ABSTRACT
Cucurbit vegetables play important role as sources of vitamins, micronutrients and income. However, their production is constrained, inter alia, by the virus diseases. Cucumber mosaic virus (CMV; Cucumovirus), Zucchini yellow mosaic virus (ZYMV; Potyvirus) and Watermelon mosaic virus (WMV; Potyvirus) are the major causal agents of devastating virus diseases of the cucurbits. Disease symptoms similar to those caused by these viruses were observed in cucurbits in the coastal lowland of Tanzania. To determine what caused these symptoms, leaf samples were collected from 223 cultivated and wild cucurbit plants and virus infections detected using Double Antibody Sandwich Enzyme-linked immunosorbent Assay (DAS-ELISA). Visual incidence of virus disease symptoms ranged from 0.0 to 90.0% but ELISA test for CMV, ZYMV and WMV revealed the range of 0 to 80%. The highest incidence of the virus infections was that of WMV (33.0%) in Cucumis sativus. The highest incidence of ZYMV and CMV were 10.4 and 13.4% in Citrullus lanatus and Cucurbita pepo, respectively. These viruses were found infecting cucurbits in single, co- and triple infections. Moreover, these viruses were also detected in the wild plants, Cucumis hystrix and Luffa aegyptiaca and in cultivated Vigna unguiculata. ZYMV was the commonest virus in wild plants. Yield losses caused by virus diseases remain undetermined for cucurbits in Tanzania but co- and triple virus infections have implication on disease severity and evolution of these viruses and may pose challenge for breeding for resistance.
Key words: Cucurbit virus diseases, DAS-ELISA, virus mixed infections.
In Tanzania, vegetables play an important role as sources of vitamins, micronutrients and income (Weinberger and Msuya, 2004). Cultivation of vegetables, including cucumber, pumpkins and watermelons is common in
areas with reliable water sources in the outskirts of Dar es Salaam city and other places in the country. It is a promising sub-sector in creation of jobs for the youths and women. Despite the importance of cucurbit crops, their production, worldwide, is constrained by many factors including virus diseases.
Cucurbits are infected by over 35 viruses (Provvidenti, 1996). Cucumber mosaic virus (CMV; Cucumovirus; Bromoviridae), Watermelon mosaic virus (WMV; Potyvirus; Potyviridae) and Zucchin yellow mosaic virus (ZYMV; Potyvirus; Potyviridae) are among viruses that cause important diseases in cultivated cucurbits (Lisa et al., 1981; Zitter and Banik, 1984). These viruses are transmitted by many aphid species in a non-persistent manner (Lisa et al., 1981; Gal-On, 2007; Gildow et al., 2008). They have a wide range of hosts but some strains, for example of CMV, may be confined to hosts in a certain family (Zitter and Murphy, 2009). CMV is reported to infect or be transmitted to over 1200 species from more than 100 plant families (Dobhal et al., 2015).
Virus diseases of cucurbits caused by CMV, ZYMV and WMV are characterized by such symptoms as mosaic, yellowing, stunted growth, fruit and leaf malformation and rugosity (Lisa et al., 1981; Zitter and Murphy, 2009). These diseases may lead to significant yield losses under field conditions. For instance, information published on the website of the Department of Agriculture and Food, Government of Western Australia, shows that when ZYMV infects cucurbits before flowering stage yield losses can be up to 100%. Rugosity and malformation of fruits, if they occur, render the fruits unmarketable.
Information on occurrence, distribution, and incidence of the viruses is required in order to develop strategies for management of diseases they cause. Likewise, one of the important aspects in management of virus diseases is to explore and understand existence of alternative hosts of the causal viruses. The viruses in alternative hosts may serve as the source inoculum for new crops even when established using virus-free seeds. Studies on specific viruses in alternative hosts in East Africa have been conducted for some crops (Tugume et al., 2008). Information on alternative hosts is useful in the management of diseases through management of these hosts. Moreover, understanding of the molecular evolution of viruses as driven by natural and other hosts is important as breeders strive to develop resistant genotypes.
While there are reports of occurrence of virus diseases of cucurbits from Africa (Lapido, 1988; Ibaba et al., 2015; Desbiez et al., 2016), such information is scanty for major virus diseases of cucurbits in Tanzania. This might be attributed to too much focus on the virus diseases of cassava and sweet potato, which are considered as the main food security crops. The virologists in the Great Lakes region have in recent years focused research in cassava mosaic disease (CMD), cassava brown streak disease (CBSD) and sweet potato virus disease complex (SPVD) at the expense of other crops such as the cucurbits which not only have nutritional values but are also depended on by many for employment and household income. To contribute to the body of knowledge on occurrence and incidence of virus diseases of cucurbit crops, cultivated and wild cucurbit plant leaf samples were collected and subjected to double antibody sandwich enzyme-linked immunosorbent assay (DAS-ELISA) to detect viruses in the same. To the best of the authors’ knowledge, this is the first report of occurrence of CMV, ZYMV and WMV in cucurbits and related wild plant species in Tanzania.
Study area
Cultivated and wild cucurbit leaf samples used in this study were collected from coastal areas in Dar es Salaam and Coast administrative regions in Eastern Tanzania in March 2016 (Figure 1). The collection was made in river valleys or in areas where irrigation allows for all time cultivation of cucurbit crops.
Selection of gardens
The gardens in the river valleys or areas where water is available for irrigation were identified near main and feeder roads. In each of the selected locations, one to two separate gardens were randomly selected from among the many small gardens that were managed by small scale gardeners. Commonly, tens of small scale gardeners cultivate vegetable crops in hired small land plots in areas that are open near water sources. These gardens are found in tandem in many valleys in the outskirts of Dar es Salaam but they may be distantly located in the Coast region. The locations of the gardens were recorded using the GARMIN global positioning system (GPS, GPS72H) and mapped using google tools (Figure 1).
Collection of leaf samples
In each of the randomly selected gardens, leaves were collected from ten plants that were sampled along the garden diagonals following an ‘X’ pattern. Five plants were collected as surveyor moved from one corner of the garden to the other. The distance from one plant to another was dictated by the size of the garden but was mostly less than 2 m. Leaf samples were collected from both symptomatic and asymptomatic plants. For each leaf sample, disease symptoms observed were recorded. The leaf samples, after detachment from the mother plants, were immediately placed in the sampling bags (ordered from DSMZ, Germany). The samples were then placed in a cool box with ice blocks. These samples were on the same day moved to a 4°C fridge at Mikocheni Agricultural Research Institute and used in enzyme-linked immunosorbent assays (ELISA) the following day.
Virus disease incidence and prevalence
Observations of disease incidence and prevalence were made on ten randomly selected plants. Incidence was estimated by dividing the number of plants with symptoms by the total number of plants on which observations were made per field. Prevalence was considered as the percentage of the fields with at least one diseased plant as assessed visually or determined by ELISA method. However, incidence of viruses computed after obtaining laboratory results are either shown per crop or plant for a given or all viruses and was computed as the number of plants confirmed by ELISA test to be infected divided by the total number of plants for the crop or wild plant under consideration.
Detection of viruses by DAS-ELISA
Enzyme-linked immunosorbent assay (ELISA) was used to confirm virus infections in the leaf samples. The antibodies used in this study were ordered from Leibniz-Institut (DSMZ, Germany). The double antibody sandwich ELISA (DAS-ELISA) was conducted following the procedures recommended by the manufacturer of the antibodies. Approximately 0.2 g of the leaf samples were put in the ELISA bags that were partitioned with filters. The samples were extracted using 3 mL of the extraction buffer that was prepared following the protocol provided by manufacturer of the antibodies. The coating antibodies (IgG; codes AS-0234, AS-0929, AS-0203 for ZYMV, CMV and WMV, respectively) were used at a dilution of 1:1000 as recommended by manufacturer. Then 200 µL of the diluted antibodies were added to the costar 96-wells flat-bottom EIA plates (Bio-Rad Laboratories (Pty) Ltd.). The plates with coating antibodies (IgG) were covered with clean aluminum foil and incubated at 37°C for 2 h. After washing as recommended, 200 µL of the extracted buffer were added to the wells in duplicate (avoiding edge wells). Appropriate positive samples (provided in the kit) were added along with healthy samples. The samples in plates were covered appropriately and placed in a 4°C fridge overnight. The conjugate antibodies used were diluted as recommended except once when dilutions were changed from 1:1000 for IgG-AP (AS-0929; CMV) to 1:1250 and from 1:500 for IgG-AP (AS-0203; WMV) to 1:600. This was done in order to optimize for the rate of colour development. Then, 200 µL of the 1 mg/ml para-nitrophenyl-phosphate in substrate buffer or a ready to use solution, alkaline phosphatase yellow liquid substrate system for ELISA (SIGMA-ALDRICH, St. Louis, USA) were used in ELISA assay final step. Where the former was used, substrate buffer was prepared as recommended by DSMZ. Results were both visually assessed and then spectrophotometric (GDMS ELISA plate Analyser; RT00300115GDM; Global Diagnostic and Medical solutions) measurement of absorbance at 405 nm was done after 30 and 120 min. In one of the tests, a problem of rapid development of colour was encountered when detecting WMV and results for that test are not reported. The sample were considered positive if there was clear development of colour and then the absorbance readings were at least three times higher than that of healthy samples.
Symptoms
A variety of symptoms were recorded on three cultivated cucurbits, namely pumpkin (Cucurbita pepo), cucumber (Cucumis sativus) and watermelons (Citrullus lanatus). Similarly, symptoms were recorded for wild cucurbits and non-cucurbit cultivated crops, which were wild cucumber (Cucumis hystrix), luffa (Luffa aegyptiaca) and cowpea (Vigna unguiculata). The symptoms observed on these plants were mosaic, leaf curling, wrinkled leaves, stunted growth, green vein banding, yellow spots, and yellow mottling (Figure 2). The commonest symptoms were yellow mottling, mosaic, rugosity or wrinkled leaves and green vein banding. Generally, disease symptoms on wild plants were not as severe as they were on cultivated cucurbits. The symptoms sometimes varied between plants but similar symptoms were observed for plants in different species (Figure 2B and C). Both symptomatic and asymptomatic wild plants from the family Cucurbitaceae were found growing near or on the edges of cultivated vegetable cucurbits (Figure 2F and E).
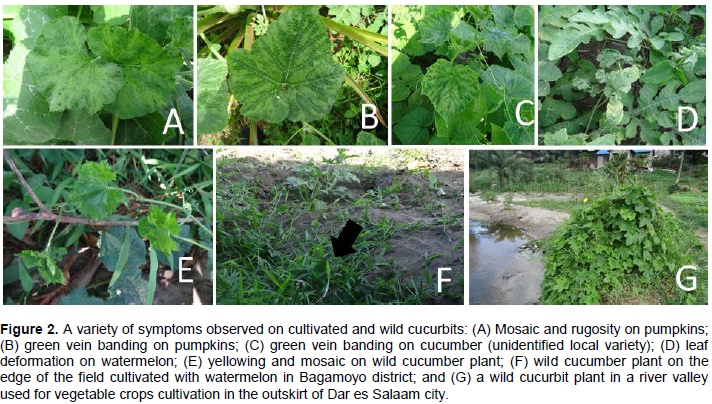
Visual assessment of virus disease incidence
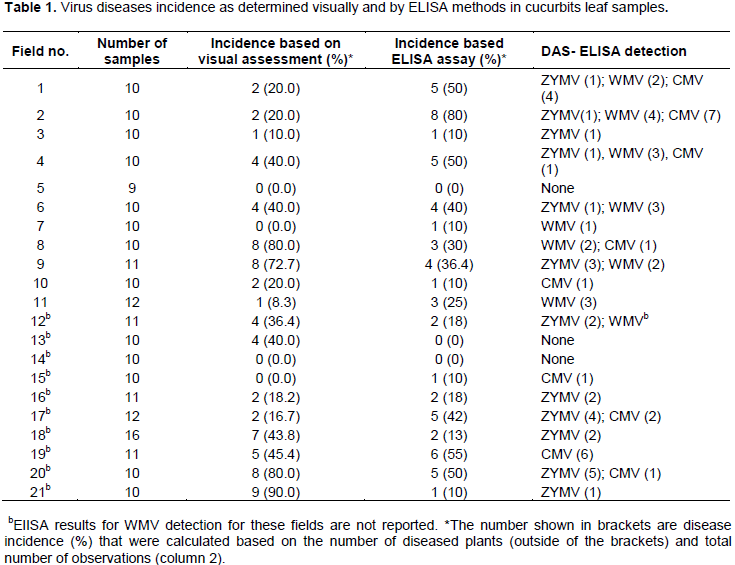
The virus disease symptoms described above were observed in both administrative regions suggesting that virus diseases of cucurbit are widely spread in the coastal areas, particularly in Dar es Salaam and Coast regions. Based on visual observations, the virus-like disease symptoms were seen on cucurbit vegetable crops in 18 out of 21 fields that were surveyed. This represented a disease prevalence of 85.7% when the same is assessed as the number of fields with at least a diseased plant over the total number of fields. The incidence of virus diseases ranged from 0.0 to 90.0% (Table 1). In five fields, the incidence of virus disease symptoms was greater than 50.0%. Visually assessed disease incidences were generally higher in cucumber and watermelon plants than in pumpkins. It was also observed that 33.0% of the wild plants exhibited conspicuous symptoms similar to those known to be caused by viruses.
Disease incidence based on the ELISA assay
Results on the general disease incidence are presented in Table 1. The same table also shows the viruses detected in the collected leaf samples. The ELISA assay was done on 223 cultivated and wild cucurbit plants for ZYMV and WMV but on only 114 plant leaf samples for CMV.
ELISA based detection of the virus infections revealed incidence levels that ranged from 0 to 80%. Similar to incidence observed under visual assessment, in five gardens, the disease incidence was higher than 50%. However, the gardens recorded as having higher incidence are not the same ones observed to have high level of incidence under visual assessment. Generally, fields that were visually assessed as being free from virus diseases were indeed confirmed by ELISA assays to be having plants that were not infected except in one farm where one plant was found to be infected (Table 1). Forty-one plants were symptomatic but tested negative for the three viruses studied.
On the contrary, 33 plant samples that were symptomless tested positive for the viruses assayed for in this study. Viruses were detected in 15, 9 and 4 C. pepo, C. sativus and C. lanatus symptomless samples, respectively. Moreover, four symptomless wild cucurbits tested positive for viruses.
Infections by plants and mixed infections
Single and mixed infections results were summarized and shown in Table 2. For plants commonly infected by a particular virus, ZYMV infections were more common in watermelons (10.4%) than in pumpkins (8.5%) and cucumber (7.5%) (Table 2). CMV was detected more in pumpkin plants (13.4%) than in cucumber (13.2%) and watermelons (3.0%).
For WMV, more infections were detected in cucumber (33.3%). The infections of WMV in pumpkins were 20.0% while in watermelon, the crop after which its name was derived, was 8.5%. Co-infections were observed in five plants (2 events in pumpkins and 3 events in cucumber) for CMV and WMV and only once for all other combinations. Infections with all three viruses were observed in only one plant (C. sativus) that was collected from Dar es Salaam region.
Infections in wild plants
The total number of wild cucurbits on which ELISA assay was done were 21. Virus infections were detected in 8 plants (38.1%). One sample of cowpea (V. unguiculata) was found growing with cucurbit crops and had symptoms of virus disease; it was treated as a weed (wild) plant and detection revealed it was infected with CMV (Table 2). Therefore, in these studies, infections with CMV, WMV and ZYMV in wild plants were about 40.0%. ZYMV was detected in 6 wild plants (28.5%). Singly, WMV was detected in one wild cucumber plant while CMV was detected in L. eagyptiaca (1 plant) and wild cucumber (1 plant). Therefore, all types of wild plants that were collected were hosts of at least one of the three viruses studied. One wild cucumber plant was found doubly infected by ZYMV and CMV.
Plant viruses are known to cause enormous crop yield losses all over the world. The damage caused by these viruses affects both the quantity and quality of the desired parts of the crops. Virus diseases of cucurbits, for instance, cause rugosity on leaves and fruits thereby making them unfit for human consumption and thus completely or to some degree reducing their marketability. Cucumber mosaic virus, ZYMV and WMV are some of the viruses that cause severe symptoms on cucurbits globally (Ibaba et al., 2015; Desbiez et al., 2016). Their occurrence has been reported from many places in the world (Massumi et al., 2007; Lisa et al., 1981; Herrera-Vásquez et al., 2013; Ibaba et al., 2015). Symptoms similar to those caused by these three viruses were observed in plants in Tanzania. The results of this study have shown that most of the symptoms observed on pumpkin, cucumber and watermelon plants in the coastal lowland areas are caused by these viruses. There were, however, some symptomatic plants that tested negative for all three viruses suggesting that there could be strains of these viruses or even other distinct viruses that infect cucurbit crops in Tanzania. This is due to the fact that distinct strains or viruses could differ serologically (Zitter and Murphy, 2009).
Both visual observations and ELISA test revealed high incidence of virus diseases in cucurbits fields in the coastal lowlands of Tanzania. There has not been any assessment of the yield losses associated with the three viruses in Tanzania but it is reasonable to surmise that the losses could be as high as in other regions of the world where such observations have been made. The incidence of virus infections may differ from location to location. In Iran and Brazil, the incidence of diseases caused by these viruses in cultivated cucurbits were found to be higher for CMV and ZYMV as compared to watermelon mosaic virus-2 (WMV-2 (Yuki et al., 2000;
Mussumi et al., 2007). In this study, albeit a few number of samples, the chance of encountering a plant infected with WMV was higher than that of encountering a cucurbit plant infected with ZYMV and CMV. On the other hand, ZYMV appears to infect wild cucurbits at higher rates than CMV and WMV. Generally, the incidence levels observed in Tanzanian coastal areas were comparable to those reported from South Africa (Ibaba et al., 2015). None of the collected wild cucurbit plants was infected with CMV. According to Zitter and Murphy (2009), some CMV strains are host specific, infecting certain hosts in the same family like the legume strain of CMV. However, given the relatively small sample size used in this study, conclusion on this matter cannot be made with regard to the lack of infections in wild cucurbits by CMV.
Most of the plants that were symptomatic tested positive for at least one of the three viruses. Interestingly, ELISA detection revealed virus infections in a considerably large number of leaf samples collected from asymptomatic plants. The absence of symptoms on plants that were found to be infected by at least one of the viruses may be explained partly by the possible genetic variation in the genotypes of the plants as farmers have different sources of planting material and also by the time at which the plants were infected. When plants are infected at their early development stage the symptoms are normally severe and may translate into high yield losses (Zitter and Murphy, 2009). The detection of viruses in asymptomatic plants is in agreement with a common understanding that conclusion on whether the plants are or are not infected with viruses can only be based on results obtained by using such means as ELISA, polymerase chain reaction, next generation sequencing and so forth.
ZYMV and WMV, but not CMV, were detected in wild plants. Although CMV was not detected in any of the wild plants, it is known to infect over 1200 plant species (Zitter and Murphy, 2009). It has been shown that the same isolate of CMV may cause severe symptoms on plants belonging to different families (Eni et al., 2013). The detection of ZYMV and WMV viruses in cultivated cucurbits and their closely related wild plants has implication on the management of these viruses. Both symptomatic and asymptomatic wild plants from the family Cucurbitaceae were observed to grow in close vicinity of cucurbit gardens (Figure 1). Since all three viruses are transmitted by aphids, there is a high chance of single or simultaneous transmission of the viruses between these hosts. Not only will the virus transmission between different hosts have implication on crop yields but may also drive the evolution of these RNA viruses, which are famously known to be prone to errors during replication (Domingo and Holland, 1994; Garcia-Arenal et al., 2001). Adaptation to different hosts may result into selection pressure (Chare and Holmes, 2004) on the virus and thus emergence of new variants, which will make it more difficult to manage the diseases through plant breeding.
Cases of co- and triple infections between a cucumovirus, CMV and the two potyviruses (ZYMV and WMV) were observed in both cultivated and wild plants. Occurrence of mixed infections of both related and unrelated viruses is common in plants (Mukasa et al., 2006; Massumi et al., 2007; Mbanzibwa et al., 2011). The interest on investigating occurrence of mixed infections might have existed for so long but it gained importance in the 1990’s following the discovery that unrelated viruses may synergize thereby causing severe symptoms on plants (Pruss et al., 1997). It was also demonstrated that co-infections between CMV (Cucumovirus) and ZYMV (Potyvirus) in cucumber cv. Delila resulted into synergism and thus breakage of resistance to CMV (Wang et al., 2004). In East Africa, studies on synergism became important following the observation that co-infection between Sweet potato feathery mottle virus (SPFMV; Potyvirus; Potyviridae) and Sweet potato chlorotic stunt virus (SPCSV; Crinivirus; Closteroviridae) results into the devastating disease of sweet potato called SPVD (Mukasa et al., 2006). Furthermore, co-infections may result into exchange of genetic materials between the co-infecting viruses resulting into a recombinant strain or virus. Indeed, a recombinant begomovirus that caused severe symptoms on cassava plants in East Africa followed co-infections and exchange of genetic material between African cassava mosaic virus (ACMV; Begomovirus; Geminiviridae) and East African cassava mosaic virus (EACMV; Begomovirus; Geminiviridae) (Deng et al., 1997; Zhou et al., 1997). In this study, the sequences of the viruses were not determined, which makes it impossible to explore recombination events in the viruses infecting cucurbits in Tanzania.
Three of the most important viruses known to cause diseases on cucurbits were detected in both symptomatic and asymptomatic cultivated and wild plants. Moreover, they were found to be widely distributed in surveyed locations. As the battle to eradicate cassava mosaic and cassava brown streak diseases continues, the virologists and plant breeders are hereby urged not to neglect the damages on cucurbits that are caused by the viruses reported herein. The cucurbits are undoubtedly among the crops whose cultivation results into creation of jobs, generation of income and improvement of health of the resources constrained persons and probably contributing to reduction of the budget for treatment of malnutrition related disorders.
Future studies should focus on unravelling genetic diversity of the isolates of these viruses in Tanzania. Information on genetic diversity is needed in order to develop management strategies for the same. There were symptomatic plants that tested negative suggesting there are other viruses which infect cucurbits. There is a need to investigate further the cause for these symptoms on plants in the coast as well as other parts of the country. This work has shown that ZYMV and WMV are infecting wild cucurbits. Therefore, any management strategies that will be developed should take this fact into consideration.
The authors have not declared any conflict of interests.
The authors acknowledge the cucurbits gardeners in the surveyed areas for the permission to access their gardens. The work was supported by Ministry of Agriculture, Livestock and Fisheries through the Mikocheni Agricultural Research Institute, Tanzania. They thank Dr. Zuberi S. Seguni (MARI, Tanzania) and Joni Kosamo (Oulu University of Applied Science) for their critical comments on the manuscript.
REFERENCES
|
Chare ER, Holmes EC (2004). Selection pressures in the capsid genes of plant RNA viruses reflect mode of transmission. J. Gen. Virol. 85:3149-3157.
Crossref
|
|
|
|
Deng D, Otim-Nape WG, Sangare A, Ogwal S, Beachy RN, Fauquet CM (1997). Presence of a new virus closely related to East African cassava mosaic geminivirus, associated with cassava mosaic outbreak in Uganda. Afr. J. Root Tuber Crops 2:23-28.
|
|
|
|
Desbiez C, Millot P, Wipf-Scheibel C, Blancard D, Chesneau T, Lecoq H (2016). First report of Pepo aphid-borne yellows virus in cucurbits in Tanzania and Mayotte. New Dis. Rep. 33:20.
Crossref
|
|
|
|
Dobhal S, Arif M, Olson J, Mendoza-Yerbafría A, Aguilar-Moreno S, Perez-Garcia M, Ochoa-Corona FM (2015). Sensitive detection and discrimination method for studying multiple infections of five major plant viruses infecting ornamental plants in nursery environments. Ann. Appl. Biol. 166:286-296.
Crossref
|
|
|
|
Domingo E, Holland JJ (1994). Mutation rates and rapid evolution of RNA viruses. In: The Evolutionary Biology of Viruses (Morse, S. S., ed.), Raven Press, New York, NY. pp. 161-184.
|
|
|
|
Eni AO, Kumar PL, Asiedu R, Alabi OJ, Naidu RA, Hughes A, Rey MEC (2013). Characterization of cucumber mosaic virus isolated from yam (Dioscorea spp.) in West Africa. Afr. J. Biotechnol. 12:3472-3480.
|
|
|
|
Gal-On A (2007). Zucchini yellow mosaic virus: insect transmission and pathogenicity - the tails of two proteins. Mol. Plant Pathol. 8:139-150.
Crossref
|
|
|
|
Garcia-Arenal F, Fraile A, Malpica JM (2001). Variability and genetic structure of plant virus populations. Ann. Rev. Phytopathol. 39:157-186.
Crossref
|
|
|
|
Gildow FE, Shah DA, Sackett WM, Butzler T, Nault BA, Fleischer SJ (2008). Transmission efficiency of Cucumber mosaic virus by aphids associated with virus epidemics in snap bean. Phytopathology 98:1233-1241
Crossref
|
|
|
|
Herrera-Vásquez JA, Córdoba-Sellés MC, Cebrián MC, Font-San-Ambrosio MI, Alfaro-Fernández A, Jordá C (2013). Viruses of Cucurbits in Panama. J. Plant Pathol. 95:435-440.
|
|
|
|
Ibaba JD, Laing MD, Gubba A (2015). Incidence and phylogeny of viruses infecting cucurbit crops in KwaZulu-Natal, Republic of South Africa. Crop Prot. 75:46–54.
Crossref
|
|
|
|
Lapido JI (1988). Viruses of vegetable crops in Africa. In Williams AO, Mbiele AL, Nkouka N (eds). Virus diseases of plants in Africa. OAU/STRC Scientific Publication, Lagos, Nigeria pp. 157-167.
|
|
|
|
Massumi H, Samei A, Hosseini Pour A, Shaabanian M, Rahimian H (2007). Occurrence, distribution, and relative incidence of seven viruses infecting greenhouse-grown cucurbits in Iran. Plant Dis. 91:159-163.
Crossref
|
|
|
|
Mbanzibwa DR, Tian YP, Tugume AK, Mukasa SB, Tairo F, Kyamanywa S, Kullaya A, Valkonen JPT (2011). Simultaneous virus-specific detection of the two cassava brown streak-associated viruses by RT-PCR reveals wide distribution in East Africa, mixed infections, and infections in Manihot glaziovii. J. Virol. Methods 171(2):394-400.
Crossref
|
|
|
|
Mukasa SB, Rubaihayo PR, Valkonen, JPT (2006). Interactions between a crinivirus, an Ipomovirus and a potyvirus in coinfected sweet potato plants. Plant Pathol. 55:458-467.
Crossref
|
|
|
|
Provvidenti R (1996). Diseases caused by viruses. In Zitter TA., Hopkins DL., Thomas CE. (eds). Compedium of cucurbit diseases. APS press. St. Paul, MN, USA. pp. 37-45.
|
|
|
|
Pruss G, Ge X, Shi MX, Carrington JC, Vance VB (1997). Plant viral synergism: the potyviral genome encodes a broad-range pathogenicity enhancer that transactivates replication of heterologous viruses. Plant Cell 9:859-68.
Crossref
|
|
|
|
Tugume AK, Mukasa SB, Valkonen JPT (2008). Natural wild hosts of Sweet potato feathery mottle virus show spatial differences in virus incidence and virus-like diseases in Uganda. Phytopathology 98:640-52.
Crossref
|
|
|
|
Wang Y, Lee KC, Gaba V, Wong SM, Palukaitis P, Gal-On A (2004). Breakage of resistance to Cucumber mosaic virus by co-infection with Zucchini yellow mosaic virus: enhancement of CMV accumulation independent of symptom expression. Arch. Virol. 149(2):379-396.
Crossref
|
|
|
|
Weinberger K, Msuya J (2004). Indigeneous vegetables in Tanzania, Significance and Prospects. AVRDC The World Vegetable Centre. Tech. Bull. 31:71.
|
|
|
|
Yuki VA, Rezende JAM, Kitajima EW, Barroso PAV, Kuniyuki H, Groppo GA, Pavan MA (2000). Occurrence, distribution, and relative incidence of five viruses infecting cucurbits in the state of São Paulo, Brazil. Plant Dis. 84:516-520.
Crossref
|
|
|
|
Zhou X, Liu Y, Calvert L, Munoz C, Otim-Nape GW, Robinson DJ, Harrison BD (1997). Evidence that DNA-A of a geminivirus associated with severe cassava mosaic disease in Uganda has arisen by interspecific recombination. J. Gen. Virol. 78:2101-2111.
Crossref
|
|
|
|
Zitter TA, Banik MT (1984). Virus Diseases of Cucurbits. Fact Sheet 732:40.
|
|
|
|
Zitter TA, Murphy JF (2009). Cucumber mosaic. The Plant Health Instructor. DOI: 10.1094/PHI-I-2009-0518-01
Crossref
|