ABSTRACT
Mineral deficiency especially that of iron and zinc has continuously emerged as a public health issue in developing countries, probably due to the over dependence on plant food sources, which contain more than enough minerals to meet the daily requirement but have a low bioavailability for physiological purposes. Experiments on in-vitro bioavailability were carried out on dry and green shelled beans. In-vitro bioavailability of iron and zinc in bean samples was determined by HCl-pepsin (HCl-P) and pepsin-pancreatin (P-P) method. The amount of the proxy bioavailable minerals were obtained by atomic absorption spectrophotometry. In both minerals there was a small but significant (P=0.009) and (P=0.0003) increase in in-vitro bioavailability after cooking. The average increase for all the varieties was 3.2 to 3.4% for iron and 1.3 to 1.6% for zinc. The two minerals were more available in cooked green shelled beans compared to dry ones. The highest difference for iron bioavailability was observed in Maharagi soja (12.9%) while lowest was in TY 3396-12 (1.4%). The highest observed for zinc was 3% in G59/1-2. Vulnerable groups who suffer from iron and zinc deficiency should be encouraged to consume green shelled beans more often in comparison to dry beans to improve their mineral uptake.
Key words: In-vitro bioavailability, green, shelled, dry beans, minerals.
In-vitro bioavailability of a mineral is generally defined as a measure of proportion of the total minerals in food or a meal that is utilized for normal body functions. For most minerals the amount that is absorbed from the gastrointestinal tract, is the major determinant of bioavailability but this varies greatly between minerals (Sandberg, 2002). Absorption is affected by a range of factors including interactions with other dietary components in the gastrointestinal tract, for example vitamin C enhances iron absorption while tannins and phytates have an inhibitory effect. Several antinutritional factors have been implicated as the main cause of reduced micronutrient availability such as phytic acid and tannins although phytic acid has a more pronouncedeffect (Zhou and Erdman, 1995; Zdunczyk et al., 1996; Antony and Chandra, 1998; Manary et al., 2000).
The quantification of bioavailability has been done by complex processes comprising isotopic elemental digestion. However, various techniques have been established by nutritionists to determine bioavailability. For instance, In-vitro studies range from measurements of solubility, extractability, and fractional dialysability to studies of the nutrient uptake in experimental animals (Miller et al., 1981; Sripriya et al., 1997; Mamiro et al., 2001). The nutrients are incubated in intestinal preparations to simulate the processes that take place in the gastrointestinal tract (Blenford, 1995). Contemporary developments have used cell culture called the Caco-2 as an in-vitro method to assess iron bioavailability in human beings (Glahn et al., 2000; Kim et al., 2011). When compared with other In-vitro methods used previously by other scientists a significant correlation (r=0.97 and P=0.0001) was found (Yun et al., 2004). The results obtained from the above methods are used as proxy to estimate bioavailability (Watzke, 1998).
Beans are naturally low in fat, with little saturated fats. The average lipid content is only 1.5% of the dry bean and unsaturated fatty acids make up 75% of the lipid material. Beans consumption does not improve only micro and macronutrient status but also they play a role in other health parameters such as improving glycemic control (Kabagambe et al., 2005), decrease the risk of coronary heart disease and lowers cholesterol levels (Bazzano et al., 2011).
Minerals and trace elements play essential roles in numerous biochemical and physiological processes in animals and man. A deficiency, an overdose or imbalances between minerals or trace elements will exert a negative effect on health or nutritional status of an individual. Generally, it is not the ingested dose of minerals and trace elements that is important to maintain balance, but rather the amount that is bioavailable (available for biological and biochemical processes in the organism). Several food components are able to form soluble or insoluble complexes with minerals and trace elements under gastrointestinal conditions. These food components thereby increase or decrease the availability for absorption in the small intestine, and thus the bioavailability of minerals and trace elements. Due to the complexity of food products, however, the relative contributions of food components to the bioavailability of minerals and trace elements are often not clear.
Although in-vivo experiments (using actual animals or human beings) are the best way to study the bioavailability of minerals and trace elements, In-vitro methods offer an appealing alternative because they are relatively simple, rapid and inexpensive (Mamiro et al., 2001). Therefore, there is a great need in human nutrition and animal nutrition for an In-vitro method, which predicts the bioavailability of minerals and trace elements in vivo. The aim of this study therefore was to compare in-vitro bioavailability of selected minerals in dry and shelled green beans for raw and cooked samples.
Bean samples
Bean seeds of 38 varieties were brought from University of Nairobi and multiplied on experimental plots at Sokoine University of Agriculture Morogoro. The objective was to harvest enough beans to perform a number of laboratory experiments on each variety. A one acre plot was cleared and seed-bed prepared. Each variety was planted on four lines 50 cm apart and 20 cm from seedling to seedling (one stand per hill). This gave on average about 4 kg per variety. All analyses were done in the Food Science and Technology Laboratory in collaboration with the Department of Soil Science. In-vitro bioavailability of minerals was determined on raw and cooked dry and shelled bean samples.
Total In-vitro bioavailable minerals
Total in-vitro bioavailable Iron and Zinc of the raw and cooked dry and green shelled bean samples was carried out by AOAC method No 968.08 and determined by atomic absorption spectrophotometry method No AOAC method 970.12, (AOAC, 1995).
In-vitro HCl-pepsin and pepsin-pancreatin mineral bioavailability
In-vitro bioavailability of iron and zinc by HCl-pepsin (HCl-P) was carried out by a method described by Kumar and Chauhan (1993). Two grams of the dried bean samples were weighed and 25 ml of HCl-Pepsin solution was added in a 100 ml conical flask. The pH was adjusted to 1.35 and the mixture was incubated at 37ºC in a metabolic shaker water bath for 90 min. At the end of 90 min, the digest was centrifuged at 3000 rpm for 45 min and the supernatant filtered through whatman filter paper no 41. The filtrated was subjected to Pepsin-Pancreatin (P-P) method as described by Miller et al. (1981). Fifteen milliliters of the filtrate was mixed in 10 ml of pancreatin bile in tyrode buffer and pH adjusted to 7.5 and incubation continued for 2 h. The digest was centrifuged at 10,000 g for 20 min and then filtered through Whatman filter paper No 41. Analysis of the filtrate for soluble iron and zinc was performed using the Atomic Absorption Spectrophotometer (Shimadzu UNICAM 919, England).
Statistical analysis
Data for the In-vitro solubility were entered in excel office 2010 computer software. Descriptive statistics was used to compare the solubility’s in raw and cooked samples and Students t-test was used to test for significant differences among the treatments at 95% confidence interval.
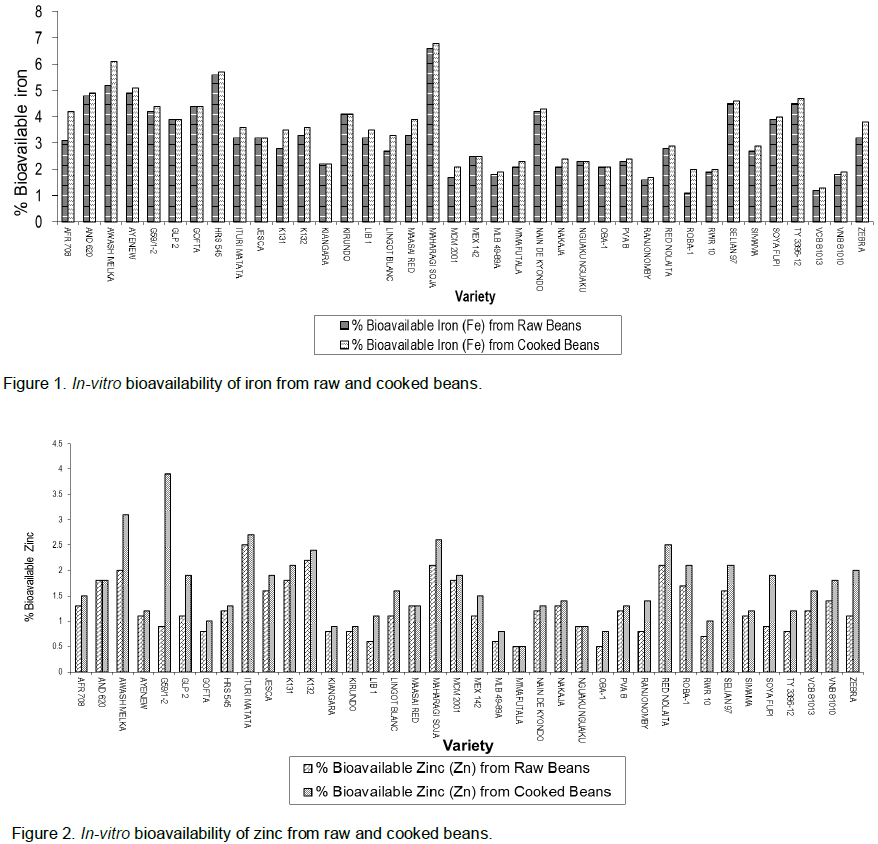
In-vitro bioavailability of iron and zinc in raw and cooked dry beans are presented in Figures 1 and 2. In both minerals there were a small but significant (P=0.009) and (P=0.0003) increase in mineral bioavailability after cooking. The average increase for all the varieties was 3.2 to 3.4% for iron and 1.3 to 1.6% for zinc.
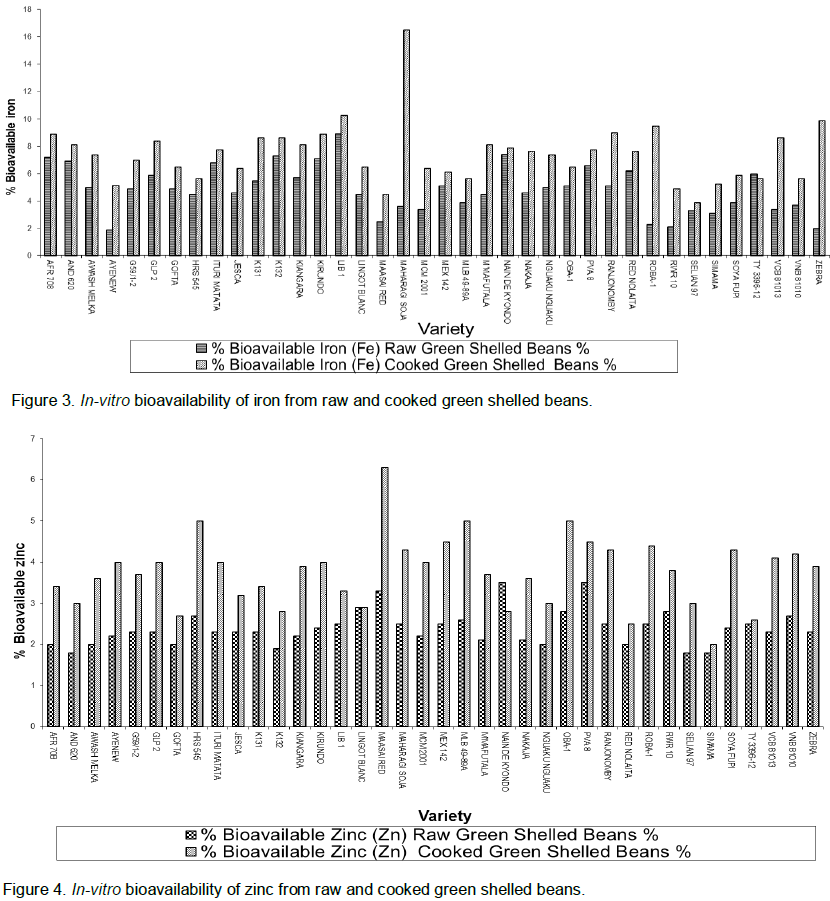
A similar trend was observed in the In-vitro bioavailability of iron and zinc in raw and cooked shelled beans (Figures 3 and 4). A relative wider gap is observed for iron and zinc bioavailability in shelled beans. In both minerals, there was a significant (P=0.000) increase in bioavailability after cooking the shelled beans. The average increase for all the varieties was 4.9 to 7.4% for iron and 2.4 to 3.6% for zinc. The highest difference for iron bioavailability was observed in Maharagi soja (12.9%) while lowest was shown by TY 3396-12 (-1.4%). The highest observed for zinc was 3% in G59/1-2.
Variety of beans displays a diverse variation in mineral bioavailability. This might probably be brought up by the variation in the content of antinutritional factors such as oxalates, lectins, hemaglutins, tannins and phytates (Sandberg, 2002). The most important antinutritional factors in the dietary minerals bioavailability are the phytates and tannins.
The bioavailability of iron, which is the amount absorbed from food can be less than one percent to over 50%. Iron is found in food in two different forms, either as heme iron, found in the haemoglobin and myoglobin of meat, poultry and fish, or as non-heme iron found in plant products. Heme iron is better absorbed (about 15 to 40%) than non-heme iron (1 to 15%) (Roughhead and Hunt, 2000). Thus, although heme iron contributes only about 10 to 15% of total iron intake but may provide substantial amount of total iron absorbed (Allen and Ahluwalia 1997, EFSA, 2010). Non-heme iron is relatively consumed in much greater quantities, but is greatly affected by both inhibitors and enhancers in the diet such that there can be up to ten fold variations in absorption rates (Gropper et al., 2005). Non-heme iron absorption from a meal containing enhancers of absorption, such as meat, fish, or chicken, is about four times greater than it would be if the major protein sources were eggs or pulses. The addition of even small amounts of meat or a source of vitamin C (ascorbic acid) substantially increases the non-heme iron absorption from the entire meal (Lynch and Cook, 1984; Baech et al., 2003; Nielsen et al., 2013). In contrast, tea, coffee or eggs decreases the absorption of non-heme iron from a meal.
Low bioavailability diets (5% of the total iron absorbed) are based mainly on cereals and root vegetables with only very small quantities of meat, fish or vitamin C-containing foods (Baech et al., 2003). Such diets often contain foods that inhibit iron absorption (maize, beans, whole-grain flour) and are dominant in many developing countries. Intermediate bioavailability diets (10% of the iron absorbed) consist mainly of cereals and root vegetables but contain some meat and some foods containing vitamin C. High bioavailability diets (15% of the iron absorbed) contain regular intakes of meat, poultry and fish (Baech et al., 2003). They also contain vitamin C-rich foods such as citrus fruits and some vegetables. A high bioavailability diet containing inhibitors of iron absorption, such as tea, coffee, cereal fibre, and dairy foods with main meals, would become an intermediate bioavailability diet.
In legume seeds, phytate is located in the protein bodies in the endosperm (Sandberg, 2002). Phytate occurs as a mineral complex, which is insoluble at the physiological pH of the intestine. It is considered antinutritional, causing reduced uptake in the human intestine of essential dietary minerals such as Fe, Zn and Ca. A dose-dependent inhibition of Fe, Zn and Ca absorption by phytate has been demonstrated in humans(Hallberg et al., 1989; Brune et al., 1992; Hurrell et al., 1992; Fredlund et al., 2002; Petry et al., 2010; La Frano et al., 2014). Inositol pentaphosphate has also been identified as an inhibitor of Fe and Zn absorption (Sandstrom and Sandberg, 1992; Sandberg et al., 1999). Furthermore, it was found that inositol tri and tetra-phosphate contribute to the negative effect on Fe absorption of processed foods containing a mixture of inositol phosphates (Sandberg et al., 1999), probably by interactions with the higher phosphorylated inositol phosphates.
Fe absorption from soya beans and soya protein products studied in single meals, using extrinsic labeling of meals with radioactive isotopes or studied by a stable isotope technique, was found to be low (Cook et al., 1981; Hurrell et al., 1992; Davidsson et al., 1994). Fe absorption from single meals based on black beans, lentils, mung beans, split beans and from two bean phenotype varieties was found to be very low, ranging from 0.8 to 1.9% (Lynch et al., 1984; Donangelo et al., 2003).
Legumes contain varying amounts of polyphenols and generally the amounts are considered higher in the coloured seeds. Beans of the species Phaseolus vulgaris were found to contain high amounts of polyphenols (Paredes-Lopez and Harry, 1989), whereas the content of polyphenols in peas (Pisum sativum) was very low. Another study which used In-vitro digestion/human Caco-2 model reported high bioavailability of iron in white compared to colored beans which was explained by presence of flavonoids in colored beans (Hu et al., 2006).
For shelled beans the percentage In-vitro bioavailability was comparatively higher than that observed in the dry beans. This is probably because the seeds were still maturing and therefore the antinutritional factors were not fully formed with regard to quantities and so the amount that was required to bind the minerals was not sufficient. Consuming green shelled beans might be rather beneficial with regard to mineral uptake than the dry beans.
Minerals from green shelled beans have been observed to be more bioavailable compared to dry beans. Vulnerable groups who suffer from micronutrient deficiencies especially iron and zinc, such as children below five years of age, pregnant and lactating mothers and the sick, should be encouraged to consume green shelled beans more often to improve their mineral uptake.
The authors have not declared any conflict of interest.
The authors would like to acknowledge the financial assistance from Pan-Africa Bean Research Alliance (PABRA) and Sokoine University of Agriculture (SUA) who facilitated for bean germplasm multiplication trials and providing all the necessary amenities to carry out the research to completion.
REFERENCES
|
Allen LH, Ahluwalia N (1997). Improving iron status through diet. The application of knowledge concerning dietary iron bioavailability in human populations. Washington DC, USAID/OMNI project.
|
|
|
|
Antony U, Chandra TS (1998). Antinutrient reduction and enhancement in protein, starch, and mineral availability in fermented flour of finger millet (Eleusine corocana). J. Agric. Food Chem. 46:2578-2582.
Crossref
|
|
|
|
|
AOAC (1995). Official methods of Analysis. Association of Official Analytical Chemists methods, AOAC 16th Edition. Washington DC.
|
|
|
|
|
Baech SB, Hansen M, Bhukhave K, Jensen M, Sorensen SS, Kristensen L, Purslow PP, Skibsted HL, Sandstorm B (2003). Non heme iron absorption from a phtate rich meal is increased by additional of small amount meat. Am. J. Clin. Nutr. 77:173-179.
|
|
|
|
|
Bazzano LA, Thomson AM, Tees MT, Nguyen CH, Winham DM (2011). Non-soy legume consumption lowers cholesterol levels: A meta-analysis of randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 21:94-103.
Crossref
|
|
|
|
|
Blenford D (1995). Bioavailability is the key to nutrient effectiveness. Food Ingredients Process. Int. 17:28-30.
|
|
|
|
|
Brune M, Rossander-Hulthe’n L, Hallberg L, Gleerup A, Sandberg AS (1992). Human iron absorption from bread:Inhibiting effects of cereal fiber, phytate and inositol phosphates with different numbers of phosphate groups. J. Nutr. 122:442-449.
|
|
|
|
|
Cook JD, Morck TA, Lynch SR (1981). The inhibitory effect of soy products on nonheme iron absorption in man. Am. J. Clin. Nutr. 34:2622-2629.
|
|
|
|
|
Davidsson L, Galan P, Kastenmayer P, Cherouvrier F, Juillerat MA, Jercberg S, Hurrell RF (1994). Iron bioavailability studied in infants: The influence of phytic acid and ascorbic acid in infant formulas based on soy isolate. Pediatr. Res. 36:816-822.
Crossref
|
|
|
|
|
Donangelo CM, Leslie RW, Sarah MK, Gianna T, David MS, Fernando EV, Cheng Z, Ross MW, Janet CK (2003). Iron and Zinc Absorption from Two Bean (Phaseolus vulgaris L.) Genotypes in Young Women. J. Agric. Food Chem. 51(17):5137-43.
Crossref
|
|
|
|
|
EFSA (European Food Safety Authority) (2010). Panel on Food Additives and Nutrient Sources added to Food (ANS); Scientific Opinion on the safety of heme iron (blood peptonates) for the proposed uses as a source of iron added for nutritional purposes to foods for the general population, including food supplements. EFSA J. 8(4):1585.
|
|
|
|
|
Fredlund K, Rossander-Hulthe’n L, Isaksson M, Almgren A, Sandberg AS (2002). Absorption of zinc and calcium: dosedependent inhibition by phytate. J. Appl. Microbiol. 93:197-204.
|
|
|
|
|
Glahn RP, Cheng Z, Welch RM (2000) Comparison of Iron Bioavailability from 15 Rice Genotypes: Studies Using an In-vitro Digestion/Caco-2 Cell Culture Model. J. Agric. Food Chem. 50(12):3586-3591.
Crossref
|
|
|
|
|
Gropper SS, Smith JL, Groff JL (2005) Advanced nutrition and human metabolism. 4th edn. Belmont CA: Thomson Wadsworth.
|
|
|
|
|
Hallberg L, Brune M, Rossander L (1989). Iron absorption in man: ascorbic acid and dose-dependent inhibition by phytate. Am. J. Clinic Nutr. 49:140-144.
|
|
|
|
|
Hu Y, Cheng Z, Heller LI, Krasnoff SB, Glahn RP, Welch RM (2006). Kaempferol in red and pinto bean seed (Phaseolus vulgaris L.) coats inhibits iron bioavailability using an In-vitro digestion/human Caco-2 cell model. J. Agric. Food Chem. 54(24):9254-61.
Crossref
|
|
|
|
|
Hurrell RF, Juilliard MA, Reddy MB, Lynch SR, Dassenko SA, Cook JD (1992). Soy protein, phytate, and iron absorption in humans. Am. J. Clinic Nutr. 56:573-8.
|
|
|
|
|
Kabagambe EK, Baylin A, Ruis-Narvarez E, Siles X, Campos H (2005). Decreased consumption of diried mature beans is positively associated with urbanization and nonfatal acute myocardial infarction. J. Nutr. 135(7):1770-1775.
|
|
|
|
|
Kim EY, Ham SK Bradke D, Ma Q, Han O (2011). Ascorbic Acid Offsets the Inhibitory Effect of Bioactive Dietary Polyphenolic Compounds on Transepithelial Iron Transport in Caco-2 Intestinal Cells. J. Nutr. 141(5):828-834
Crossref
|
|
|
|
|
Kumar A, Chauhan BM (1993). Effects of phytic acid on protein digestibility (In-vitro) and HCL – Extractability of minerals in Pearl millet sprouts. J. Cereal Chem. 70:504-506.
|
|
|
|
|
La Frano MR, de Moura FF, Boy E, Lönnerdal B, Burri BJ (2014). Bioavailability of iron, zinc, and provitamin A carotenoids in biofortified staple crops. Nutr. Rev. 72:289-307.
Crossref
|
|
|
|
|
Lynch SR, Beard JL, Dassenko SA, Cook JD (1984). Iron absorption from legumes in humans. Am. J. Clinic Nutr. 40:42-47.
|
|
|
|
|
Mamiro PS, Van Camp J, Mwikya SM, Huyghebaert (2001). In-vitro extractability of calcium, iron and zinc in Finger millet and kidney beans during processing. J. Food Sci. 66:1271-1275.
Crossref
|
|
|
|
|
Manary MJ, Hotz C, Krebs NF, Gibson RS, Westcott JE, Arnold T, Broadhead RL, Hambidge KM (2000). Dietary phytate reduction improves zinc absorption in Malawian children recovering from tuberculosis but not in well children. J. Nutr. 130:2959-2964.
|
|
|
|
|
Miller DD, Schricker BR, Rasmussen RR, Van Campen D (1981). An In-vitro method for estimation of iron availability from meals. Am. J. Clinic Nutr. 34:2248-2256.
|
|
|
|
|
Nielsen AVF, Tetens I, Meyer AS (2013). Potential of Phytase-Mediated Iron Release from Cereal-Based Foods: A Quantitative View. Nutrients. 5(8):3074-3098.
Crossref
|
|
|
|
|
Paredes-Lopez O, Harry GI (1989). Changes in selected chemical and antinutritional components during tempeh preparation using fresh and hardened common beans. J. Food Sci. 54:968-970.
Crossref
|
|
|
|
|
Petry N, Egli I, Zeder C, Walczyk, T and Hurrel R (2010). Polyphenols and phytic acid contribute to the low iron bioavailability from common beans in young women. J. Nutr. 140:1977-1982.
Crossref
|
|
|
|
|
Roughhead ZK, Hunt JR (2000). Adaptation in iron absorption: iron supplimentation reduces non-heme iron but not heme iron absorption from food. Am. J. Clinic Nutr. 72:982-989.
|
|
|
|
|
Sandberg A (2002). Bioavailability of minerals in legumes. Br. J. Clinic Nutr. 88:S281-S285.
Crossref
|
|
|
|
|
Sandberg AS, Brune M, Carkssin NG, Hallberg L, Skoglund E, Rossander-Hulthen L (1999). Inositol pphosphates with different number of phosphate groups influence iron absorption in humans. Am. J. Clin. Nutr. 70:240-246.
|
|
|
|
|
Sandstorm B, Sandberg AS (1992). Inhibitory effects of inositol phosphates on zinc absorbtion in humans. J. Trace Elem. Health Sis. 6:99-103.
|
|
|
|
|
Sripriya G, Antony U, Chandra TS (1997). Changes in carbohydrate, free amino acids, organic acids, phytate and HCl extractability of minerals during germination and fermentation of finger millet (Eleusine coracana). Food Chem. 58(2):345-350.
Crossref
|
|
|
|
|
Watzke H (1998). Impact of processing on bioavailability examples in foods. Trends Food Sci Tech. 9: 320-327.
Crossref
|
|
|
|
|
Yun S, Habicht JP, Miller DD, Glahn RP (2004). An In-vitro digestion\Caco-2 cell culture accurately predicts the effects of ascorbic acid and polyphenolic compounds on iron bioavailability in humans. J. Nutr. 134:2717-2721.
|
|
|
|
|
Zdunczyk Z, Frejnagel S, Krefft B (1996). Effect of faba beans coat with different phenolics content on the use of protein by rats. Pol. J. Food Nutr. Sci. 46:91-102.
|
|
|
|
|
Zhou JR, Erdman J (1995). Phytic acid in Health and disease. Crit. Rev. Food Sci. Nutr. 35:495-508.
Crossref
|
|