ABSTRACT
The aim of this study was to characterize cotton cultivars based on growth data and on antioxidant enzymes activity, in order to identify better-adapted cultivars to water-stress conditions. Nine genotypes were submitted to 7 days of water suppression starting at 45 days after seedling emergence (phase R1). The essay was carried out in greenhouse conditions, where plants were grown in 5 L vases filled with sandy soil previously fertilized as recommended for the crop. A completely randomized experimental design adopted in a 9×2 (genotypes × water treatment) factorial scheme and 4 replications. Cultivar classifications were performed by multivariate analysis, using canonical variable and UPGMA cluster analysis. The following growth traits were recorded: Plant height, leaf stalk diameter, number of leaves and root length and weight. Antioxidative activities (SOD, CAT and APX) were also tested by spectrophotometry. Water stress affected all genotypes with different response level. The genotypes adapted to semiarid environment showed better capacity to grow under water limitation, and also better performance of antioxidative enzymes in order to avoid cellular damages. BRS 286, CNPA 7MH and CNPA 5M were better adapted to drought period and regarded as the best cultivars to use in cotton breeding aiming tolerance to water stress.
Key words: Gossypium hirsutum L., antioxidative enzymes, water stress.
Abbreviation:
SOD, Superoxide dismutase; APX, ascorbate peroxidase; CAT, catalase; EDTA, ethylenediamine tetra-acetic acid; NBT, nitrobluetetrazolium; ROS, reactive oxigen species; ¹O2, oxigen singlet; O2•, superoxide; OH•, hydroxil radical; H2O2, hydrogen peroxide; O2, molecular oxigen; CV, canonical variables; UPGMA, unweighted pair group method with arithmetic mean; CCC, cophenetic correlation coefficient.
Plants submitted to environmental stresses respond with several physiological and biochemical chain reactions in order to minimize or avoid the cell damages. Drought is one of the more critical environmental problems because it leads to losses in several crops, worldwide. Damages took place in varied level, depending on genotype,
phenological stage and mainly, intensity and length of dry period. At cell level, when stress signs are perceived by cell, an excessive amount of ROS is generated, such as ¹O2, O2•-, H2O2 and OH•, which lead to oxidative damage at various levels, depending on the tolerance mechanisms of genotype (Sekmen et al., 2014; Farooq et al, 2009). Excessive ROS may cause cell death due to the inactivation of enzymes essential to defense metabolism, and also damages in organelles and membranes due to degradation of proteins, lipids and nucleic acids. The cytotoxic action of ROS is controlled by combined action of plant defense machinery, which includes both enzymatic and non-enzymatic systems. In the enzymatic, the most known involves the actions in cascade started by SOD, which starts the neutralization process and acts as catalyzer of O2•- dismutation into H2O2 and O2. Subsequently, CAT promotes H2O2 inactivation, which is mainly produced during photorespiration process, converting it into H2O and O2. Finally, APX acts in H2O2 decomposition by using ascorbate as reductor, donating electrons to formation water and monodehydroascorbate (Barbosa et al., 2014; Shigeoka et al., 2002; Alscher et al., 2002).
In growth aspect, water stress affects the plant in all phase of development, especially before blooming and the fruit development. Symptoms are often phenotypically apparent, affecting plant height, canopy and root systems due to inhibition of cell expansion (Baldo et al., 2009; Ball et al., 1994). Physiologically, water suppression leads to changes in cell division and expansion. Consequently, other processes are triggered such as reduction in photosynthesis and respiration rates, delay in nutrients absorption and assimilation, losses in turgidity and stomatal conductance, senescence and leaf shedding, among others (Farooq et al., 2009). The response of plant to water stress tolerance depends on several factors, among which are photosynthetic metabolism, genetic inheritance and water suppression period.
Gossypium L. is a malvaceae with more than fifty identified species. Many of them have wide variability to growth in semiarid environment. In Brazil, the types, Gossypium hirsutum subsp. latifolium Hutch, a short cycle-herbaceous and annual and var. Marie-galante Hutch, a late cycle-arbustive and perennial are the main accepted by growers (Freire and Vidal Neto, 2013). The genotypes from M. galante types have broad adaptation to dry environments, although they show less fiber yield than latifolium ones.
Drought tolerance in herbaceous cotton is genotype-dependent, and reduction in canopy, plant height and boll numbers are often seen in sensible plants. The effect of water stress is most critical in reproductive phase, mainly in boll and fiber developments (Baldo et al., 2009; Ball et al., 1994).
Baldo et al. (2009) evaluated cotton genotypes submitted to 45 days of water suppression and observed severe reduction on plant height, stem diameter, number of leaves, number of leaves and reduction of 25% on boll yield, as well. Batista et al. (2010) corroborated these results when evaluated cotton genotypes submitted to 23 days of water suppression. The authors also observed losses on fiber quality, although elongation on root system was verified in tolerant genotypes, as a defense response to desiccation.
In Brazil, the main commercial cultivars growth in Savanna region have limited yield in environments with water irregularities. Considering the world climatic changes, mainly those related to water availability, the development of cultivars tolerant to drought is a valuable strategy adopted by several plant breeder in several countries. Since 1970's, the Brazilian Agricultural Research Corporation (Embrapa) coordinates a robust cotton breeding program, focusing on the development of high yield cultivars, with broad environmental adaptation. Annually, several genotypes are tested by using biochemical and agronomic traits in order to identify promising parental for further use in dialelic crossing aiming tolerance to drought. Several top lines have been obtained by this procedure, revealing expressive production and broad adaptation to semiarid environment, in field conditions (Cavalcanti et al., 2016). As the genetic improvement is a dynamic process, it is necessary that new materials could be periodically evaluated in order for further use in hybridization platforms.
In this work, we evaluated the response to water stress in nine Brazilian cotton cultivars, based on growth analysis and in activity of antioxidative enzymes, during early growth.
Genetic resources and experimental design
The experiment was carried out in greenhouse, in Campina Grande, PB (7º13’50”S, 35º52’52”W, 551m), from July to September 2015. Seeds of nine cultivars were grown in 5L vessels filled with sandy soil previously fertilized with urea (20 Kg ha-1), single super-phosphate (60 Kg ha-1), and potassium chloride (30 Kg ha-1). Two seedlings were maintained per vessel, all daily watered, maintaining moisture near field capacity. The water requirements of plants were estimated according to Almeida et al. (2015).
Treatments were established at R1 phase (45 days after emergence), represented by the beginning of flowering (Marur and Ruano, 2001). Plants were submitted to regular watering (control) and water suppression (stressed treatment) for 7 days. A factorial-completely randomized design was adopted with four replications. Temperature and relative air humidity data were daily collected during assay.
After stresses period, the following data were collected: plant height, main stem diameter, total number of leaves, main root length and fresh weight of root mass. The main characteristics of cultivars used in this work are shown in Table 1.
Biochemical analysis
Biochemical assays were based on activity of antioxidative enzymes. Leaf tissues were collected at the end of water suppression, in control and stressed plants. A crude extract (25%) was obtained from fully expanded leaves, by using monobasic phosphate buffer (100 mM) and EDTA (0.1 mM) (pH 7.0). The activity of antioxidative enzymes (SOD, CAT and APX) was estimated by spectrophotometry (Thermo Scientific-Biomate).
SOD procedure was followed as described in Bulbovas et al. (2005), with minor modifications. The reaction (2.0 ml: 100 mM of monobasic potassium phosphate (pH 7.8), 1 mM EDTA, 13 mM methionine, 75 mM of nitrobluetetrazolium (NBT), 1 nM of riboflavin and 40 µl of plant extract) was exposed to fluorescent light (75 W) for 15 min and read at 560 nm. APX methodology was followed as described in Nakano and Asada (1981). The reaction (1.5 ml: 50 mM of monobasic phosphate buffer and 0.1 µM of EDTA (pH 6.0), 0.5 mM of ascorbate, 1 mM of H2O2 and 75 µM of plant extract. The activity was determined by oxidation of sodium ascorbate during 1 min, at 290 nm. Measurement was carried out using the molar extinction coefficient of 2.8 M-1cm-1. The CAT activity was estimated following Beers Júnior and Sizer (1952) methodology (1.5 ml: 100 mM of monobasic phosphate buffer and 0.1 M of EDTA (pH 7.0), 20 mM of H2O2 and 50 µM of the plant extract). The activity was determined by H2O2 degradation in 1 min, at 240 nm. Measurement was carried out using the molar extinction coefficient of 36 M-1cm-1.
Multivariate statistical analysis
Growth and biochemical data were submitted to Lilliefors test in order to verify the normality. Then, data were submitted to variance analysis, by using F test (p≤0.05). Tukey test (p≤0.05) was used to mean comparisons. For multivariate analysis, we use only statistically significant data from stressed treatment. Dissimilarity measurement between genotypes were based on Mahalanobis method (D2) in which 9 × 9 matrix served as basis to UPGMA clustering method and also to estimate the relative contribution of traits to differentiation of genotypes (Cruz et al., 2012). The adjustment of hierarchical method was based on cophenetic correlation coefficient (Sokal and Rohlf, 1962).
The CV were estimated through transformation of original data into a set with equivalent dimension of uncorrelated data (Cruz et al., 2012). The first CV often explains the maximal amount of variance in the data set and its direction. The scores corresponding to the CVs were calculated from the correlation matrix. The first two CV scores were used to group the genotypes in dispersion graphic. Cluster analysis was performed using the software GENES, version 2013.5.1(Cruz, 2013).
Nine cotton cultivars were submitted to water suppression at beginning of flowering and evaluated as tolerance based on growth traits and activity of antioxidative enzymes. The symptoms of water stress were verified from the fourth day on late cultivars (developed to Cerrado environment), however, at the end of the seventh day, all plants had developed significant growth alterations (Figure 1), particularly on height, number of leaves and root length.
Statistically significant differences (p≤0.01) were verified by analysis of variance (F test) in genotypes and treatments for most growth traits but no significant effect on G x TH interaction was seen for stem diameter or root mass weight, meaning that the behavior of genotypes were similar in both treatments.
Table 2 shows the means obtained for plant height (PH), number of leaves (NL) and root length (RL). Cultivars showed different responses to plant height and number of leaves during water suppression period. FMT 701, CNPA 7MH, FMT 705, FMT 966 and BRS 286 showed minor losses, under to 15% in relation to control plants.
Taking into account the behavior of root system in plants from stressed treatment, we found that the most cultivars showed adjustment to drought condition because they were able to deep their roots into the soil, especially CNPA 7MH, CNPA 5M and 286. As this trait is associated with a morphological mechanism of drought tolerance, these results agree with genealogy of these three cultivars, once they were generated from drought resistance parents and are all adapted to semiarid environment (Carvalho et al., 2014).
However, the behavior of FMT 705 and FMT 701 are inedited because both were developed to Brazilian Cerrados (Freire and Vidal Neto, 2013), and no record about drought tolerance have been found, so far. According to reports in literature, the impact of water stress in cotton crop depends not only on duration but also on physiological phase that takes place (Baldo et al., 2009; Plaut et al., 1996). Roots are the first tissues that detected the dissection signals and provide a prompt response when soil moisture decreases. Plaut et al. (1996) submitted cotton plants to several level of water suppression (20, 40, 60, 80 and 100% pot capacity) at flowering stage (42 to 70 days) and reported an expressive increase in root length density in all moisture contents above 20% in the two deepest soil segments (24 and 36 cm).
No hydrotropism was seen and dry matter production by roots was less severely inhibited than that by shoots. The authors concluded that, even at soils moisture content equivalent to a Ψm of 0.1 MPa, the rate of root growth was sufficient to reach a wetted soil layer at 36 cm. Ball et al. (1994) reported that a short water suppression (5 days) in cotton plants during boll development (55 to 65 days) lead to reduction in leaf expansion up to 61%, while root system is decreased in about 50%. These values are varied, depending on level of tolerance of cultivar, but reduction in growth is a natural strategy in order to minimize the transpiration and, consequently, save water for further use in
physiological processes (Baldo et al., 2009).
As to antioxidative enzyme analyses, statistically significant differences were found in variance analysis to genotypes, water treatments and G x TH (p<0.01) to all three enzymes evaluated, meaning that the antioxidative machinery of cultivars responded differently to water treatments.
The individual behavior of each cultivar to SOD, CAT and APX is presented in Figure 2. These enzymes act in cascade events in order to avoid cell damages caused by ROS production, in response to oxidative stresses.
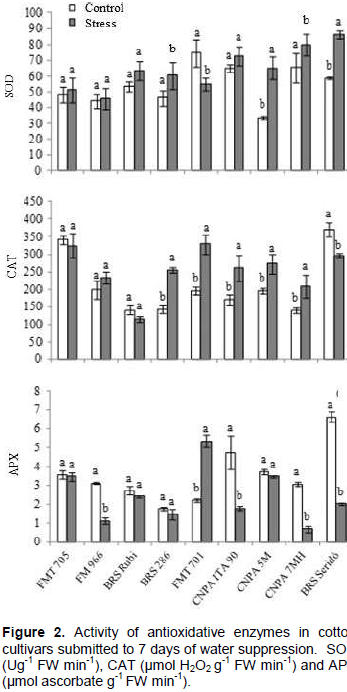
We found an input of 85, 52, 30 and 19% in SOD activity to CNPA 5M, BRS Seridó, BRS 286 and CNPA 7MH, respectively, in stressed treatment. The availability of SOD in cells of these genotypes contributed to better adjustment in defense processes, generating reasonable amounts of CAT, so that, in the end of the antioxidant chain complex, the APX production was normalized (CNPA 5M and BRS 286 in both treatments) or minimized (CNPA 7MH and BRS Seridó). These cultivars, therefore, were more adjusted to water stress, based on conditions of this assay. We also found that FMT 705, FM 966, BRS Rubi and CNPA ITA 90 did not show enough SOD input to trigger the first process of antioxidative complex, so that, the next steps involving neutralization of H2O2 by CAT and APX, were probably circumstantial, and not directly involved with water stress response.
Finally, the behavior of FMT 701 was different from all other cultivars, with low activity of SOD in stressed treatment and a high input of APX (about 135%), at end of antioxidative process. This reaction suggests that the sum of peroxides not neutralized by CAT, overloaded the cell machinery, generating an expressive input of APX. Besides, as peroxidase also generated by photorespiration process, we suggests that the increase seen here may be associated to this event, whose metabolic pathway naturally generates enough ROS. This could explain the over-expression of CAT and APX in this cultivar.
Several works are available in literature reporting the role of antioxidative enzymes in response to water stress. The input of SOD is often reported as a response to drought tolerance in plants due to better ability to eliminate super-oxide from cells (Sekmen et al., 2014; Cataneo et al., 2010; Rahman et al., 2004). As a consequence, the levels of H2O in cells are increased, and further neutralized by CAT and APX, which convert H2O2 into H2O2 +½ O2 and H2O2 into H2O + R(O)2, respectively. The reduction of H2O2 level will contribute to minimize the cell damages caused by oxidative stresses.
Sekmen et al. (2014) submitted two cotton cultivars (tolerant and sensitive to drought), during 10 days of water suppression, from 21 days of emergence. They found an input of 71 and 57% in SOD activity, in tolerant and sensible plants of stressed treatment, indicating that tolerant plants have better adjustment to eliminate O2-. When this elimination is limited by cell machinery, the superoxides may form hyperoxides in the cell leading to cell damages and further physiological changes.
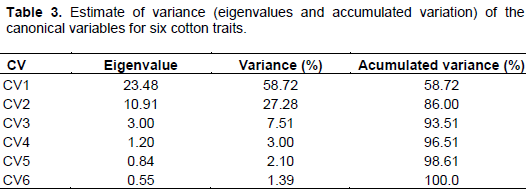
In order to estimate the genetic divergence among cultivars, CV analysis was performed using six statistically significant traits from stressed treatment. Table 3 displays the eigenvalues, individual and accumulated variance (%) associated with CV. The first two CV explained 86% of total variance (CV1 = 58.72%; CV2 = 27.28%), indicating that the most variability may be summarized in these two components and genotypes may be plotted in a two-dimensional graphical dispersion.
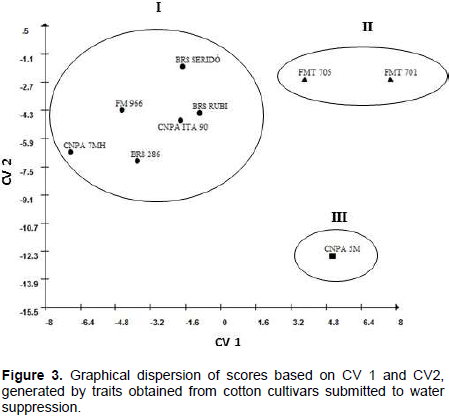
Three groups were formed with clear distinction as to drought tolerance: 1: represented by six annual cultivars: Two mid-cycle and developed for Cerrado region (FM 966 and CNPA ITA 90) and three earliness and developed for semiarid conditions (BRS 286, BRS Rubi, BRS Seridó and CNPA 7MH). It worth noting that, although FM 966 and CNPA ITA 90 are not recommended for dry regions, both have broad environmental adaptation, inherited from Deltapine Acala 90, an annual cultivar developed in USA West (Longenberger, 2008); 2: Represented by FMT 705 and FMT 701, both late cycle cultivars, developed for Cerrado region, with high technological input (Freire and Vidal Neto, 2013; Anselmo et al., 2009); and 3: represented only by CNPA 5M, a perennial type, with broad adaptation to dry environments (Souza and Silva, 1994).
The relative contribution of traits to the genetic divergence was estimated based on S.j statistical (Singh, 1981). The analysis showed that APX, number of leaves, root length and plant height were the most contributive traits, with 53, 15, 13 and 12%, respectively.
In order to corroborate the results seen by graphical dispersion, an UPGMA clustering analysis was also performed, based on dissimilarity coefficient ≥ 80% and CCC = 0.79 (p<0.01). Three groups were formed (Figure 4), with identical clustering seen in Figure 3, indicating consistency of both multivariate methods as to genetic divergence of cotton cultivars.
Drought is a phenomenon established in tropical and semiarid environments and leads to different levels of damage in several crops. The improvement focused on drought tolerance is a relevant goal in many breeding programs around the world. Plants with ability to adjust the solutes in order to avoid the cell damages faced by
water suppression are robust candidates to breeding focusing on yield and environmental adaptations.
Cotton is a fiber crop of significant importance to textile industry. The Brazilian breeding program coordinates by Embrapa have made efforts in order to develop competitive cultivars, to attend the national and international fiber markets. Previous knowledge of genetic background of parents is quite useful in order to shorten the selection procedures, often requested for development of cultivars. The results showed here are contributive to assist in cotton breeding and provide information about promising candidates for further improvement to environment with water limitation.
Based on climatic changes verified in several Brazilian regions, especially in water availability, we suggest the use of cotton cultivars from Groups I and II in dialelic crossings in order to combine yield and drought tolerance for further selection of promising top lines with broad yield stability and environmental adaptation.
The authors have not declared any conflict of interests.
SOD, Superoxide dismutase; APX, ascorbate peroxidase; CAT, catalase; EDTA, ethylenediamine tetra-acetic acid; NBT, nitrobluetetrazolium; ROS, reactive oxigen species; ¹O2, oxigen singlet; O2•, superoxide; OH•, hydroxil radical; H2O2, hydrogen peroxide; O2, molecular oxigen; CV, canonical variables; UPGMA, unweighted pair group method with arithmetic mean; CCC, cophenetic correlation coefficient.
REFERENCES
|
Almeida LLS, Nobre RG, Souza LP, Barbosa JL, Elias JJ (2015). Crescimento do algodoeiro colorido pós-poda em solos com distintas PSTs e doses de esterco. RVADS 10(4):06-11.
|
|
|
|
Alscher RG, Erturk N, Heath LS (2002). Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 53(372):1331-1341.
Crossref
|
|
|
|
|
Anselmo JL, Costa DS, Leal JF (2009). Ensaio de competição de cultivares de algodoeiro em Chapadão do Sul - MS. In: Congresso Brasileiro do Algodão, Campina Grande. Anais, Embrapa Algodão. pp. 1567-1571.
|
|
|
|
|
Baldo R, Scalon SPQ, Rosa YBCJ, Mussury RM, Betoni R, Barreto WS (2009). Comportamento do algodoeiro cultivar delta opal sob estresse hídrico com e sem aplicação de bioestimulante. Cienc.Agrotec. 33:1804-1812.
Crossref
|
|
|
|
|
Ball RA, Oosterhuis DM, Mauromoustakos A (1994). Growth dynamics of the cotton plant during water-deficit stress. Agron. J. 86:788-795.
Crossref
|
|
|
|
|
Barbosa MR, Silva MMA, Willadino L, Ulisses C, Camara TR (2014). Geração e desintoxicação enzimática de espécies reativas de oxigênio em plantas. Cienc. Rural. 44(3):453-460.
Crossref
|
|
|
|
|
Batista CH, Aquino LA, Silva TR, Silva HRF (2010). Crescimento e produtividade da cultura do algodão em resposta a aplicação de fósforo e métodos de irrigação. Rev. Bras. Agric. Irrigada. 4(4):197-206.
Crossref
|
|
|
|
|
Beers Júnior RF, Sizer IW (1952). A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 195(2):133-140.
|
|
|
|
|
Bulbovas P, Rinaldi MCS, Delitti WBC, Domingos M (2005). Variação sazonal em antioxidantes em folhas de plantas jovens de Caesalpinia echinata Lam. (pau-brasil). Rev. Bras. Bot. 28(4):687-696.
Crossref
|
|
|
|
|
Carvalho LP, Farias JFC, Lima MMA, Rodrigues JIS. (2014). Inheritance of different fiber colors in cotton (Gossypium barbadense L.). Crop Breed. Appl. Biotechnol. 14(4):256-260.
Crossref
|
|
|
|
|
Cataneo AC, Chamma KL, Ferreira LC, Déstro GFG, Sousa DCF (2010). Atividade de superóxido dismutase em plantas de soja (Glycine max L.) cultivadas sob estresse oxidativo causado por herbicida. Rev. Bras. Herbicidas. 4(2):23-31.
Crossref
|
|
|
|
|
Cavalcanti JJV, Vasconcelos UAA, Vasconcelos WS, Santos RC, Farias FJC, Araújo GP, Assunção JH (2016). Combining ability estimates for agronomic and morphological traits in cotton under water stress. In: World Cotton Research Conference – 6. Goiana: Brazilian Cotton Institute. Abstracts. P 131.
|
|
|
|
|
Cruz CD (2013). GENES - a software package for analysis in experimental statistics and quantitative genetics. Acta Sci. Agron. 35(3):271-276.
Crossref
|
|
|
|
|
Cruz CD, Regazzi AJ, Carneiro PCS (2012). Modelos biométricos aplicados ao melhoramento genético. Viçosa: UFV. 514p.
|
|
|
|
|
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA (2009). Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 29:185-212.
Crossref
|
|
|
|
|
Freire EC, Vidal Neto FC (2013). Melhoramento Genético do Algodoeiro. In: Vidal Neto FC, Cavalcanti JJV. (Ed.) Melhoramento Genético de Plantas no Nordeste - Brasília, DF: Embrapa. p. 49-83.
|
|
|
|
|
Longenberger PS (2008). Evaluation of chlorophyll fluorescence as a tool for the identification of drought tolerance in upland cotton (Dissertation).Texas A&M University: Texas. 129p.
|
|
|
|
|
Marur CJ, Ruano O (2001). A reference system for determination of cotton plant development. Rev. Oleaginosas Fibrosas 5(2):313-317.
|
|
|
|
|
Nakano Y, Asada K (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidases in spinach chloroplast. Plant Cell Physiol. 22(5):867-880.
|
|
|
|
|
Plaut Z, Carmi A, Grava A (1996). Cotton root and shoot responses to subsurface drip irrigation and partial wetting of the upper soil profile. Irrig. Sci.16:107-113.
Crossref
|
|
|
|
|
Rahman SML, Mackay WA (2004). Superoxide dismutase and stress tolerance of four tomato cultivars. Hortic Sci. 39(5):983-986.
|
|
|
|
|
Sekmen AH, Ozgur R, Uzilday B, Turkan I (2014). Reactive oxygen species scavenging capacities of cotton (Gossypium hirsutum) cultivars under combined drought and heat induced oxidative stress. Environ. Exp. Bot. 99:141-149.
Crossref
|
|
|
|
|
Singh D (1981). The relative importance of characters affecting genetic divergence. Indian J. Genet. Plant Breed. 41(2):237-245.
|
|
|
|
|
Shigeoka S, Ishikawa T, Tamoi M, Miyagaw Y, Takeda T, Yabuta Y, Yoshimura K (2002). Regulation and function of ascorbate peroxidase isoenzymes. J. Exp. Bot. 53(372):1305-1319.
Crossref
|
|
|
|
|
Sokal RR, Rohlf FJ (1962).The comparison of dendrograms by objective methods. Taxonomy 11(1):30-40.
Crossref
|
|
|
|
|
Souza JG, Silva JV (1994). Fenologia e fisiologia do algodoeiro arbóreo após a seleção para acúmulo de amido nas raízes. Rev. Bras. Fisiol. Veg. 6(2):145-148.
|
|