ABSTRACT
The objective of this study was to evaluate the effect of organic-matter incorporated into the soil on population densities of the causal agents of the dry rot disease of yam, under field conditions. The experiment was performed in a natural infested area with a mixed population of Pratylenchus coffeae and Scutellonema bradys, in Quebrangulo county (Alagoas state, Brazil) in a randomized block design with five treatments and five replicates. The sources of organic matter used as soil amendments were: coconut husk powder, castor bean cake, cattle manure and chicken manure. Non amended soil was used as a control. Nine months after planting, the tubers were harvested. No statistical differences were found among disease incidence, yam production and nematode population densities in the soil. However, the application of chicken manure reduced P. coffeae population in tubers.
Key words: Dioscorea spp., Scutellonema bradys, Pratylenchus coffeae, nematode management.
Yam (Dioscorea spp.) is a monocotyledonous plant of the family Dioscoreaceae, comprising more than 600 species in the genus, mostly grown in Africa (Dioscorea cayenensis), the Caribbean, Mexico and Southeast Asia (Dioscorea alata, Dioscorea esculenta, Dioscorea composita, Dioscorea dumetorum and Dioscorea rotundata) and South America (D. cayenensis) (Cazé, 2002).
In 2014, African countries produced 65.7 million tons of tubers equivalent to 96.4% of the world production (68.2 million tons). Among South American countries, Brazil ranks second, with 25.5 thousand hectares and an estimated production of 247 thousand tons (FAO, 2015). According to Santos et al. (2011), the Northeastern region is the largest producer of yams in Brazil with approximately 15 thousand ha and production of 200 thousand tons (average yield of 10.5 t ha-1), mainly cultivated in the states of Paraíba, Pernambuco, Bahia and Alagoas. Among the constraints to yam production in Brazil, dry rot disease caused by Scutellonema bradys, Pratylenchus coffeae and Pratylenchus brachyurus causes the greatest damage to this crop (Moura, 2006).
In Alagoas, mixed populations are found in most yam-growing areas with an incidence ranging between 0.2 and 85% (Muniz et al., 2012).The first symptoms of the disease are light yellow lesions below the outer skin of the tubers, turning to a dark brown to black color as the disease progresses. External cracks arise in the tubers’s skin and complete deterioration may occur during storage. The damage caused by the nematode is confined to sub-epidermal, peridermal and parenchymatous tissues extending to 1-2 cm into the tuber, although sometimes deeper (Kwoseh et al., 2002; Bridge and Starr, 2007). Above-ground symptoms are not apparent (Bridge and Starr, 2007). The most successful method for preventing nematodes’ damages remains in the use of nematode-free seed tubers in nematode-free land (Bridge and Starr, 2007), but the difficulty to obtaining healthy propagative material turn this technique unfeasible (Moura, 2006).
According to Gowen et al. (2005) and McSorley (2011), an extensive range of organic materials have shown efficiency in reducing nematode populations in a number of pathosystems. Organic materials as agro-industrial and animal wastes may act as nutrient sources and improve water-holding capacity of the soil, increasing plant growth. Higher organic content in soils also stimulates the activity of plant-parasitic nematodes’ antagonistic organisms. Furthermore, decomposition of residues results in the accumulation of specific compounds in the soils which may be nematicidal (Bridge, 1996). However, information on the use of organic materials in the management of plant-parasitic nematodes that affect yam crops is limited (Santos et al., 2009; Osei et al., 2013).
Thus, the aim of this work was to evaluate the effect of some animal and agro-industrial wastes that are available in the Northeast region of Brazil, to manage nematodes under field conditions.
The experiment was conducted on February 2013, on a farm located in Quebrangulo county, AL (9° 15’ 50.9” S; 36° 26’ 11.7” W), previously grown with yam and naturally infested with dry rot disease nematodes. The experiment was performed in a randomized block design with five treatments and five replicates, in plots consisting of four ridges 3.50 m long, considering the two central ridges as useful area. Seed tubers weighing approximately 250 to 350 g each were selected from a field known to be free of the dry rot disease (based on the absence of symptoms and after randomly sampling tubers to test presence of plant-parasitic nematodes), and planted at a 1.20 x 0.35 m spacing.
Previous to planting, a composite soil sample of four sub-samples in each plot was collected, using a zig-zag pattern, in the two central rows to evaluate the initial nematode populations. The nematodes were extracted, according to the centrifugal-flotation technique (Jenkins, 1964), in aliquots of 100 cm3 of soil and the nematodes were quantified with the aid of Peter’s counting slides (Astel®, Botucatu-SP, Brazil), under a light microscope. The identification of Pratylenchus species was done according to Gonzaga et al. (2012). In addition, the chemical and physical properties of soil were determined (Table 1), as well as the chemical analysis of the sources of organic matter (Table 2).
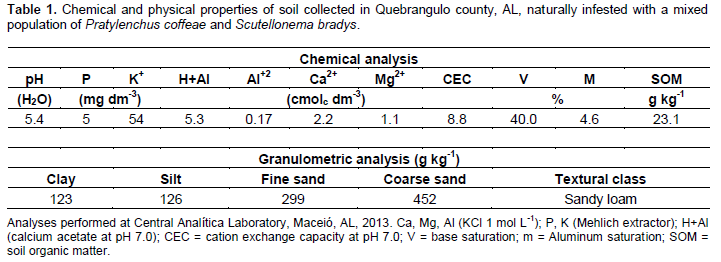
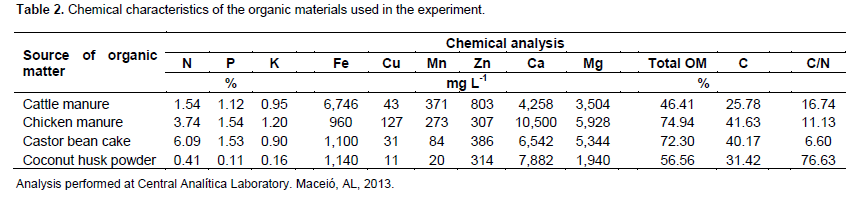
Soil treatments included: 1) untreated soil (control), 2) coconut husk powder (from coconut epicarp) - 37 t ha-1, 3) castor bean cake – 2.5 t ha-1, 4) cattle manure - 10 t ha-1 and 5) chicken manure - 4 t ha-1. These dosages were determined based on the results of the chemical analysis of soil and the nutrient content of the sources of organic materials, using a reference of 150 kg ha-1 nitrogen. Due to the high C/N ratio of coconut husk powder, a supplemental fertilizer with ammonium sulfate (10 g/plant) was applied to this treatment. Mineral fertilization with NPK 16-00-20 (20 g/plant) was made 70 days after planting, according to soil testing analysis.
The evaluation of the initial nematode populations showed the presence of P. coffeae and S. bradys with average of 12.0 to 25.0 specimens/100 cm3 of soil among plots. There were no statistical differences, indicating the uniformity of the nematode populations in the field (Table 3). These low nematode population levels could be attributed to the stressful conditions due to the dry season. At the end of the experimental period, there was no significant difference among disease incidence, tuber production and nematode population densities in soil due to the use of different sources of organic matter. However, the application of chicken manure reduced P. coffeae population in tubers (Table 3).
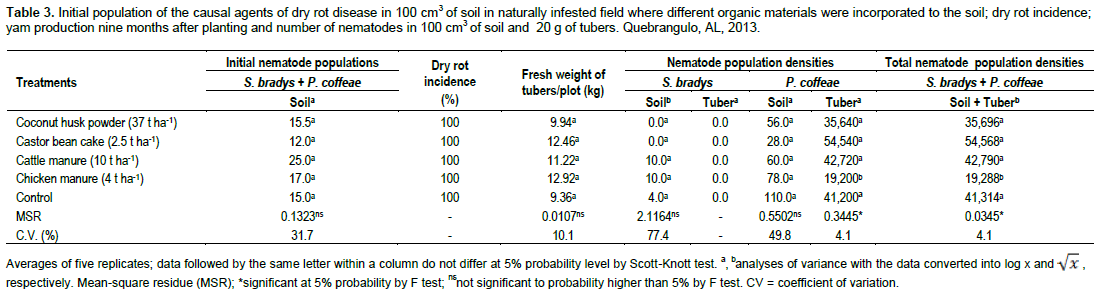
Despite the efficiency of chicken manure in controlling nematode populations (Ferraz et al., 2010; Abdel-Dayem et al., 2012), negative results have also been observed. Examples can be found in Brazil, with Meloidogyne javanica and Meloidogyne incognita on bananas (Vilas Boas et al., 2004a; Vilas Boas et al., 2004b), and in the United States with Heterodera glycines in soybean crop (Donald et al., 2013). However, no published report on this source of organic matter was found regarding P. coffeae or S. bradys on yam plants.
In Nigeria, Adesiyan and Adeniji (1976) observed that application of cattle manure to the soil at a rate of 1.89 t ha-1, increased tuber yield of D. alata, and significantly reduced the population density of S. bradys. The differences in the current results may possibly be related to the yam species and the implementation conditions on each experiment. Although, the mentioned work had been done under field conditions, the soil was artificially infested with the nematode. In addition, the authors reported that the average nematode population per 50 g of tuber peelings at harvest was 1,410 specimens which correspond to approximately 28 individuals per gram of tissue. Although, the application of the treatment significantly reduced the nematode population, according to Bridge et al. (2005), populations of S. bradys in excess of 20 nematodes/g of tuber peelings are necessary to produce external symptoms of damage. In the present work, up to 2,727 specimens of P. coffeae per gram of tuber peelings were recorded.
In Brazil, Santos et al. (2009) assessed the effect of antagonistic plants used as green manure and organic wastes to control nematodes in yam (D. cayenensis), under field conditions. The authors observed incidence of dry rot disease of 36.35% in the first year of cropping and 21.88% in the second, with the use of cattle manure. However, data on the initial nematode populations in the area, the rate of manure application, and the disease incidence from the control plants, which prevent comparisons between results were not shown. With respect to by-products from the processing of coconut fruits, the data on its use in the management of plant-parasitic nematodes are scarce, and when assessed for controlling M. javanica in banana (Vilas Boas et al., 2004a) and tomato crops (Dallemole-Giaretta et al., 2010), only in the second case was observed a favorable result.
Silveira et al. (2002) detected high quantity of microorganisms in coconut coir fiber (from coconut mesocarp) reported as agents of biocontrol for several pathogens, among these are Trichoderma species. According to Meyer et al. (2000), culture filtrate from T. virens contained extracellular factors that inhibited egg hatch and second-stage juvenile mobility of M. incognita.
Concerning the use of castor bean cake to control plant-parasitic nematodes, some researches have been already published. For example, this organic material has been applied to the soil for management of Meloidogyne species in sugarcane (Dinardo-Miranda and Fracasso, 2010), and tomato crops (Lopes et al., 2009; Roldi et al., 2013). According to Rich et al. (1989) the nematicidal activity of this product was attributable to the chemical compound, ricin, a natural occurring lectin capable of inhibiting protein synthesis (Audi et al., 2005). The results obtained in the present work are not in accordance with this. The difference between the reports shown in the literature and the current work could be due to variations in nematode species, host plant, chemical composition of the organic amendments as well as the rate and time of application, and the environmental factors like temperature, microbial community and soil type. In addition, the present work involved a mixed nematode population.
The use of organic materials as cattle manure, chicken manure, castor bean cake and coconut husk powder did not reduce the incidence of dry rot of yams under field conditions. However, the application of chicken manure reduced P. coffeae population in tubers.
The authors have not declared any conflict of interests.
The first author thanks the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for granting a master’s degree scholarship.
REFERENCES
|
Abdel-Dayem EA, Erriquens F, Verrastro V, Sasanelli N, Mondelli D, Cocozza C (2012). Nematicidal and fertilizing effects of chicken manure, fresh and composted olive mill wastes on organic melon. Helminthologia 49(4):259-269.
Crossref
|
|
|
|
Adesiyan SO, Adeniji MO (1976). Studies on some aspects of yam nematode (Scutellonema bradys). Ghana J. Agric. Sci. 9:131-136.
|
|
|
|
Audi J, Belson M, Patel M, Schier J, Osterloh J (2005). Ricin poisoning: A comprehensive review. J. Am. Med. Assoc. 294:2342-2351.
Crossref
|
|
|
|
Bridge J (1996). Nematode management in sustainable and subsistence agriculture. Annu. Rev. Phytopathol. 34:201-225.
Crossref
|
|
|
|
Bridge J, Coyne DL, Kwoseh C (2005). Nematode parasites of tropical root and tuber crops (excluding potatoes). In: Plant parasitic nematodes in subtropical and tropical agriculture. Luc M, Sikora RA, Bridge J (Eds.). 2nd ed. CAB International, Wallingford. pp. 221-258.
Crossref
|
|
|
|
Bridge J, Starr JL (2007). Yams (Dioscorea spp.). In: Plant nematodes of agricultural importance: a color handbook. Bridge J, Starr JL (Eds.). Academic Press, San Diego. pp. 79-83.
Crossref
|
|
|
|
Cazé FJ (2002). Clonagem do inhame (Dioscorea sp.) pormétodos biotecnológicos. II Simpósio nacional sobre as culturas do inhame e do taro, 23 a 26 de setembro de 2002, João Pessoa, PB, Brasil. 1:113-126.
|
|
|
|
Coolen WA, D’Herde CJ (1972). A method for the quantitative extraction of nematodes from plant tissue. State Agricultural Research Center, Ghent. 77 p.
|
|
|
|
Dallemole-Giaretta R, Freitas LG, Zooca RJF, Podestá GS, Caixeta LB, Ferraz S, Lopes EA (2010). Associação de Pochonia chlamydosporia, Bacillus cereus e fibra de coco no controle de Meloidogyne javanica em tomateiro. Nematol. Bras. 34(1):18-22.
|
|
|
|
Dinardo-Miranda LL, Fracasso JV (2010). Efeito da torta de mamona sobre populações de nematoides fitoparasitos e a produtividade da cana-de-açúcar. Nematol. Bras. 34(1):68-71.
|
|
|
|
Donald PA, Allen PB, Tyler DD, Sistani KR, Tewolde H, Walker ER (2013). Effect of broiler litter application to soybean crop infested with soybean cyst nematode. Nematropica 43(1):24-34.
|
|
|
|
FAO (2015). Food and Agriculture Organization of the United Nations. Statistics Division.
View
|
|
|
|
Ferraz S, Freitas LG, Lopes EA, Dias-Arieira CR (2010). Manejo sustentável de fitonematoides. Ed. UFV. Viçosa. 306 p.
|
|
|
|
Gonzaga V, Santos JM, Soares PLM (2012). Chave ilustrada para a identificação das seis espécies de Pratylenchus mais comuns no Brasil. 7 p.
View
|
|
|
|
Gowen SR, Quénéhervé P, Fogain R (2005). Nematode parasites of bananas and plantains. In: Plant parasitic nematodes in Subtropical and Tropical Agriculture. Luc M, Sikora RA, Bridge, J (Eds.). 2nd ed. CAB International, Wallingford. pp. 611-643.
Crossref
|
|
|
|
Jenkins WR (1964). A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Dis. Rep. 48(9):692.
|
|
|
|
Kwoseh C, Plowright RA, Bridge J (2002). The yam nematode: Scutellonema bradys. In: Plant resistance to parasitic nematodes, Starr JL, Cook R, Bridge J (Eds.). CAB International, Wallingford. pp. 221-228.
Crossref
|
|
|
|
Lopes EA, Ferraz S, Dhingra OD, Ferreira PA, Freitas LG (2009). Soil amendment with castor bean oilcake and jack bean seed powder to control Meloidogyne javanica on tomato roots. Nematol. Bras. 33(1):106-109.
|
|
|
|
McSorley R (2011). Overview of organic amendments for management of plant-parasitic nematodes, with case studies from Florida. J. Nematol. 43(2):69-81.
|
|
|
|
Meyer SLF, Massoud SI, Chitwood DJ, Roberts DP (2000). Evaluation of Trichoderma virens and Bulkholderia cepacia for antagonistic activity against root-knot nematode, Meloidogyne incognita. Nematology 2(8):871-879.
Crossref
|
|
|
|
Moura RM (2006). Principais doenças do inhame-da-costa no nordeste do Brasil. Anais da Academia Pernambucana de Ciência Agronômica 3:180-199.
|
|
|
|
Muniz MFS, Silva EJ, Castro JMC, Alencar LMC, Rocha FS, Gonzaga V (2012). Intensity of dry rot disease of yam in the state of Alagoas, Brazil. Nematropica 42(2):198-200.
|
|
|
|
Oostenbrink M (1966). Major characteristics of the relation between nematodes and plants. Mededelingen Landbouwhogeschool, Wageningen. 46 p.
|
|
|
|
Osei K, Otoo E, Danso Y, Adomako J, Agyeman A, Asante JS (2013). Organic soil amendments in nematode management in yam production. Nematropica 43(1):78-82.
|
|
|
|
Rich JR, Rahi GS, Opperman CH, Davis EL (1989). Influence of the castor bean (Ricinus communis) lectin (ricin) on motility of Meloidogyne incognita. Nematropica 19(1):99-103.
|
|
|
|
Roldi M, Dias-Arieira CR, Severino JJ, Santana SM, Dadazio TS, Marini PM, Mattei D (2013). Use of organic amendments to control Meloidogyne incognita on tomatoes. Nematropica 43(1):49-55.
|
|
|
|
Santos ES, Lacerda JT, Carvalho RA, Cassimiro CM (2009). Produtividade e controle de nematóides do inhame com plantas antagônicas e resíduos orgânicos. Tecnol. Ciênc. Agropecu. 3(2):7-13.
|
|
|
|
Santos ES, Lacerda JT, Matias EC, Barbosa MM (2011). Cultivo do inhame em base agroecológica. EMEPA-PB, João Pessoa. 60 p.
|
|
|
|
Silva FAZ (2014). ASSISTAT – Statistical Assistance, Version 7.7 beta. Universidade Federal de Campina Grande, Brazil.
View
|
|
|
|
Silveira EB, Rodrigues VJLB, Gomes AMA, Mariano RLR, Mesquita JCP (2002). Pó de coco como substrato para produção de mudas de tomateiro. Hortic. Bras. 20(2):211-216.
Crossref
|
|
|
|
Vilas Boas LC, Cares JE, Tenente RCV (2004a). Efeito de diferentes adubações orgânicas em cultivares de bananeira, visando o controle de Meloidogyne javanica em microparcelas sob condições de campo. XI Talento Estudantil. Embrapa Recursos Genéticos e Biotecnologia, Brasília.
View
|
|
|
|
Vilas Boas LC, Cares JE, Tenente RCV, Silva Neto SP (2004b). Efeito de diferentes materiais orgânicos em bananeira no controle de Meloidogyne incognita, sob condições de casa de vegetação. XI Talento Estudantil. Embrapa Recursos Genéticos e Biotecnologia, Brasília.
View
|