Full Length Research Paper
ABSTRACT
Understanding the habitat preference of the Stone Partridge (Ptilopachus petrosus) is a crucial step for its conservation and management across different ecosystems. This study was focused on the environmental factors and the vegetation data of the species habitat which were collected from 60 plots surveyed along 6 perpendiculars transects. Binary logistic regression was used to determine the relationship between the Stone Partridge and the environmental variables. Phytoecological data were analysed using a Detrended Correspondence Analysis (DCA) to identify the different plant communities from which Shannon and Pielou indices have been measured. The analyses identified the proximity of the rocks as the environmental variables which determine the Stone Partridge presence. Three plant communities were associated with the Stone Partridge presence. The plant families most represented were the Poaceae followed by Leguminosae-Papilionoideae, Leguminosae-Caesalpinioideae, Rubiaceae, Combretaceae, Euphorbiaceae, Asteraceae, Leguminosae-Mimosoideae and Cyperaceae. The phanerophytes and therophytes were the life forms most represented. The Shannon diversity indices for the plant communities varied from 2.12 at 4.53 bits. The identification of preferred habitats are a valuable tool for managers interested in the conservation of this species, however constant monitoring that identifies changes in the habitats is required to identify detrimental habitat change.
Key words: Ptilopachus petrosus, Logistic regression, plant communities, conservation, Benin.
INTRODUCTION
The consequences of anthropogenic and climatic disturbances on animal communities and their environment have been the focus of conservation research in recent decades (Cardinale et al., 2012; Dirzo et al., 2014). Suitable habitat for animals unable to adapt to anthropogenic change is decreasing due to habitat loss and fragmentation (Fischer and Lindenmayer, 2007; Gehring and Swihart, 2003), harvesting or poaching (Gangaas et al., 2013; Martin and Caro, 2013; Carter, 2017), pollution of rivers and water bodies (Jepson et al., 2016; Desforges et al., 2016), mortality linked to human-animal conflict (J?drzejewski et al., 2017), climate change (Brodie and Pearson, 2016; Descamps et al., 2017) and emerging diseases caused by fungi and parasites (Weinstein et al., 2017). Between 1970 and 2005, it is estimated that the global population of African fauna within protected areas fell by around 60% on a continental scale (Gandiwa et al., 2016; Murn et al., 2016). This is especially true for birds, whose populations have collapsed in West Africa (Howland et al., 2016; Bakker et al., 2016; Amoussou et al., 2012; Ayanlade and Proske, 2016) and in the case of the Stone Partridge, a species thought to be restricted to woody ecosystems near hills and rocky outcrops (Fretwell and Trathan, 2009).
In Benin, the Stone Partridge is mostly found in the centre and the northern of the country, with fairly low numbers in most of their habitats. This species is declining due to poaching and the effects of climate change (Bass and Chakrabarty, 2014). Yet the Stone Partridge has a special place in Beninese culture as it is used for medicinal and magical purposes (Codjia et al., 2021). Although the species has been included in various phylogenetic studies and is listed in various site-specific species lists, it has not been the focus of any surveys. There is thus a requirement for conservation and management purposes of a better understanding of the habitat requirements of the Stone Partridge. This study describes the preferred habitats of the Stone Partridge through a phytoecological survey across the species range in Benin.
MATERIALS AND METHODS
Study area
This study was undertaken in the Republic of Benin (Benin). The Republic of Benin is situated in West Africa between the latitudes 6°10’N and 12°25’N and longitudes 0°45’E and 3°55’E (Figure 1). It is bordered by the republics of Togo in the west, Nigeria in the east, Atlantic Ocean in the south, Burkina Faso and Niger in the north. It covers a land area of 112,622 km2. The country hosts roughly 11 million people, with a population growth rate of 2.71% (World Bank, 2019). The mean annual rainfall ranges from 900 to 1100 mm. The mean monthly temperature ranges from 25 to 35°C and values of the relative air humidity range from 81% in August to 26% in February (Adam and Boko, 1993). Natural vegetation types include riparian forests, woodlands, tree/shrub savannahs and grass savannahs. The communal land is dominated by secondary and disturbed vegetation types including fallow, savannah patches, degraded woodland and riparian forest (Adomou, 2005).
Bird survey data
In each of the 3 climatic zones of Benin, a visual confirmation was made of the presence / absence of the species through the natural vegetation and agricultural lands, following perpendicular transects, NNE-SSW (25-225 grades) and ESE-WNW (125 –325 grades) (Figure 2). The transects extended up to 1 km. A series of circular plots of 706.5 m² (15 m radius) were placed along each of the two transects. Plots were centred on the transects and were placed at regular intervals of 200 m on the NNE – SSW and ESE – WNW transects, respectively. Two perpendicular transects were randomly selected from each climate zone where Stone Partridge were observed and cited in literature.
Before going to the field, it was important to define the routes to be maked. For this, information system Geographic ArcGis 10.1 was used to delineate the transects. Once these trails were traced, the geographical coordinates of the fixed plots were recorded with a GPS in order to optimize the orientation on the ground. A total of 60 plots were surveyed along 6 perpendicular transects.
In each plot, the signs of presence of the Stone Partridge were first looked for. These signs of the presence correspond to droppings, feathers, traces or direct observations of individuals. This species is rare and very difficult to observe in the wild because of its great discretion. The droppings are often the main signs of the presence of the species. Usually, they are located in their resting places.
As soon as an indication of the presence of the Stone Partridge is detected along these transects, its location is recorded with GPS. Phytoecological surveys were undertaken to determine the plant communities in Stone Partridge habitat according to the sigmatist method of Braun-Blanquet (1932) and the environmental predictor variables (distance to farmland, presence of rock-outcrops, the distance to water, slope, presence of predators, canopy cover and bushfire) were collected. Thereafter, the distance to farmland, water and slope was derived based on plots waypoints using Euclidean distance in the spatial analysis extension of ArcGIS 10.1 software.
Phytoecological inventory method
Phytoecological surveys were undertaken to determine the plant communities in Stone Partridge habitat according to the sigmatist method of Braun-Blanquet (1932). 60 circular plots of 706.5 m² (15 m radius) were used to collect vegetation composition in the four main vegetation types: woodland, shrub/tree savannah, grass savannah and fallow cultivated areas. Tree, shrub and herbaceous plant sampling were carried out through plots (Weber et al., 2000). In each plot, we recorded: (i) the vegetation type; (ii) the exhaustive species list, (ii) the percentage of cover for each species, (iii) the number of species as well as the percentage cover for each vegetation layer; (iv) the soil texture; (v) the topographic position (slope, bottom slope, mid-slope, top of slope or summit).
The plant species were assigned coefficients of abundance-dominance (Braun-Blanquet, 1932) which is the expression of the relative space occupied by all individuals of each species. The average coefficients of cover (ACC) used were:
5: species covering 75 to 100% of the survey area (ACC) = 87.5%
4: species covering 50 to 75% of the survey area (ACC) =62.5%
3: species covering 25 to 50% of the survey area (ACC) =37.5%
2: species covering 5 to 25% of the survey area (ACC) =15%
1: species covering 1 to 5% of the survey area (ACC) =3%
+: species covering 0 to 1% of the survey area (ACC) =0.5%.
Data analysis
Environmental factors determining the presence of the Stone Partridge
Binary logistic regression was used, an appropriate statistical tool to determine the influence of explanatory variables on response variables when the latter take a dichotomous form (Peng et al., 2002). The logistic model predicts the Logit of the response variable (Y) from the explanatory variables (X). The Logit is the natural logarithm (ln) of the ratio of the probabilities (π) that Y occurs and the probabilities (-π) that Y does not occur. The model is specified as follows:
where 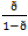 is the “Odd”; β0 is the y-intercept; β1, β2, …, βk are the regression coefficients of the independent variables x1i, x2i,…, xki.
is the “Odd”; β0 is the y-intercept; β1, β2, …, βk are the regression coefficients of the independent variables x1i, x2i,…, xki.
The explanatory variables (x) were the seven environmental variables described earlier. Stepwise regression was applied from the full model to select the best model based on the most significant variables. Before running the logistic regression, multivariate correlation analysis was performed to verify collinearity between explanatory variables. The significance of the parameters of the logistic regression was evaluated by the likelihood ratio c², the deviation test and the statistics of Hosmer- Lemeshow and Wald. All statistical analyses were performed with R version 4.0.5.
Plant community ordination, classification and indicator species determination
An ordination technique (Detrended Correspondance Analysis, DCA) was performed to identify possible gradients in and between communities that allow the description of the characteristics of the Stone Partridge habitat.
Floristic analysis of the plant communities inhabited by the Stone Partridge
Plant community species richness: Species richness was determined by counting the number of species recorded in the plots describing each plant community. The total number of species recorded per plant community was computed and estimated the species richness for woody and herbaceous plant species separately.
Plant community diversity: Shannon index was used as a measure of α-diversity, which combines species richness with relative abundance (Shannon and Weaver, 1949). It was estimated as:
where pi is the relative abundance of the species i in a given plant community and S the species richness of the community.
Plant community evenness: The evenness of a plant community was calculated using Pielou evenness (Pielou, 1969) defined as:
E = H’/log2S
where S is the total number of species per plant community and H’ is the value of the Shannon index. E values range from 0 (dominance of few species in the community) to 1 (evenly distribution of plant species in the community).
Classification of the plant community by life forms
Life forms were assigned to species using those defined by Raunkiaer (1934): Therophyte (Th), Hemicryptophyte (Hec), Chamaephyte (Ch), and Phanerophyte (Ph).
RESULTS
Environmental factors determining the presence of the Stone Partridge
The presence (pa) of the Stone Partridge seems to show a correlation with the proximity of the rocks (Figure 3). The overall evaluation of the logistic regression model and the Hosmer-Lemeshow goodness test revealed that the model provided adequate form to the data with Omnibus tests of significant coefficients and a 92% correct prediction. The results show that only one variable significantly influenced the presence of the species: the proximity of rocks/hill significantly increases the likelihood of the Stone Partridge being present (Table 1). The birds presence was not clearly related to the distance from the fields, the water and the presence of predators.
Floristic composition of plant communities
A total of 210 plants species belonging to 49 families were encountered within plants communities associated with the Stone Partridge. The most species rich families were Poaceae (45 species, 21.43%) followed by Leguminosae-Papilionoideae (31 species, 14.76%), Leguminosae-Caesalpinioideae (11 species, 5.24%), Rubiaceae (10 species, 4.76%), Combretaceae (9 species, 4.29%), Euphorbiaceae (8 species, 3.81 %), Asteraceae and Leguminosae-Mimosoideae (7 species each, 3.33 %), and Cyperaceae (6 species, 2.86%).
The Stone Partridge habitat typology
The detrended correspondance analysis ordination diagram (Figure 4) of the plot data identified three plant communities associated with the Stone Partridge: (i) Saxicolous savannah, (ii) Open-forest and (iii) Farmland/Fallows. The dominant plant species in each habitat were Acacia macrostachya and Detarium for Saxicolous savannah, Anogeissus leiocarpa and Burkea africana in Open-forests, Vitellaria paradoxa and Annona senegalensis for Farmland/Fallows.
Specific diversity of plants communities
Table 2 presents the ecological parameters of the different plant communities. The highest species richness (143 species) was obtained for farmland/fallows dominated by V. paradoxa and A. senegalensis, while the lowest (92 species) was obtained for saxicolous savannah with A. macrostachya and Detarium microcarpum. The Shannon diversity index (H) varied between 2.12 bits (Open-forest) and 4.53 bits (Saxicolous savannah). Pielou’s equitability index was the highest for saxicolous savannah and lowest for open-forest.
Life forms composition of plant communities in Stone Partridge habitat
The dominant life-form in communities were therophytes (annuals) with 38.57% (81 species) followed by phanerophyte (large shrubs and trees) 33.57% (71 species), hemicryptophytes (herbaceous perennials), chamaephytes (woody plants with resting buds on or near the ground) and geophyte (plants with underground storage organs like buls) 9.05% each (19 species each) and epiphytes 0.48 % (1 species). Figure 5 shows the raw and weighted spectra according to life-form in each plant community inhabited by Stone Partridge. The therophytes species were the most abundant in raw and weighted spectra in each community.
DISCUSSION
The study showed that the Stone Partridge were strongly associated with the farmland and fallows habitats, followed by the open forest habitat, and hardly at all with the saxicolous savannah habitats. However, the presence of the rocky outcrops is essential to the Stone Partridge habitat (BirdLife International, 2021). These habitats in turn are associated with different plant communities (Jacobi et al., 2007). The preferred habitats that are farmland and fallows are characterised by V. paradoxa and A. senegalensis plant species. These results are similar to those of Aleza et al. (2015). The soil has a sandy-silty texture and is topographically associated with slopes. The three types of plant communities identified are distributed along a topographic gradient due to the presence of hills. The variability of grazed plant groups is caused by climatic and topographical factors and by the influence of human activities (Dawar et al., 2021). From a topographical point of view, the studied stations present a difference since they belong to different topographic levels: the saxicolous savannahs are on the side of the hills while the fields/fallow are at the bottom of the slope. Studies have shown that the disturbance gradient due to topography is large enough to determine the distribution of Stone Partridge habitats. Despite the negligible number of studies carried out to date on the habitats of Stone Partridge in Benin, all the results obtained through this study are consistent with those of the already existing bibliography (Hanle et al., 2020).
The presence of signs of the presence of the Stone Partridge in fallow land is explained by the fact that they go there to feed on grasses, roots, fallen fruits and fungi (Khan et al., 2021).
Patterns of floristic diversity of Stone Partridge habitat
The floristic composition of plant communities shows that the dominance of the families Poaceae, Leguminosae-Papilionoideae, Leguminosae-Caesalpinioideae, Rubiaceae, Combretaceae, Euphorbiaceae, Asteraceae, Leguminosae-Mimosoideae and Cyperaceae. These observations are consistent with the results obtained by previous studies in the same area (Oumorou et al., 2010; Houessou et al., 2013). The marked presence of therophytes is not surprising as the sites are under strong anthropogenic pressure. However, the predominance of therophytes is a favourable situation for Stone Partridge because its diet mainly consists of seeds (Carroll, 1994).
CONCLUSION
Monitoring of the species habitat can contribute to the conservation as well as management of the Stone Partridge population in the Benin. In this study, three plant communities inhabited by the Stone Partridge were explored. Given the use of the species by local communities for food as well as medicinal purposes, and concerns regarding population declines, it was found that the species was frequently found in anthropogenically modified habitat (farmlands), and especially in association with rocky outcrops. Further monitoring is required to further understand land use patterns influencing the distribution of this totemic species in Benin: the move from community managed land to large-scale commercial agriculture may have negative consequences for the species in the future.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGMENTS
The authors thank Dr Harriet M. Ndofor-Foleng and Dr Alan TK Lee for their helpful comments and English language editing during the preparation of this manuscript. They also extend their appreciation to Dr Etienne Monsoundé Dossou for the exported data and the rural population for their collaboration. They appreciate the grant from the Deutscher Akademischer Austauschdienst DAAD to carry out this research.
REFERENCES
|
Adam S, Boko M (1993). Le Bénin. Les éditions Flamboyant/EDICEF 6 p. |
|
|
Adomou AC (2005). Vegetation patterns and environmental gradients in Benin: implications for biogeography and conservation. PhD thesis Wageningen University, Wageningen. |
|
|
Aleza K, Villamor GB, Wala K, Dourma M, Atakpama W, Batawila K, Akpagana K (2015). Woody species diversity of Vitellaria paradoxa CF Gaertn traditional agroforests under different land management regimes in Atacora district (Benin, West Africa).International Journal of Biodiversity and Conservation 7(4):245-253. |
|
|
Amoussou LL, Lougbégnon TO, Djossa BA, Kidjo FC, Awessou B, Mensah GA (2012). Analyse de la pression anthropique et son effet sur la biodiversité des sites à ériger en réserves de faune au Sud-Bénin. Bulletin de la Recherche Agronomique du Bénin (BRAB) NSpécial Elevage et Faune pp. 28-34. |
|
|
Ayanlade A, Proske U (2016). Assessing wetland degradation and loss of ecosystem services in the Niger Delta, Nigeria. Marine and Freshwater Research 67(6):828-836. |
|
|
Bakker P, Schmittner A, Lenaerts JTM, Abe?Ouchi ABiD, van den Broeke MR, Yin J (2016). Fate of the Atlantic Meridional Overturning Circulation: Strong decline under continued warming and Greenland melting. Geophysical Research Letters 43(23):12-252. |
|
|
Bass AE, Chakrabarty S (2014). Resource security: Competition for global resources, strategic intent, and governments as owners. Journal of International Business Studies 45(8):961-979. |
|
|
BirdLife International (2021). Species factsheet: Ptilopachus petrosus. |
|
|
Braun-Blanquet J (1932). Plant Sociology: The Study of Plant Communities. McGray Hill Edition: New York, London. |
|
|
Brodie J, Pearson RG (2016). Ecosystem health of the Great Barrier Reef: time for effective management action based on evidence. Estuarine, Coastal and Shelf Science 183:438-451. |
|
|
Cardinale BJ, Duffy JE, Gonzalez A, Hooper DU, Perrings C, Venail, P, Naeem S (2012). Biodiversity loss and its impact on humanity. Nature 486(7401):59-67. |
|
|
Carroll JP (1994). Family Odontophoridae (New World quails). |
|
|
Carter CS (2017). The role of oxytocin and vasopressin in attachment. Psychodynamic Psychiatry 45(4):499-517. |
|
|
Codjia CS, Onyimonyi AE, Lougbégnon TO, Codjia JT (2021). Meat and magic: traditional use of the Stone Partridge Ptilopachus petrosus in Benin. Ostrich pp. 1-7. |
|
|
Dawar K, Fahad S, Jahangir MMR, Munir I, Alam SS, Khan SA, Ishaq Mian A, Datta R, Saud S, Banout J, Adnan M, Ahmad MN, Khan A, Dewil R, Habib-ur-Rahman M, Ansari MJ, Danish S (2021). Biochar and urease inhibitor mitigate NH3 and N2O emissions and improve wheat yield in a urea fertilized alkaline soil. Scientific Reports 11(1):1-11. |
|
|
Descamps S, Aars J, Fuglei E, Kovacs KM, Lydersen C, Pavlova O, Strøm H (2017). Climate change impacts on wildlife in a High Arctic archipelago-Svalbard, Norway. Global Change Biology 23(2):490-502. |
|
|
Desforges JPW, Sonne C, Levin M, Siebert U, De Guise S, Dietz R (2016). Immunotoxic effects of environmental pollutants in marine mammals. Environment International 86:126-139. |
|
|
Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJ, Collen B (2014). Defaunation in the Anthropocene. Science 345(6195):401-406. |
|
|
Fischer J, Lindenmayer DB (2007). Landscape modification and habitat fragmentation: a synthesis. Global Ecology and Biogeography 16(3):265-280. |
|
|
Fretwell PT, Trathan PN (2009). Penguins from space: faecal stains reveal the location of emperor penguin colonies. Global Ecology and Biogeography 18(5):543-552. |
|
|
Gandiwa E, Heitkönig IM, Eilers PH, Prins HH (2016). Rainfall variability and its impact on large mammal populations in a complex of semi-arid African savanna protected areas. Tropical Ecology 57(2):163-180. |
|
|
Gangaas KE, Kaltenborn BP, Andreassen HP (2013). Geo-spatial aspects of acceptance of illegal hunting of large carnivores in Scandinavia. PloS one 8(7):e68849. |
|
|
Gehring TM, Swihart RK (2003). Body size, niche breadth, and ecologically scaled responses to habitat fragmentation: mammalian predators in an agricultural landscape. Biological conservation 109(2):283-295. |
|
|
Hanle J, Singhakumara B M, Ashton MS (2021). Complex small-holder agriculture in rainforest buffer zone, Sri Lanka, supports endemic birds. Frontiers in Ecology and Evolution 9:57. |
|
|
Houessou LG, Teka O, Imorou IT, Lykke AM, Sinsin B (2013). Land use and land-cover change at" W" Biosphere Reserve and its surroundings areas in Benin Republic (West Africa). Environment and Natural Resources Research 3(2):87. |
|
|
Howland BW, Stojanovic D, Gordon IJ, Radford J, Manning AD, Lindenmayer DB (2016). Birds of a feather flock together: using trait-groups to understand the effect of macropod grazing on birds in grassy habitats. Biological Conservation 194:89-99. |
|
|
Jacobi CM, Do Carmo FF, Vincent RC, Stehmann JR (2007). Plant communities on ironstone outcrops: a diverse and endangered Brazilian ecosystem. Biodiversity and Conservation 16(7):2185-2200. |
|
|
J?drzejewski W, Puerto MF, Goldberg JF, Hebblewhite M, Abarca M, Gamarra G, Schmidt K (2017). Density and population structure of the jaguar (Panthera onca) in a protected area of Los Llanos, Venezuela, from 1 year of camera trap monitoring. Mammal Research 62(1):9-19. |
|
|
Jepson PD, Deaville R, Barber JL, Aguilar À, Borrell A, Murphy S, Law RJ (2016). PCB pollution continues to impact populations of orcas and other dolphins in European waters. Scientific Reports 6(1):1-17. |
|
|
Khan AR, Khan AA, Iqbal J, Khan AM, Hassan Z, Aatif HM, Shahzad U (2021). Impact of Feeding Habits on Population Ecology and Breeding Biology of Grey Francolin (Francolinus pondicerianus) in District Bhakkar, Punjab, Pakistan. Pakistan Journal of Zoology pp. 1-7. |
|
|
Peng CYJ, Lee KL, Ingersoll GM (2002). An introduction to logistic regression analysis and reporting. The Journal of Educational Research 96(1):3-14. |
|
|
Martin A, Caro T (2013). Illegal hunting in the Katavi-Rukwa ecosystem. African Journal of Ecology 51(1):172-175. |
|
|
Murn C, Mundy P, Virani MZ, Borello WD, Holloway GJ, Thiollay JM (2016). Using Africa's protected area network to estimate the global population of a threatened and declining species: a case study of the Critically Endangered White?headed Vulture Trigonoceps occipitalis. Ecology and Evolution 6(4):1092-1103. |
|
|
Oumorou M, Sinadouwirou T, Kiki M, Kakaï RG, Mensah GA, Sinsin B (2010). Disturbance and population structure of Vitex doniana Sw. in northern Benin, West Africa. International Journal of Biological and Chemical Sciences 4(3). |
|
|
Raunkiaer C (1934). The Life Forms of Plants and Statistical Plant Geography. Clarendon Press, Oxford. |
|
|
Shannon CE, Weaver W (1949). Mathematical theory of communication. Chicago: University of Illinois. |
|
|
Weinstein AM (2017). An update overview on brain imaging studies of internet gaming disorder. Frontiers in Psychiatry 8:185. |
|
|
Weber HE, Moravec J, Theurillat JP, (2000). International Code of Phytosociological Nomenclature. 3rd edition. Journal of Vegetation Science 11(5):739-768. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0