Root-knot nematodes (Meloidogyne javanica) interferes with the production of tomato. The indiscriminate use of chemical nematicides brings negative consequences to society. The aim of this study was to investigate the effect of crambe extract on the control of M. javanica, with different modes and application times, in tomato. Studies were conducted during the 2014 cropping season at the climatized greenhouse located at the Biological Cotrol Complex and Protected Cultivation. Prof. Dr. Mário César Lopes, belongs to State University of West Paraná- UNIOESTE, campus Marechal Cândido Rondon, Paraná, Brazil. The experiment was laid out in a factorial design. The roots of tomato seedlings were immersed in the extract, with different modes of application (roots + soil, roots + leaf, roots + soil + leaf), in four different times of application (before inoculation; during the inoculation; after inoculation; and weekly until 45 days after inoculation). Seven days after the transplant of tomato plants was performed, evaluations were made for the inoculation of 2.500 eggs of M. javanica (and 513 J2) per pot, and 45 days after inoculation. For egg mass, times after inoculation and weekly were efficient, with greater reduction by way of root + leaf and root + soil, respectively. The reduction of total root-knots was efficient for the weekly time by way of root + soil and root + soil + leaf, and lesser than 41 and 47.75% compared to the control. In the repetition of the experiment, for modes by root + soil + leaf in weekly applications, eggs mass and total root-knots were lesser than 60.95 and 27.95% compared to the control. Although, other methods and application times also present positive results in reducing J2 and eggs per gram of root and per 100 cm3 of soil, the weekly time by way of root + soil + leaf confirmed their results in a repetition of the experiment with reduced population M. javanica.
Tomato (Solanum lycopersicum L.) is the most popular vegetable in Brazil (Belan et al., 2011) and its area under cultivation has increased within decade, from 60 thousands há in 2005 to 6 5 thousands in 2014 (IBGE,
2015). Nematodes pose a threat to tomato production. Root-knot nematodes (Meloidogyne species) causes annual estimated losses of $118 billion to crops in the world (Atkinson et al., 2012).
Root-knot nematodes are found in tropical and temperate regions and are among the most damaging pathogens worldwide (Trudgill and Blok, 2001). This genus makes drastic changes in root development, to induce and keep on the giant cells, which are power supply to the nematodes (Caillaud et al., 2008). Giant cells are the result of cellular hypertrophy and hyperplasia and symptoms are associated with root-knots and reduction in volume of the root system, and consequently, the plant soak up less water and nutrients, resulting in lesser crop yields (Ferraz and Monteiro, 2011). When the second stage juvenile (J2) infects a root and establishes its feeding site, a reprogramming of the cells correlated with gene activity in the metabolism, protein synthesis, cell division and transport, and signal transduction takes place (Gheysen and Fenoll, 2002).
The plant does not allow a passive penetration of the pathogen, but it activates defense system, including protein-RP, phytoalexins, phenolic compounds and other (Molinari, 1996; Stangarlin et al., 2011). For activation of plant defense mechanisms in nematode control, abiotic and biotic inducers can be used (Salgado and Silva, 2005).
Chemical nematicides are used to control nematodes, but its continued use can reduce its efficiency, making nematodes resistant, in addition to negative impacts on the environment and society (Javed et al., 2008; Zhang et al., 2012). Therefore, there is an increasing demand for development of new natural nematicides, aiming a partial or total replacement of chemical nematicides (Aissani et al., 2013). The tomato crop is too dependent on the use of pesticides, thus it is necessary to study alternative actions, and understand the applicability and plant extracts mode of action, so these can be used efficiently in the management of diseases (Schwan-Estrada, 2009). Studies on the use of secondary plant metabolites against root-knot nematodes have been reported by several workers (Dias-Arieira et al., 2003; Lopes et al., 2005; Oka et al., 2006).
Plant extracts have been used in countless modes and application times for the control of nematodes, with protective or healing effect, induction of resistance or direct effect (Chinnasri et al., 2003; Molinari and Loffredo, 2006; Javed et al., 2008). However, several studies have shown nematode control efficiency correlated with glucosinolates in plants, particularly, plants belonging to the family Brassica (Walker, 1996; Wu et al., 2011; Aissani et al., 2013). The crambe (Crambe abyssinica Hochst) belongs to the Brassicaceae family and it contains glucosinolates (Lazzeri et al., 1994). However, there are few studies involving crambe in control of nematodes, as well as studies involving activation of resistance mechanisms in Brassica. Therefore, considering the demand of consumers for safe food associated with beneficial effects caused by the compounds present in Brassicas and few crambe studies, the study aimed to evaluate the crambe extract effect on control of Meloidogyne javanica, with diferent modes and application times in tomato.
The experiment was conducted at the climatized greenhouse located at the Biological Cotrol Complex and Protected Cultivation of Prof. Dr. Mário César Lopes, belonging to State University of West Paraná- UNIOESTE, campus Marechal Cândido Rondon, Paraná, Brazil. The experiment was conducted between the periods of April and June, 2014 and it was repeated in October to December, 2014. The experiment was conducted in a greenhouse and the pots were arranged in a completely randomized design (CRD) on benches at a mean temperature of 22°C, with five replications.
The hydroalcoholic extract of 250 mg L-1 (70% ethanol and 30% water) was used and stored at the ambient temperature, protected of light for 15 days (Loguercio et al., 2005), evapored and resuspended in water. The crambe leaves were collected at vegetative stage (35 days after emergence, DAE), were dried at 45°C for 48 h and gound. Tomato seedlings, Santa Cruz Kada, were used for 25 days after sowing (DAS), and transplanted in pots with 3 L capacity, filled with sterilized soil by autoclaving at 120°C/1 atm for 1 h, in the mixture 3:2:1 (soil: sand: organic matter). Later, a soil sample was sent for chemical analysis, for later correction.
The inoculum was obtained from infected tomato plants cultivated in greenhouse and identified based on perineal region setup (Hartman and Sasser, 1985). The variables were analyzed 45 days after inoculation, concomitantly with the end of the experiment.
Data were collected based on the number of eggs and J2 per 100 cm3 of soil and grams root, number of root-knots and egg mass in the roots. After separation of the roots from the soil, they were washed and dried at room temperature to fresh root mass weighing, with subsequent storage of roots and soil in plastic bags, with temperature at 4°C.
The centrifugal flotation method in sucrose solution was used for the extraction of eggs and soil nematodes (Jenkins, 1964). Extraction of M. javanica eggs and J2 from infested tomato roots was based on the method described by Freitas et al. (2007). The eggs and J2 retained on the 400 mesh sieve were transferred to Peters blade and quantified by optical microscope.
To check for egg mass in the roots, these egg mass were stained with Phloxine B to 15 mg L-1 for 20 min, then washed to remove excess dye (Taylor and Sasser, 1978), dried with paper towel and counted root-knots with egg mass and no mass eggs, with table magnifying aid. The total root-knots was obtained by the sum of root-knots egg mass and no mass eggs.
The experiment was laid out in a factorial design (3×4 + 1). The extract was first applied by way of the root system and later in three different modes in the plant: soil, leaf and soil + leaf in four application times: (1) previously inoculation (three days after transplanting); (2) during inoculation (seven days after transplanting); (3) after inoculation (one week after inoculation); and (4) weekly until 45 days after inoculation (including all application times mentioned earlier) and an additional treatment: inoculated and untreated control.
The plants were treated with fungicide (Trifloxystrobin + Tebuconazole (75 + 150 g, i.a. ha-1) due to the presence of Stemphylium leaf, caused by the fungi Stemphyllium species, 15 days after transplanting, in a interval of 3 days between the application of the extract treatment, and the second fungicide application was made 15 days after the first application.
The extract was first applied by way of the root system in all plants except for the control, at transplanting time and seedlings roots were immersed for a period of
3 seconds and transplanted in the pots; later, the plants with treatment by way of the leaves had their powdered leaves in the adaxial face until point of dripping. Not to come into contact with the ground, the pots were covered with aluminium foil and journal. Order for the spray does not come into contact with other plants; the pots were removed and isolated. Treated plants received 30 ml of extract by way of soil per pot, corresponding to 1% v/v. All treatments were performed in the morning. Seven days after the transplant, the inoculation of 2.500 eggs of
M. javanica. (and 513 J2) per pot was performed.
Data analysis was performed Lilliefors normality test at 5% probability. In the absence of normality, test Q was used; therefore, all the data were with four replications. Even in the absence of normality, data from egg mass and J2 and eggs per 100 cm
3 of soil were transformed to
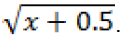
Eggs and J2 per g of root data were transformed into log (x+1). Thereafter, the analysis of variance were carried out and means related to treatment were compared to Tukey test médium. When the additional treatment was significant in the analysis of variance, an additional test was used, Dunnett. Statistical software genes (Cruz, 2006) was used. The experiment was repeated once to confirm the best application times. It was conducted using a factorial design (3x2+1). Being the extract applied by way of the root system and in three different parts of the plant, the application times were: after inoculation (14 DAT) and weekly until 45 days after inoculation. After inoculation and weekly showed greater reduction in the population of
M. javanica and it was repeated for confirmation.
In the application times after inoculation and weekly, the M. javanica egg mass was lesser than in the control (Table 1). After inoculation, the root+leaf and root+ soil+ leaf were lesser than the control in 24.50 and 34.17%, respectively. Already, for the weekly time, the way with less egg mass were root+soil with 27.33% and root+soil+leaf with 42.77% compared to the control. Although these do not differ from each other, the latter presented 15.44% less egg mass than by way of root+soil and 8.60% below the root+soil+leaf after inoculation time. Thus, successive applications and from the beginning of cultivation until the end, increase the tomato protection, for induction of resistance mechanisms and/or direct effect.
Leaf applications of plant extracts can induce systemic nematicide action, with fast and cheap control, and have lesser toxicity, such substances that act systemically can be released via root exudates, changing its composition and giving greater protection to plants (Lopes et al., 2005). In this study, extract applications after inoculation by way of root+leaf, may have presented systemic action, however, the control was better when associated with the application ways. Already, successive applications of the extract by way of root+leaf, may have released exudates that stimulated the hatching and penetration, favoring the development of nematode within the root. According to Dias-Arieira et al. (2003), roots exudates may inhibit or stimulate the emergence of juvenile nematodes.
Oka and Cohen (2001), found that foliar applications of BABA (DL-α-Amino-n-butyric acid) against Meloidogyne in wheat and barley (Hordeum vulgare L.), reduced the number of mass eggs, but higher concentrations of BABA was needed to induce resistance in plants, not showing symptoms of phytotoxicity.
As shown in Table 2, only by way of root+soil and root+soil+leaf in weekly applications differed from the control to reduce root-knots on tomato roots by 41 and 47.75%, respectively, and these did not differ between itself. Foliar applications did not differ from the control in all tested times. These results corroborate the ones found by Lopes et al. (2005), in which leaf applications leaves and seeds extracts of Mucuna pruriens (L.) or Ocimum basilicum L. leaves extracts did not affect the height and fresh weight of the shoots of tomato, as well as the number of eggs per root system and the number of root-knots induced by M. javanica, but the application of the extracts in soil only reduced the number of nematode eggs. It is believed that the degradation by soil microflora may have affected the efficiency of extracts applied to the soil; in addition, the amount of used extract may have been low. This may ocurr in this study for the application by way of soil, leading to the need for repeated applications.
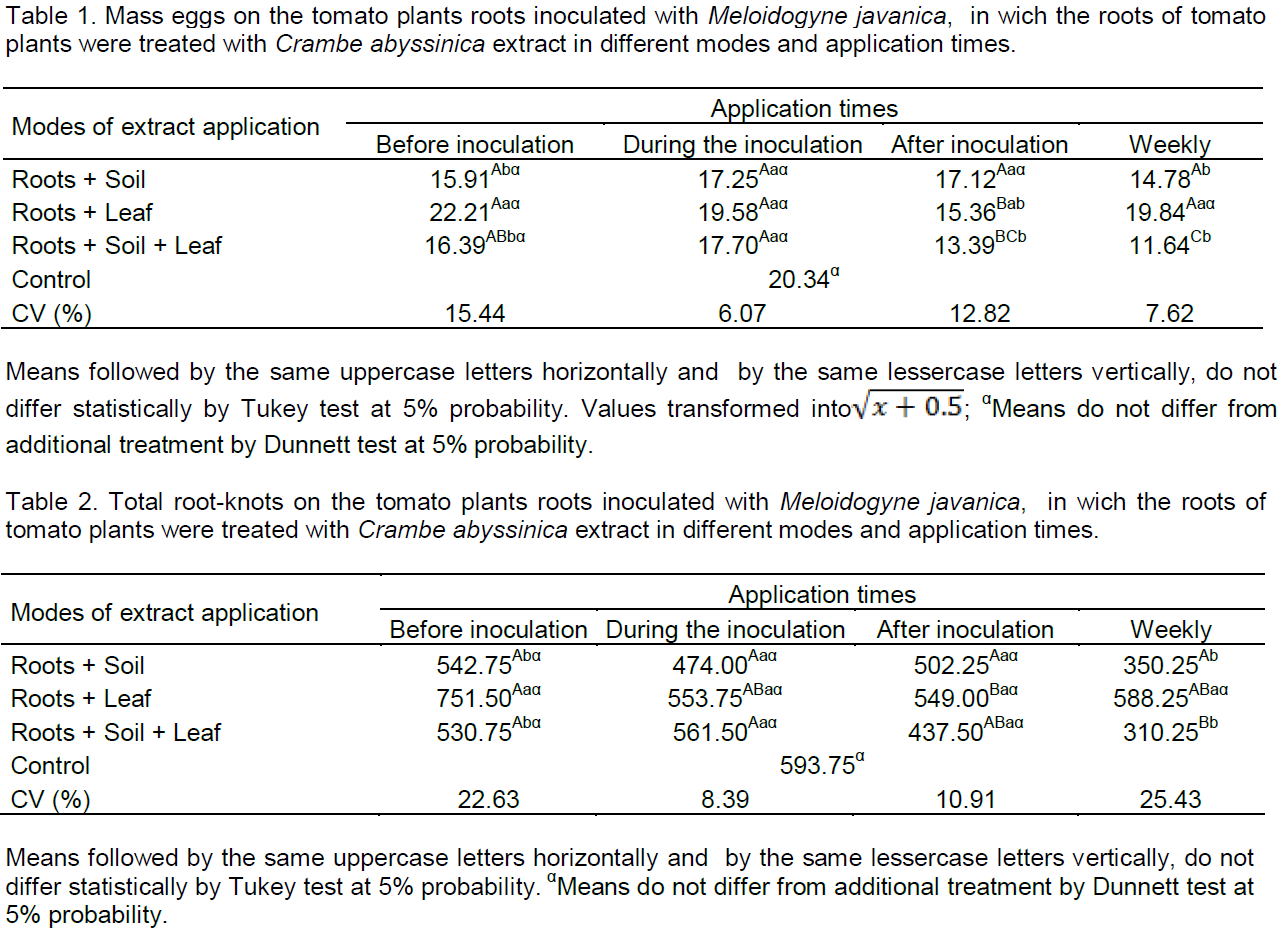
According to Almeida et al. (2012), extracts of different plant species such as neem (Azadirachta indica), nettle (Fleurva aestuans), and castor bean (Ricinus communis) prepared by different methods were applied in the soil and promote the number of root-knots reduction on inoculated tomato plants with M. Javanica eggs/juveniles, making it less attractive to nematodes and reducing parasitism, in a nematicide way, reinforcing the hypothesis that plant metabolites may exhibit biocidal activity. At the time after inoculation, there was no difference between modes of application and the control. However, at this same time, there was a reduction in the mass number of eggs by way of root+leaf and root+soil+leaf, so it can be concluded that the extract has acted in reproduction, once feeding sites were induced. According to Campos et al. (2011), M. javanica can penetrate at the roots and form feeding sites, however, it has not been ensured that it will reproduce, because the nutritional status influence the reproduction. Continuous stimulation of the nematode for induction and maintenance of giant cells is necessary, otherwise the cells be atrophied (Reddigari et al., 1985).
The times before inoculation and during the inoculation did not reduce total egg mass and root-knots (Tables 1 and 2). This may be due to the tomato seedlings being in adaptation period and/or there was no recognition of elicitor and signaling in the plant. Another hypothesis may be due to the inoculum M. javanica, in which different stages of embryogenesis enables the hatching of juveniles along the experiment conduction time.
According to Salgado et al. (2007), regardless of the time, the Acibenzolar-S-Methyl (ASM) application did not differ from control for number of root-knots, reproduction factor and population of Meloidogyne exigua in coffee. The authors attributed this to the inoculum, wich present different stages of embryogenesis, recommending the use of juveniles (J2) rather than eggs, once the penetration phase and early formation of roots feeding site could coincide with the phase of maximum effect ASM. However, Silva et al. (2002) developed a work with ASM applications each 7 days in tomato plants inoculated only with eggs and observed reduction of egg mass and root-knots of Meloidogyne spp.
Times that show effect on egg mass (Table 1) was after inoculation and weekly and on total root-knots (Table 2) weekly. Therefore, these times were repeated (Table 3) for confirmation of the effect on
M. javanica in tomato.
The lowest mass number of eggs was observed only in root+soil+leaf when applied weekly, with reduction of 60.95% compared to the control (Table 3). Thus, can be concluded that there was resistance induction associated with the direct effect on nematodes. Applications of plant extracts of Tagetes patula by way of leaves and soil, activate defense mechanisms in tomato roots against Meloidogyne incognita (Franzener et al., 2007).
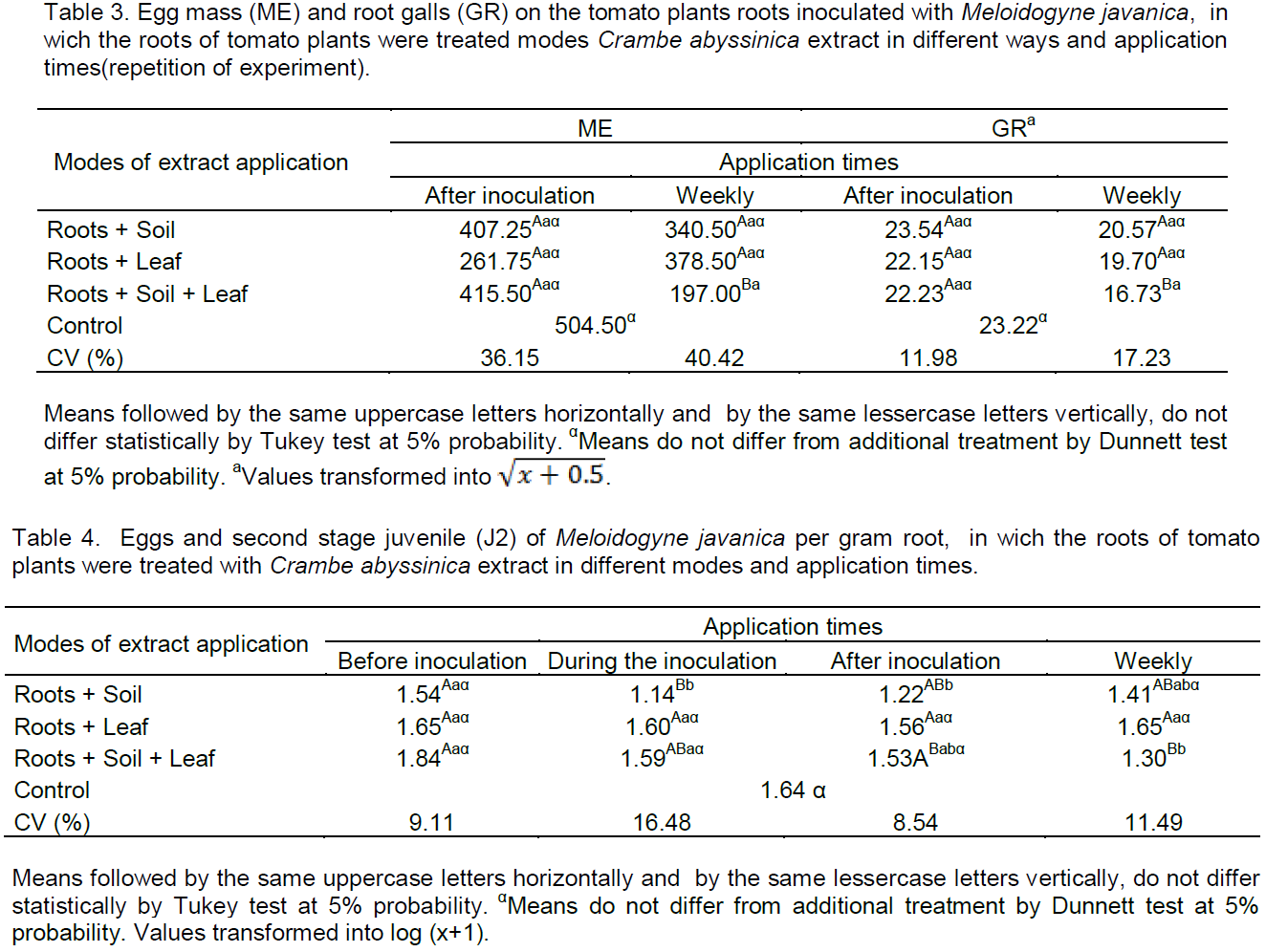
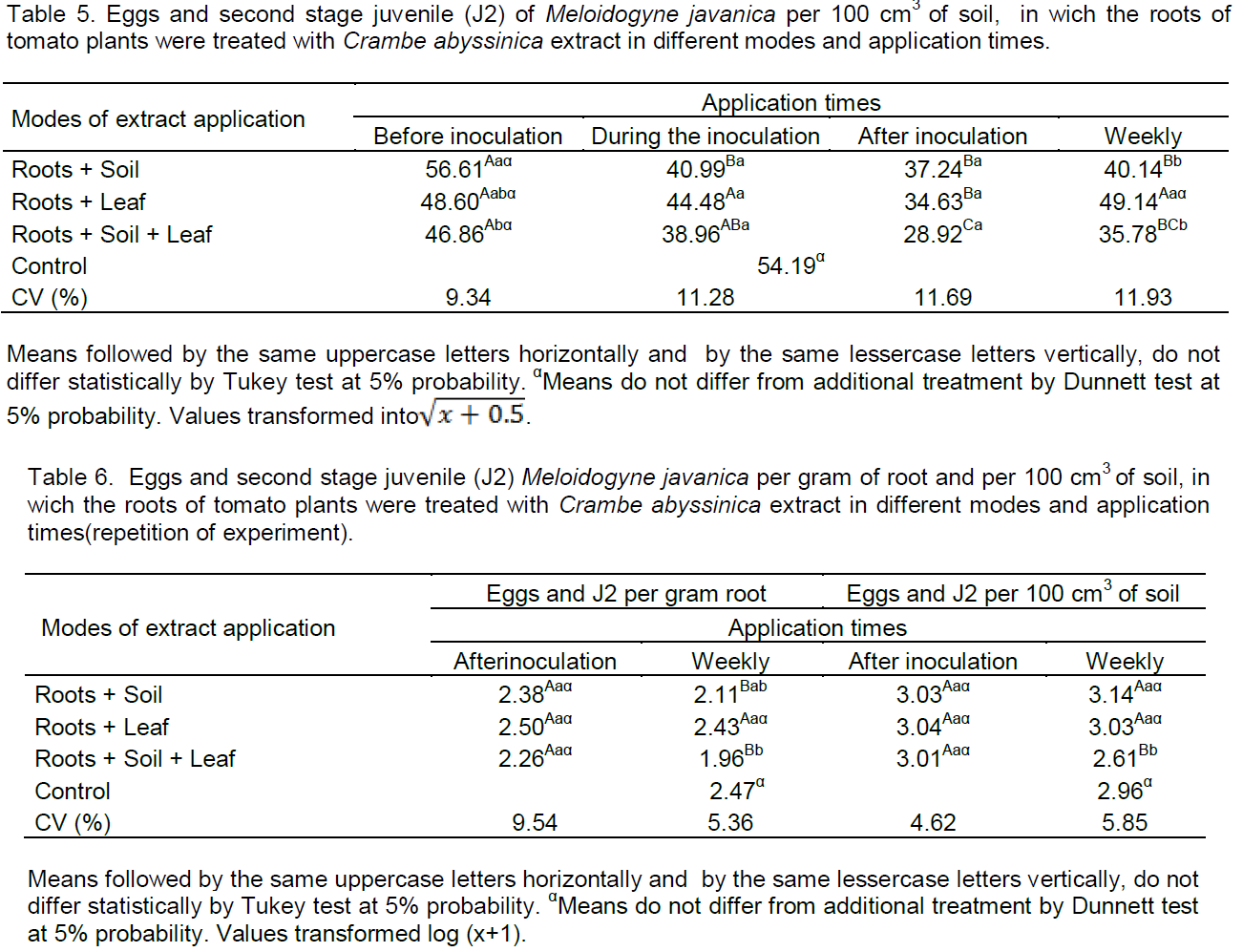
In Table 3, the total number of root-knots by way of root+soil+leaf when applications were weekly, was lesser than 27.95% when compared with the control. Thereby confirming that the crambe extract should be applied from the start of cultivation until the end, and all modes of application, thus, join the possible effects which generated resistance induction and/or direct effect. According to Williamson and Kumar (2006), resistance mechanisms can occur in plants against nematodes, such as cell death near the front end of nematodes, affecting the development of feeding sites, as can occur after the beginning of power, promoting or atrophying an abnormal development of the feeding site.
Allyl isothiocyanate and allyl nitrile derivatives of glucosinolates present in Brassicas and the important in the control of soil pathogens were evaluated for their persistence, that is, expression of biological activity in the soil (Borek et al., 1995). These were influenced by several factors, resulting in irreversible chemical conversion or absorption to soil constituents. The allyl isothiocyanate and allyl nitrile presented an average of 97.1 and 80% of transformation in the first 10 days, respectively. Allyl isothiocyanate was negatively affected by organic carbon and total nitrogen, an increase in temperature of 10 to 25°C adversely affected the useful life. In temperature of 20°C, the useful life was about 4 days, but was positively affected by increased humidity. Since the disappearance of allyl nitrile in soil occurred more quickly in moist soil, there was higher concentrations in organic carbon and low temperatures. Thus, the quick dissipation of both compounds in the soil has important implications for the control of pathogens in soil and must provide multiple spraying or soil application, therefore, isothiocyanates have various reactions with nucleophilic groups, alcohols, phenols, esters, thiols, thiophenols, ammonia, amines, hydroxyamines, and hydrazines (Borek et al., 1995). Perhaps, for that reason, applications in soil must be repeated in this study.
According to Table 4, root+soil had presented less J2 and eggs of M. javanica per g of the root at the time of inoculation, and lesser than 30.49% compared to control. However, the time after inoculation by way of root+soil did not differ statistically with a reduction of 25.61% compared to the control. Despite the fact that the weekly time did not differ statistically from the time of inoculation, it did not differ from the control. These results may be due to the action of the extract on hatching eggs and accordingly J2 is least in the roots. The mechanisms involved in the control of M. javanica by way of root+soil in weekly applications must be related to nutrition, because they had less egg mass and root-knots, not affecting the penetration of J2 as well as root+leaf and root+soil+leaf ground sheet after inoculation, which reduced the reproduction, but did not reduce the penetration.
Acording to Rocha and Campos (2004), exudates cell can act as inhibitors or stimulants to the hatching, as well as nematostáticos or nematicides, since the effect on the enzyme acetylcholinesterase leads the J2 to reduce their movements. So, maybe nematostatic effect for some period occurred in times of inoculation and after inoculation by way of root+soil, but when there was penetration, the nutritional status of the root were enough to develop feeding and reproduction sites.
Corroborating with work for the time after inoculation with application by way of root+soil, Mateus et al. (2014) concluded that Verbena officinalis L. and Erythrina mulungu M. extracts were applied to the soil once after tomato inoculation with M. incognita eggs, and the number of eggs per root system was reduced, interfering in the multiplication of the nematode.
According Rahman et al. (2011), growing population of M. javanica in grapevine roots treated with Brassica seeds flour for 2 to 3 consecutive years, has increased the susceptibility, which was, probably, due to allelochemicals absorption by roots, leading to a higher population of J2 in the roots.
The applictions by way of root+leaf does not differ from the control in any of the studied appliction times to J2 and M. javanica eggs per gram root (Table 4). Already, by way of root+soil+leaf differ from the control only for the weekly application, below 20.73%, this treatment can have an effect on hatching eggs and/or inhibitory effect of penetration. The reduction of J2 within the root, reduces forming of feeding site and lesser reproduction. The penetration of J2 of sedentary endoparasitic nematodes may occur, but the plant does not host the nematode, for other events must occur for parasitism success (Faria et al., 2003).
Aqueous extract of Cajanus cajan L., Origanum vulgare L., Mucuna aterrima P. and Momordica charantia L. promoted a reduction in the number of eggs per root system; the extract was applied in the soil on the day of Rotylenchus reniformis infestation eggs and consecutively to every 15 days up to 60 days (Gardiano et al., 2011). Corroborating with this study, in which applications extracts by way of soil and foliar, consecutively, presented nematode control.
In Table 5, application times during the inoculation and after inoculation did not differ each other, and they presented less J2 and eggs per 100 cm3 of soil compared to the control. The weekly application time showed differences for modes of application, and by way of root+soil and root+soil+leaf with smaller population in the soil, lesser than 25.93 and 34% compared with the control, respectively. Perhaps, a repellent effect and/or nematostatic is the mechanism involved, by direct effect of the extract or by means of induction exudates roots. Biochemical changes occurring in plants after being induced resistance may result in changes in the nature of exudates and can attract or repel nematodes (Salgado and Silva, 2005).
The applications by way of root+soil showed no difference between the tested times, except prior to inoculation with the highest population in the soil. Already, for modes root+leaves, the only time that differed with less population in the soil was after inoculation and 36.09% lesser than that compared to the control. By modes of root+soil+leaf, times have reduced the number of J2 and eggs per 100 cm3 of soil were in inoculation lesser than 28.10% after inoculation lesser 46.63 and 33.97% compared to the control, but did not differ statistically from the time after inoculation. 33.97% lesser than the compared to the control, but did not differ statistically from weekly time after inoculation.
Based on the smaller population in the soil, the application times wich had a greater effect were: after inoculation and weekly, despite the time of inoculation also have been shown to be effective, modes with further reduction was not in this. In the repetition of the experiment (Table 6), the modes that differ from the control J2 and to eggs per gram of ground were root+soil and soil+root+leaf applied weekly, whereas root+soil was intermediate and 14.57% lesser compared with the control, while by way of root+soil+leaf presented less J2 and eggs per gram of root and 20.65% lesser compared to the control. The population of J2 and eggs per 100 cm3 of soil was lesser than the control only by way of root+soil+leaf when applied weekly, with reduction of 11.82%.
Differences were observed between the periods of April to June and in repetition of the experiment in October to December. To J2 and eggs per gram root, the applications by way of roots+soil in both times tested, there were diferences. The penetration reduced by way of root+soil+leaf in weekly applications was confirmed in the repeat experiment. For J2 and eggs per 100 cm3 of soil, the efficiency by modes of root+soil+leaf and absence of control for root+leaf weekly applications, was confirmed in the repeat experiment. The differences observed between the periods of April and June and repeat experiment in October and December may be due to difference in temperature between them. In the periods of October to December, temperatures were higher, with an average of 24.8°C favoring the reproduction; already in the the periods of April to Jun, the average temperature was 19.3°C. These temperature data are from Automatic Surface Observing Weather Station.
In a complementary way, the incorporation of mustard (Brassica juncea) biomass and seeds flous in soil reduced the density of M. javanica of the grapevines, but a lesser increase in the population after the suppession by the treatments was found, requiring annual treatment for several consecutive years to reduce the population of M. javanica and improve the grape production, once that the population increase is associated with the eggs hatching, and the longevity of compounds present in Brassicas, as isothiocyanates, which depend on a number of factors such as soil moisture (Rahman and Somers, 2005).
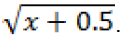 Eggs and J2 per g of root data were transformed into log (x+1). Thereafter, the analysis of variance were carried out and means related to treatment were compared to Tukey test médium. When the additional treatment was significant in the analysis of variance, an additional test was used, Dunnett. Statistical software genes (Cruz, 2006) was used. The experiment was repeated once to confirm the best application times. It was conducted using a factorial design (3x2+1). Being the extract applied by way of the root system and in three different parts of the plant, the application times were: after inoculation (14 DAT) and weekly until 45 days after inoculation. After inoculation and weekly showed greater reduction in the population of M. javanica and it was repeated for confirmation.
Eggs and J2 per g of root data were transformed into log (x+1). Thereafter, the analysis of variance were carried out and means related to treatment were compared to Tukey test médium. When the additional treatment was significant in the analysis of variance, an additional test was used, Dunnett. Statistical software genes (Cruz, 2006) was used. The experiment was repeated once to confirm the best application times. It was conducted using a factorial design (3x2+1). Being the extract applied by way of the root system and in three different parts of the plant, the application times were: after inoculation (14 DAT) and weekly until 45 days after inoculation. After inoculation and weekly showed greater reduction in the population of M. javanica and it was repeated for confirmation.