 4 kg sand (Dasgupta, 1988). The formalin treated soil was covered with polythene sheet for 48 h and then exposed for 48 h for aeration before setting experiment. The plastic trays (12² ´ 8²) were filled with the sand. The experiment was carried out in Complete Randomized Design with three replications. Three hundred harvested seeds of each treatment including control were sown in plastic trays (100 seeds/tray) maintaining equal distances among the seeds. Plants were watered as when necessary for maintaining proper moisture. Randomly selected 10 seedlings were uprooted carefully from each tray and washed thoroughly with running tap water. Data was recorded for each treatment at 14 days after sowing (DAS) on different parameters. Vigour Index (VI) was computed using the following formula of Baki and Anderson (1973): Vigor index = (Mean shoot length + Mean root length) x % Germination
Blotter method for seed health test was carried out following blotter method to detect seed borne pathogens associated with seed sample (ISTA, 1996). Three layers of blotting paper (Whatman filter No.1) soaked in water and were kept at the bottom of a 9.0 cm dia. plastic Petri dish and there after 25 seeds were kept on filter paper. Four hundred harvested seeds of each treatment were taken randomly from each sample. The petri dishes containing seeds were incubated at 20±2°C under alternating cycles of 12 h near Ultra Violet light and darkness for 7 days. Incubated seeds were examined under stereo-binocular microscope to record the incidence of different seed borne fungi. Each seed borne infection was recorded and expressed in percentage (Agarwal et al., 1989).
All recorded data on different parameters were analysed statistically using MSTAT-C computer program and treatment means were evaluated for significance using DMRT following Gomez and Gomez (1984).
Highest (86.93%, 87.30% at 95 DAT) reduction of disease severity of brown spot disease was observed with foliar spray of Proud (0.1%) followed by (85.38%, 84.13%) with Proud (0.05%) and (83.45%, 84.13%) with BAU-Biofungicide (2%) among three counting period in two successive years 2012 and 2013 (Table 1). No statistical significant difference of disease severity was found between Proud and BAU-Biofungicide. These findings of this trial were in accordance with the observation of Razu and Hossain (2015). They reported that BAU-Biofungicide (2%) and Tilt 250EC (0.1%) were applied as foliar spray for 3 times of rice cv. BRRI Dhan49 under field condition. The lowest 13.93, 14.51 and 14.87% disease severity of brown spot were recorded at 70, 80 and 90 DAT as foliar spray of BAU-Biofungicide (2%) as well as 15.39, 15.73 and 16.41% disease severity were found in Proud 0.1%. Gupta et al. (2013) conducted a field experiment with three rice varieties viz., Basmati-370, Jaya and PC-19 during 2011 to 2012 with application of propiconazole and azoxystrobin which significantly reduced the disease severity of brown spot (69, 73 and 70%) of all the varieties as compared to their respective controls (Table 2).
.png)
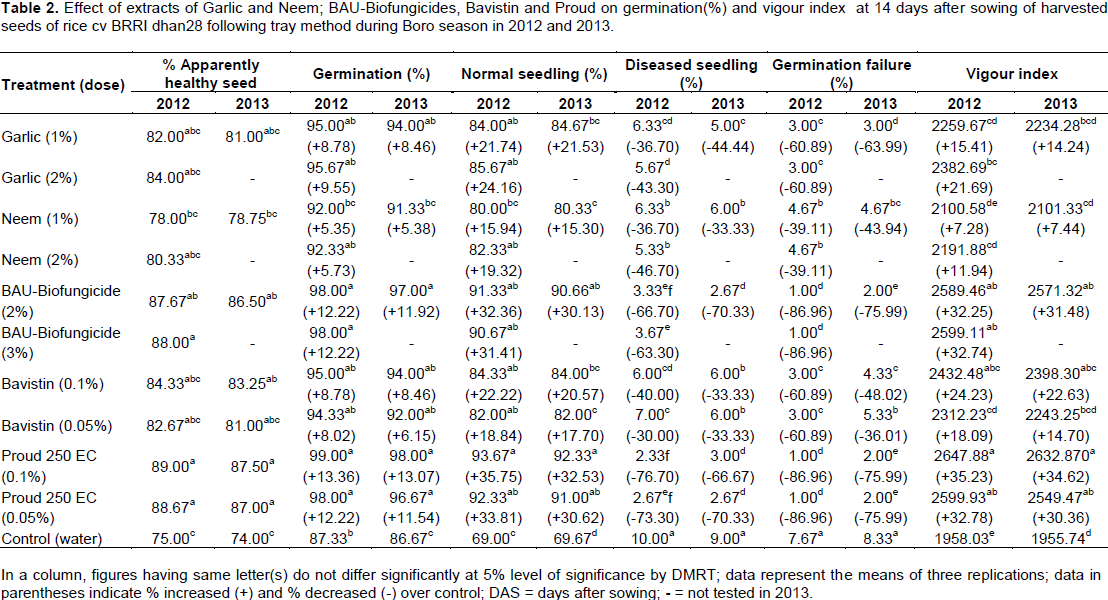
Highest (89.00%, 87.50%) apparently healthy seed was recorded in Proud (0.1%) followed by (88.67%, 87.00%) with Proud (0.05%) and (87.67%, 86.50%) with BAU-Biofungicide (2%). In germination test, Proud (0.1%) showed the highest (99.00%) germination, while maximum (93.67%) normal seedling was recorded in Proud (0.1%). Higher increase (35.75 and 33.81%) of normal seedlings was found with Proud (0.1 and 0.05%) as well as in BAU-Biofungide (2%) having (32.36%) over control in harvested seeds in 2012. Maximum (76.70%) reduction of diseased seedling was found in Proud (0.1%) followed by Proud (0.05%) and BAU-Biofungicide (2%). In case of germination failure, maximum (86.96%) reduction was found with BAU-Biofungicide (2%) and Proud (0.1 and 0.05%) in 2012. Vigour index appeared with the highest increase (35.23%) with Proud (0.1%), while (32.74%) increased in BAU-Biofungicide (2%) compared to control (Table 3). The results were supported by Ora et al. (2011). They showed the better performance in terms of lowest pathogenic incidence, rotten seed, dead seed, seed germination and seedling vigour index. Mahmud and Hossain (2013) alsoevaluated the efficacy of BAU-Biofungicide (2 and 3%), garlic (1 and 2%) and neem extracts (1 and 2%), Bavistin (0.1 and 0.05%) and Proud (0.1 and 0.05%) for controlling brown spot of rice cv. BR11. BAU-Biofungicide (3%) showed the highest germination (98.00%) along with maximum 93.00% normal seedling among the treatments. Highest 78.50% of apparently healthy seed was recorded in Potent (0.1%) followed by BAU-Biofungicide (3%) (74.33%), while minimum (47.75%) was in control. Biswas et al. (2008) reported that Trichoderma treated rice seeds showed maximum germination (92%) and increased shoot and root length 21 and 25%, respectively. Hossain et al. (2015) also reported that 28.25, 18.31 and 17.79% vigour index were increased over control when wheat seeds of Kanchon variety were treated with BAU-Biofungicide (2.5%), Bavistin (0.1%) and garlic (1%), respectively at 10 days after sowing. This result is in accordance with the findings of Hasan et al. (2005) and Chowdhury et al. (2005).
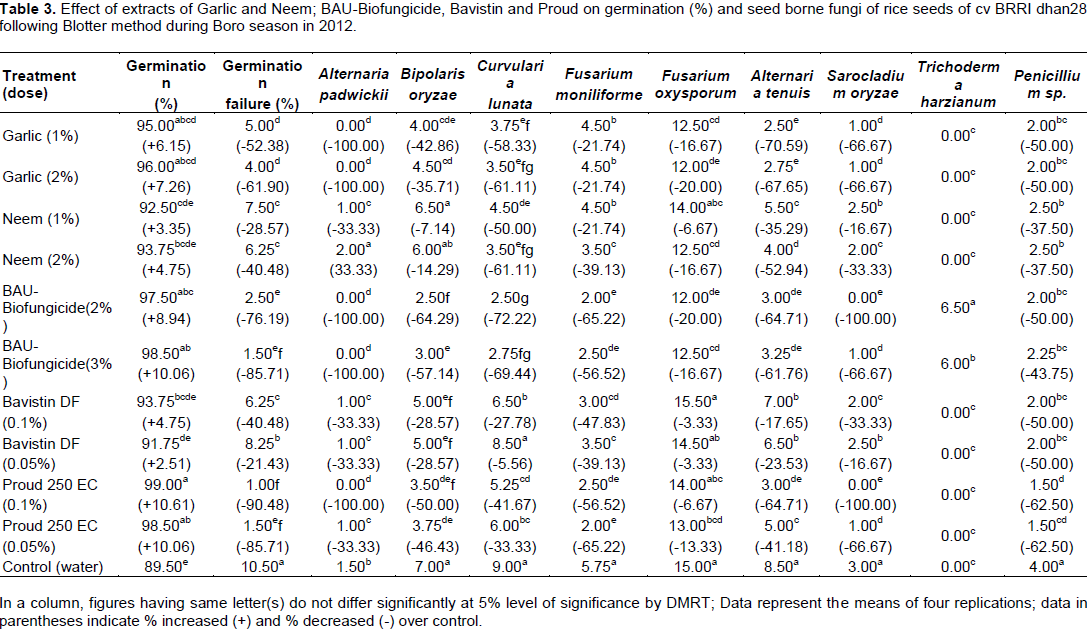
Plant extracts, BAU-Biofungicide and fungicides on health status of rice seeds were evaluated by standard blotter incubation test and the seeds were found to be associated with 9 different seed borne fungi viz., Alternaria padwickii, Alternaria tenuis, B. oryzae, Curvularia lunata, Fusarium moniliforme, Fusarium oxysporum, Sarocladium oryzae, Trichoderma harzianum and Penicillium sp. in 2012 and 2013 (Tables 3 and 4).
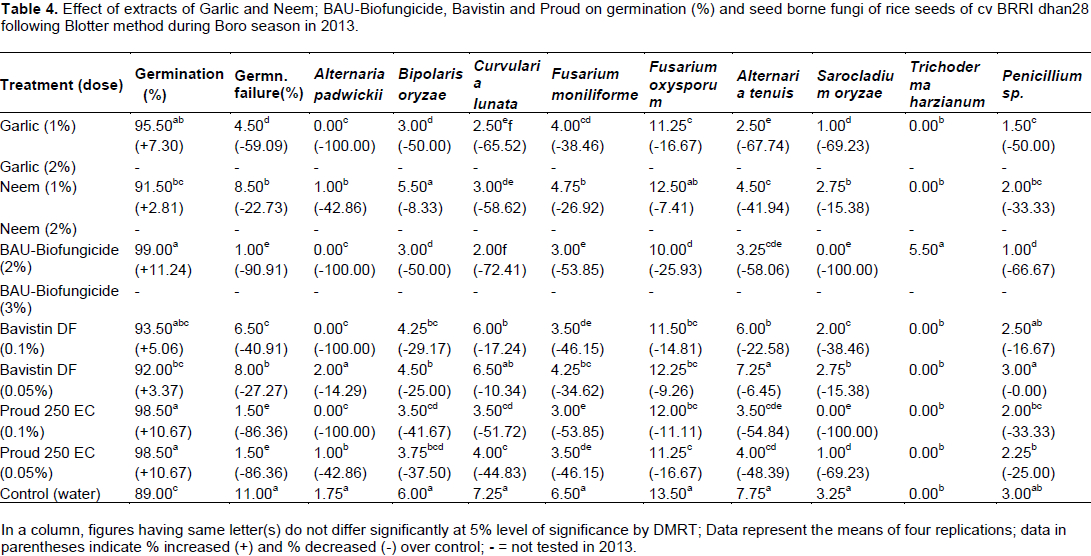
Hundred percent reduction of seed-borne infection of A. padwickii was found with BAU-Biofungicide (2 and 3%) and Proud (0.1%) and garlic (1 and 2%). Highest (64.29%) reduction of seed-borne infection of B. oryzae was obtained in harvested seeds by spraying plots with BAU-Biofungicide (2%) over control followed by BAU-Biofungicide (3%) having (57.14%) reduction. Elham et al. (2011) evaluated the efficacy of T. harzianum against B. oryzae. T. harzianum inhibited the growth of rice brown spot pathogen. Farid et al. (2002) also found that four fungicides viz., Bavistin, Hinosan, Tilt 250 EC and Dithane M-45 were evaluated against B. oryzae. Cent percent mycelial growth inhibition was obtained with Dithane-45, while Tilt 250 EC inhibited (95.58%) mycelial growth inhibition at concentration of 500 ppm. Higher reduction (72.41%) of seed-borne infection of C. lunata was recorded in BAU-Biofungicide (2%) followed by BAU-Biofungicide (3%) and garlic (2%). T. harzianum inhibited pathogenic fungus C. lunata to a great extent in rice seed as also reported by Sagar et al. (2005). Jat and Agalave (2013) showed that Trichoderma species inhibited the growth of seed borne pathogn C. lunata. Maximum (25.93%) reduction of seed-borne infection of F. oxysporum was found in BAU-Biofungicide (2%) followed by garlic (2%) and Proud (0.1%) over control as shown in Tables 3 and 4. Patale and Mukadam (2011) reported that T. harzianum inhibited the growth of F. oxysporum and later overgrew the test fungus. Nisa et al. (2011) also evaluated carbendazim, hexaconzol, bitertanol, myclobutanil, mancozeb, captan and zineb and extracts of Allium sativum, Allium cepa and Mentha arvensis for their effect on the inhibition of mycelial growth and spore germination of F. oxysporum. Highest (65.22%) reduction of F. moniliforme was found with BAU-Biofungicide (2%) and Proud (0.05%) over control. These findings were in accordance with the observation of Jat and Agalave (2013). They observed that Trichoderma species inhibited the growth of seed borne infection of F. moniliforme. Sagar et al. (2005) and Ahmad and Mukesh (2002) reported that T. harzianum was effective in reducing seed borne infection of F. moniliforme and increased seedling vigour and seed germination. Maximum inhibition in mycelial growth was observed in hexaconozole at 1000 ppm. Hundred percent reduction of seed-borne infection of S. oryzae was found with BAU-Biofungicide (2%) and Proud (0.1%), while garlic (1%) showed (69.23%) reduction (Table 3 and 4). Kalaiselvi and Panneerselvam (2015) reported that T. harzianum was found to be most effective with 96% inhibition against S. oryzae over control after 7th day of incubation by dual culture. Highest (66.67%) reduction of seed-borne infection of penicillium sp was recorded in BAU-Biofungicide (2%) followed by Proud (0.1%) in Tables 3 and 4.
Patale and Mukadam (2011) also found that three species of Trichoderma showed antagonistic activity against P. notatum. These findings were also supported by Jat and Agalave (2013). T. harzianum was associated with B. oryzae infected seeds and when kept for few days for incubation it overgrew B. oryzae. T. harzianum was associated even one year preservation of seeds of BAU-Biofungicide sprayed plot.
Highest net profit Tk 69578.00 was achieved in case of foliar spray of Proud (0.1%) followed by Tk 66695.00 with foliar spray of BAU-Biofungicide (2%). The benefit-cost ratio (BCR) 2.87:1 was found in Proud (0.1%), while BAU-Biofungicide (2%) showed 2.78:1 (Table 5). Hasan et al. (2014) reported that BAU-Biofungicide and Bavistin were found to have in controlling tikka disease of groundnut under field condition. They obtained benefit-cost ratios by 2.64:1 and 2.30:1 in application of BAU-Biofungicide (3%) and Bavistin (0.1%), respectively as foliar spray. Hossain (2012) applied BAU-Biofungicide and Tilt for controlling biological control of leaf blight of wheat under field condition. The benefit-cost ratio (2.16:1) was achieved in BAU-Biofungicide (2.5%) (seed treatment plus foliar spray), while by Bavistin 0.1% + Tilt 0.1% was 2.33:1.
Proud (0.1 and 0.05%) showed profound effect in controlling brown spot disease of rice, while BAU-Biofungicide (2%) was found to have in reducing disease severity as well as reduced cost of production with BCR and protecting seed borne pathogens in filed as they were applied as foliar spray. Highest benefit-cost ratio (BCR) 2.87:1 was found in Proud (0.1%) which is close to BAU-Biofungicide (2%) spray (2.78:1). It was evident that BAU-Biofungicide (2%) pronounced significant effect in increasing germination of seeds, seedling growth and vigor index and enhanced grain yield of harvested seeds in BAU-Biofungicide sprayed plot.
The authors have not declared any conflict of interests
The author gratefully acknowledge the funding authority, Director of World Assembly of Muslim Youth, Dawah Program, Dhaka, Bangladesh
REFERENCES
|
Agarwal PC, Mortensen CM, Mathur SB (1989). Seed-born diseases and seed health testing of rice. Technical Bulletin No. 3, Phytopathlogical Paper No. 30, CAB International Mycological Institute (CMI) Kew, Surrey, UK. pp. 58-59.
|
|
|
|
Ahmad S, Mukesh S (2002). Evaluation of fungal and bacterial antagonists against Fusarium nioniiiforme causing foot rot disease of rice. Ann. Agric. Res. 23:319-321.
|
|
|
|
|
Baki AA, Anderson JD (1973). Vigour determination of soybean seed by multiple criteria. Crop Sci. 13:630-633.
Crossref
|
|
|
|
|
Barnwal MK, Kotashthane A, Magculia N, Mukherjee PK, Savary S, Sharma AK, Singh HB, Sing US, Sparks AH, Varier M, Zaidi N (2013). A review on crop losses, epidemiology and disease management of Brown spot to identify research priorities and knowledge gaps. Eur. J. Plant Pathol. 136(3):443-457.
Crossref
|
|
|
|
|
BBS (2014). Bangladesh Bureau of Statistics. Ministry of Planning. Government of the People's Republic of Bangladesh, Dhaka, Bangladesh.
|
|
|
|
|
Biswas SK, Ratan V, Srivastava SSL, Ramesh S (2008). Influence of seed treatment with biocides and foliar spray with fungicides for management of Brown leaf spot and Sheath blight of paddy. Indian Phytopathol. 61(1):55-59.
|
|
|
|
|
BRRI (2004). Modern Rice Cultivation. Bangladesh Rice Research Institute. Gazipur. pp. 6-27
|
|
|
|
|
Chowdhury MMH (2012). Evaluation of quality status and management Truthfully Labeled Seed (TLS) of rice in Bangladesh, PhD Thesis, Bangladesh Agricultural University, Mymensingh.
|
|
|
|
|
Chowdhury SP, Hasan MM, Alam S, Chowdhury AN, Alam MS (2005a). Effect of plant extracts on seed borne fungi of lentil (Lensesculenta moench). Bangl. J. Crop Sci. 16(2):197-205.
|
|
|
|
|
Cumagun CJR (2012). Managing Plant Diseases and Promoting Sustainability and Productivity with Trichoderma. J. Agric. Scic. Technol. 14:699-714.
|
|
|
|
|
Dasgupta MK (1988). Principles of Plant Pathology. Applied Publisher Pvt. Ltd. New Delhi p. 706
|
|
|
|
|
Elham K, Sadravi M, Naeimi S, Khosravi V (2011). Biological control of rice brown spot with native isolates of three Trichoderma. Braz. J. Microb. 43(1):297-305.
|
|
|
|
|
Fakir GA (2004). An annotated list of seed borne diseases in Bangladesh. Seed Pathology Centre, Department of Plant Pathology. BAU, Mymensingh.
|
|
|
|
|
Fakir GA, Mia MAT (2004). The quality of Farmer saved rice seeds in Bangladesh. National workshop on seed health improvement. BARC, Dhaka.
|
|
|
|
|
Farid A, Khalequzzaman KM, Islam N, Anam MK, Islam MT (2002). Effect of fungicides against Bipolaris oryzae of rice under in vitro condition. Pak. J. Plant Pathol. 1(1):4 -7.
Crossref
|
|
|
|
|
Gomez KA, Gomez AA (1984). Statistical Procedures for Agricultural Research 2nd Edition. Kohn Wiley and Sons, New York. pp. 207- 215
|
|
|
|
|
Gupta V, Shamas N, Razdan VK, Sharma BC, Sharma R, Kaur K, Chapal Johnand DJ, Kumar A (2013). Foliar application of fungicides for the management of brown spot disease in rice (Oryza sativa L.) caused by Bipolaris oryzae. Afr. J. Agric. Res. 8(25):3303-3309.
|
|
|
|
|
Haq Mainul, Mia MAT, Rabbi MF, Ali MA (2008). Incidence and severity of rice diseases and insect pests in relation to climate change. International Symposium on Climate Change and Food Security in South Asia. Dhaka, Bangladesh. pp. 1-37
|
|
|
|
|
Hasan MM, Chowdhury SP, Alam S, Hossain B, Alam MS (2005). Antifungal effect of plant extracts on seed-borne fungi of wheat seeds regarding seed germination, seedling health and vigour index. Pak. J. Bio Sci. 8(9):1284-1289.
Crossref
|
|
|
|
|
Hasan MM, Islam MR, Hossain I, Shirin K (2014). Biological Control of Leaf Spot of Groundnut. J. Biosci. Agric. Res. 01(02):66-78.
|
|
|
|
|
Hossain I (2011). BAU-Biofungicide: Unique Eco-friendly Means and New Dimension of Plant Disease Control in Bangladesh. Leaflet published from the Dept. of Plant Pathology, Bangladesh Agricultural University, Mymensingh. pp. 8-11
|
|
|
|
|
Hossain M (2012). Biological control of leaf blight (Bipolaris Sorokiniana) of wheat. PhD Thesis, Bangladesh Agricultural University, Mymensingh.
|
|
|
|
|
Hossain MM, Hossain I, Khalequzzama KM (2015). Effect of seed treatment with Biological control agent against Bipolaris leaf bight of wheat. Inter. J. Sci. Res. Agric. Sci. 2(7):151-158.
|
|
|
|
|
IRRI (1996). Standard Evaluation System of Rice, 4th ed. International Rice Research Institute, P.O. Box 933,1099 Manila, Philippines. P 52
|
|
|
|
|
ISTA (1996). International rules for Seed Testing. Seed Sci. Technol. 4:3-49.
|
|
|
|
|
Jat JG, Agalave HR (2013). Antagonistic properties of Trichoderma species against oilseed-borne fungi. Sci. Res. Rep. 3(2):171-174.
|
|
|
|
|
Kalaiselvi S, Panneerselvam A (2015). In-vitro evaluation of fungicides and two species of Trichoderma against sarocladium oryzae causing sheath rot of paddy (oryza sativa l.). World J. Pharm. Res. 4(2):1200-1206.
|
|
|
|
|
Kamal MM, Mia MAT (2009). Diversity and pathogenicity of the rice brown spot pathogen, bipolaris oryzae (breda de haan) shoem. In Bangladesh assessed by genetic fingerprint analysis. Bangladesh J. Bot. 38(2):119-125.
|
|
|
|
|
Lore JS, Thind TS, Hunjan MS, Goel K (2007). Performance of different fungicides against multiple diseases of rice. Indian Phytopathol. 60:296-301.
|
|
|
|
|
Mahmud H, Hossain I (2013). Eco-friendly management of brown spot (Bipolaris oryzae) of rice and quality seed production. 10th International Congress of Plant Pathology. The Chinese Society for Plant Pathology. P 89.
|
|
|
|
|
Mia MAT, Ahmad MU, Sharma NR, Ali A, Miah SA (1990). Antifungal activity Antifungal activity of some plant extracts. Bangladesh J. Bot. 19(1):5-10.
|
|
|
|
|
Nisa TU, Wani AH, Bhat MY, Pala SA, Mir RA (2011). In-vitro inhibitory effect of fungicides and botanicals on mycelia growth and spore germination of Fusarium oxysporum. J. Biopest. 4:53-56.
|
|
|
|
|
Ora NAN, Faruq MT, Islam N, Aktar, Rahman MM (2011). Detection and Identification of Seed Borne Pathogens from Some Cultivated Hybrid Rice Varieties in Bangladesh. Middle-East J. Sci. Res. 10(4):482-488.
|
|
|
|
|
Padamanabhan SP (1973). Great Bengal Famine. Ann.-Res. Phytopathol. pp. 11-24
Crossref
|
|
|
|
|
Patale SS, Mukadam.DS (2011). Management of plant pathogenic fungi by using Trichoderma species. Biosci. Dis. 2(1):36-37.
|
|
|
|
|
Razu MAU, Hossain I (2015). Eco-friendly management of rice diseases. Inter. J. Appl. Sci. Biotechnol. 3(1):80-88.
Crossref
|
|
|
|
|
Sagar SD, Hegde YR, Kulkarni S, Rao MSL (2005). Biocontrol of seed mycoflora of rice. Ann. Biol. 21:217-220.
|
|
|
|
|
USDA (2015). World Rice Production. The United States Department of Agriculture.
|
|
|
|
|
USDA/Foreign Agricultural Services (2014). World Agricultural production pp. 10-14.
|
|