ABSTRACT
A field study was conducted at Debre Zeit Agricultural Research center station under rain fed condition with the objectives to determine the effect of seed priming for improving chickpea variety productivity and to determine the effectiveness of seed priming treatment and variety on stand establishment. The experimental factors were laid out in RCBD with three replications. The study indicated that all the phenological and growth traits significantly differed as a result of priming treatment and variety. With respect to yield and yield related traits, only seed yield kgha-1, harvest index (%), seeds plant-1 and seeds pod-1 were significantly affected by the main effect. While, the rest of yield related traits responded differently due to variety alone. However, all variables studied in the field were not significantly affected by the interactions of the main effects. Improvement made due to main effect hydro and osmopriming was statistically similar for all phenological traits; seeds plant-1 and seeds pod-1 was considerably improved as a result of osmopriming than hydropriming. However, plant height, stand count at emergence and at harvest, seed yield kg ha-1 were substantially increased by 7, 10, 12 and 15%, respectively as a result of water priming over the control. Therefore, from present study, it can be concluded that hydropriming can step-up economical benefit of chickpea growing farmers.
Key words: Chickpea, hydropriming, osmopriming, productivity, seed priming.
Chickpea (Cicer arietinum L.) is 98 to 99% self-pollinated (Singh, 1987); diploid species having basic chromosome number of 16 and belongs to family Leguminasae (Poehlman and Sleper, 1995). It is one of the cool season food legume crops of Ethiopia which is mainly grown in the central, Northern and Eastern high land area of the country where the mean annual rainfall and altitude, respectively range from 700-2000 and 1400-2300 m. a. s.l. (Geletu, 1994).
Ethiopia shares 2% among the most chickpea producing countries following India (64%), Turkey (8%) and Pakistan (7%) (ICRISAT, 2004). Within 2002 and 2004, the global chickpea production was 8.0 million tons from an area of 10.1 million hectares giving an average productivity of 0.79 ton ha-1 (ICRISAT, 2006).
Two types of chickpea are cultivated in the world viz. the Desi and the Kabuli types. Both types of chickpea are grown in Ethiopia whilst unimproved local Desi variety being the most widely is grown in all places (Muehlbauer and Abebe, 1997).
In Ethiopia, most of the production is used for domestic consumption. Chickpea seed can be eaten as green vegetable (eshet), roasted (kollo), boiled (nifro) and dry vegetable. The flour of chickpea is also used to make ‘shimbra asa’, popular dish during fasting time. Flour of roasted chickpea seeds (shiro) are used to make shiro wot (sauce) and taken with injera (bread). Chickpea can also improve the soil fertility through biological nitrogen fixation and intercropped with cereals. Despite this fact, however, the yield of chickpea in Ethiopia is still low which can be attributed to biotic and abiotic factors (Geletu and Yadeta, 2002).
The major abiotic stresses contributing to the greatest source of variation of seed yield in chickpea are cold, heat and drought. Out of these, drought is the major and usual limiting factor as chickpea is essentially grown after the rainy season on residual soil water, which often exposes the crop to terminal drought (Geletu and Yadeta, 2002). This synonym has been described as in the semi-arid tropics, crops often fail to establish quickly and uniformly, leading to decreased yield because of low plant populations (Clark et al., 2001)
Despite chickpea plant can produce extra vegetative growth (in a favorable moisture regime) to cover available space, poor plant stands and stunted growth are often a major cause of low seed yields in semiarid environments (Saxena, 1987).
Resource-poor farmers often lack the means to optimize seed bed conditions before sowing and they are particularly at risk from adverse weather after sowing. Moreover, for farmers who grow annual crops from seed, good stand establishment is of paramount importance because patchy stands due to uneven germination result in low yields and often crop failure (Khan et al., 2008 Therefore, one way of improving productivity of chickpea in drought prone area is seed priming. Priming is a procedure that partially hydrates seed, followed by drying of seed, so that germination processes begin, but radical emergence does not occur. It includes soaking seed in water or osmotic solution, and intermixture with porous
matrix material (Ghana and William, 2003).
Improvement of seed quality by physiological treatments is a simple, easy and impressive approach to enhance seed performance and agricultural productivity (Basu, 1994). For most crops mean yield increases due to priming range from zero to more than 200%, with an overall average increase of 30% (Harris, 2004).
On-farm priming of seeds of a range of tropical and sub-tropical crops have been tested as a means to promote rapid germination and emergence and to increase seedling vigor and hence yield (Harris, 2004).
It has been reported that seed priming trials in western India increased chickpea yield and those of other rain fed crops (Harris et al., 1999). Therefore, the present study was initiated with the objectives to determine the effect of seed priming for improving chickpea varieties productivity and to determine the effectiveness of seed priming treatment and variety on stand establishment.
Description of the experimental site
The experiment was carried out at Debre Zeit Agricultural Research Center on station. The center is located at 47 km South East of Addis Ababa at 8° 44´N Latitude and 38° 58´ E Longitude at an elevation of 1980 m.a.s.l. The mean annual rain fall recorded at the station is 851 mm and the average annual minimum and maximum temperatures are 8.9 and 28.3°C respectively (Wikipedia, 2012).
Experimental material, treatments and design
The experiment was conducted on six chickpea varieties released at different year (Table 1). The seed of six chickpea varieties produced in the same production year were obtained from National Chickpea Improvement Program, Debre Zeit Agricultural Research Center. The two priming treatments viz. water and 0.5% KH2PO4 were applied for 8 h. In addition, in one sample no treatment was applied. The primed treatments were prepared in distilled water. After 8 h soaking, primed seed of each variety was dried slowly at ventilated room.
Experiment
Experimental design was two factors factorial in RCBD (chickpea varieties and priming media) with three replications. Seeds were sown in about 5 to 7 cm depth with a density of 33 seeds m-2. Each plot consisted of four rows with 4 m length and, spaced 30 cm apart between rows and 10 cm space between each plant within the rows. And data were taken from two central rows of net area of 2.4 m2.
Phenological data
Days to 50% emergence: It was recorded as the number of days taken from sowing up to 50% of seedling emergence from each plot.
Days to 50% flowering: The date on which 50% of the total plants flowered in the plot was recorded. The number of days taken to 50% flowering was computed from the date of sowing till 50% of the plants attained flowering.
Days to 90% physiological maturity: It was recorded as the number of days from the day of planting to the date when 90% of the plants attained physiological maturity in each plot.
Growth parameters
Plant height (cm): Plant height was measured in centimeters from ground level to the plant tip at physiological maturity using ten plants taken randomly from each plot.
Stand count at emergence and at harvest: Total numbers of plants in the two central rows were counted at emergence and at physiological maturity from the entire net area of each plot.
Yield and yield associated characters
Ten plants in the two central rows were taken randomly from each plot in all cases for yield and yield associated character data collection.
Number of primary branches per plant: Branches that grow from the main stem are primary branches. Number of primary branches per plant was obtained by dividing total number of primary branches from ten plants by ten.
Number of secondary branches per plant: Branches which grow from Primary branches are secondary branches. Numbers of secondary branches per plant were obtained by dividing total number of secondary branches from ten plants by ten.
Number of pods per plant: Number of pods per plant was computed by dividing total number of pods obtained from ten plants by ten.
Number of seeds per plant: Number of seeds per plant was calculated by dividing total number of seeds from ten plants by ten.
Number of seeds per pod: Number of seed(s) per pod was recorded by dividing total number of seeds from ten plants by total number of pods from ten plants.
100 seed weight (g): It was taken by weighing 100 seeds drawn randomly from the grain yield obtained from each experimental plot.
Above ground biomass (yield kg ha-1): The weight in grams was recorded by weighing the total above ground biomass harvested from the two central middle rows from each experimental plot after air dried and was converted to get biomass yield per hectare.
Seed yield (kg ha-1): Seed yield obtained in grams from each experimental plot’s central two rows (2.4 m2) and was converted to get seed yield per hectare.
Harvest index (%): It was calculated as a ratio of total seed yield to total above ground biomass yield harvested from the two middle rows.
Data analysis
The collected data were subjected to statistical analysis as per the design using Statistical Analysis System (SAS, 2001) computer software. Where significant differences were detected, the mean separations were carried out using the least significant differences (LSD) at 0.05 level of probability. Linear correlations between yield and yield associated traits were calculated using SAS computer software.
Phenological parameters
The results on phenological data as influenced by pre-sowing invigoration chickpea seed treatments as presented in Table 2.
Days to 50% emergence
The difference between primed and non-primed seed for days to 50% emergence was significant (Table 2). Osmopriming found to decrease days to 50% emergence by17% as compared untreated while, hydro priming reduced days to 50% flowering by 16%. However, seed treated with water and 0.5% KH2PO4 recorded almost the same number of days for days to 50% of emergence. This is because primed seed will require little further imbibitions before the germination process starts, thus less time is required for the seed to germinate (Murungu and Madanzi, 2010). In such a way that it might have reduced the number of days required for seedling emergence.
Emergence enhancement in osmoprimed seed may be attributed to metabolic repair processes, a buildup of germination metabolites or osmotic adjustments during priming treatment (Bray et al., 1989). This finding agreed with Arif et al. (2008) who reported that seed priming hastened and improved days to 50% emergence of soybean seed with deionized water and PEG-8000 (300 g/L) by priming for 6 h. In agreement with this finding, several other reports showed improved and early seedling emergence in common bean as a result of water priming (Harris, 1996; Harris et al., 1999).
Variety also responded differently to the priming media at p<0.05. Among all varieties, least days were taken to emerge for DZ-10-4 variety but it was not significantly different from Arerti and Habru. In addition, DZ-10-11, Akaki and Natoli varieties had similar effect for days to 50% emergence and these were (Desi type) delayed for days to 50% emergence as compared to the Kabuli type of chickpea varieties used for this study. The interaction between the two factors (variety × priming medium) for days to 50% emergence was not significant. It was in conformity with the finding of Finch-savage et al. (2004) who reported that the variable response in maize seed to priming may be explained by the different varieties used, during seed soaking which may be amplified by wet conditions in poorly draining soils and high temperature during sowing. However, in the present study poor drainage during sowing was not limiting factor.
Days to 50% flowering
The days to 50% flowering differed significantly due to invigoration seed treatments. Water treatments took significantly lesser days for 50% flowering by 2.5 days compared to unprimed seed which may be due to better early and faster emergence. While, the seeds treated with 0.5% KH2PO4 and untreated recorded almost equal number of days for 50% flowering (Table 2). This findings is as per with Musa et al. (1999) who found that seed priming resulted in earlier crop flowering on chickpea. Narayanareddy (2008) also found that seed water hydration decreased days to 50% flowering by two days over untreated control. The advantages of increased rate of emergence could be correlated with early flowering and early harvest observed in the present study. A similar observation in advancement of flowering was also reported by Basu (1999) in maize and rice. There were also significant differences among the varieties response for days to 50% flowering (Table 2). The least days taken for Habru 50% flowering whereas the highest days taken for Arerti 50% flowering. Next to Arerti, DZ-10-4 and DZ-10-11 respectively showed delay in 50% flowering. But the variety Akaki and Natoli had similar days for 50% flowering (Table 2). The interaction of variety x priming was not significantly different for days to 50% flowering.
Days to 90% physiological maturity
The results on days to 90% physiological maturity as influenced by pre-sowing invigoration seed treatments are presented in Table 2. With respect to days to 90% physiological maturity, priming treatment and variety significantly (P < 0.01) affected time to 90% physiological maturity but there were no significant interactions observed. Both hydro and osmo invigoration seed treatments took significantly lesser days to physiological maturity compared to untreated control. The seeds treated with 0.5% KH2PO4 recorded lesser number of days to physiological maturity and it was harvested three days earlier than unprimed one. However, hydro and osmopriming failed to show significant differences between themselves for days to 90% physiological maturity. Primed chickpea seed had lesser days for days to 90% physiological maturity than non-primed seed, agreeing with the findings of Musa et al. (2001) who found earlier maturity in chickpea by soaking seeds for 8 h in water in which the primed one harvested 3 to 7 days earlier than unprimed one. Harris et al. (1999) also found that seed priming resulted in earlier crop maturity on chickpea. In general, the enhanced phenology in the chickpea due to priming is associated with faster emergence and reduction of imbibition periods (Harris et al., 1999).
Regarding the responses of varieties to days to 90% physiological maturity, Natoli attained physiological maturity earlier than any other variety and followed by Habru while, Arerti had the longest days for days to 90% physiological maturity. However, DZ-10-11 and Akaki attained physiological maturity equally where as an intermediate result was recorded for DZ-10-4 variety for physiological maturity (Table 2). This finding is in conformity with the findings of Murungu and Madanzi (2010) who found difference in response among wheat variety as a result of seed treatments.
Growth and growth related parameters
Plant height (cm)
The data on plant height (cm) at harvest as influenced by pre-sowing invigoration seed treatments are presented in Table 2. Significant differences were observed (p<0.01) due to invigoration seed treatments and varieties for plant height at harvest but there was no interaction effect for seed priming x variety. Water treated seeds were significantly higher by 2.8 cm or 7% for plant height at harvest over untreated control. However, the seeds treated with 0.5% KH2PO4 and unprimed recorded statistically similar plant height at harvest.
The enhanced plant height as a result of seed priming might be due to cell enlargement and increase in normal cell division (Karivaratharaju and Ramakrishnan, 1985). The better crop growth due to seeds treatment may also be attributed to the fact that treatments activate the synthesis of proteins, RNA, free amino acids and soluble sugars in the first phase of germination which could be advantages for subsequent phases of growth (Jyotsna and Srivastava, 1998). The enhancement of plant height may also be due to the improvement and faster plant emergence in invigorated seeds which might have created cooperative competition among the plants for light and resulted in taller plants. The result is in agreement with the result of Harris et al. (1999), who observed taller plants with seed invigoration. Similar findings were also observed by Musa et al. (2001) in chickpea upon hydro seed priming.
Effects of variety on the plant height was highly significant (p<0.01) in which Habru and Akaki produced the tallest and the shortest plant height, respectively among the varieties used for the study (Table 2). The two Kabuli type, namely DZ-10-4 and Arerti had similar plant height at harvest but both of these recorded higher plant height at harvest than all the varieties except Habru. In general Kabuli type of chickpea varieties attained higher plant height at harvest than the Desi type of chickpea varieties tested currently.
Stand count at emergence and at harvest
The results of both stand count at emergence (p<0.05) and at harvest (p<0.01) were observed significantly different as affected by seed treatment, variety though their interactions were not significant at p<0.05. Water primed seeds recorded an increase of 10 and 12% over the control for stand count at emergence and stands count at harvest respectively. However, there was no significant difference between osmopriming and unprimed for both stand count at emergence and at harvest (Table 2).
This outcome is in agreement with other authors’ findings. For example, Musa et al. (2001) reported that numbers of plants at harvest were increased by 10% due to water seed priming in chickpea. Similar result was also reported by Manigopa et al. (2007) in which seed priming increased the number of plants at harvest by 12.8% in chickpea (Cicer arietinum L.).
With respect to effect of variety for stand count at emergence and harvest, the Variety DZ-10-4 differed significantly from all other varieties tested for the study except Akaki variety. Stand count at emergence and at harvest for the varieties Arerti, Habru, DZ-10-11, Akaki and Natoli were all at par (Table 2).
Yield and yield associated parameters
Seed yield and biomass yield
The difference between primed and non-primed seed plots for seed yield kg ha-1 was significant at p<0.05 and varieties were also responded differently with respect to seed yield and biomass yield kg ha-1 at p<0.01. The interaction between the two factors namely seed priming x variety for improvement of chickpea seed yield was not significant (Table 3). Seed yield obtained from the primed seed plots were significantly higher than non-primed seed plots. Seed yield was enhanced with water and 0.5% KH2PO4 priming treatments by 15 and 3% respectively as compared to unprimed. In addition, seed priming by water showed an increase of 11% seed yield improvement over that of 0.5% KH2PO4 seed priming. However, seed yield obtained with osmopriming as well as that of the control were not different statistically (p>0.05) (Table 3).
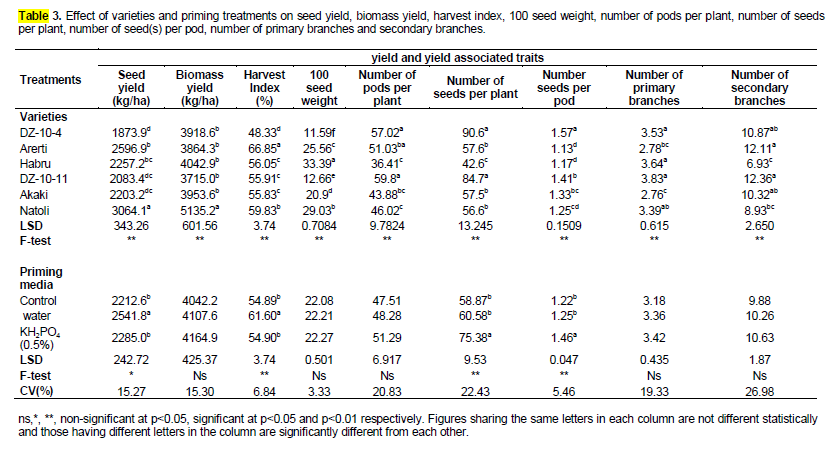
The improvement in yield of primed seed plots may be due to early and improved emergence and early floral initiation in the priming treatments that ultimately resulted in the higher yield. It had been also hypothesized that faster emergence from seed priming would result in better resource utilization and allow more time for optimal growth and grain filling resulting in higher yield (Murungu and Madanzi, 2010). In addition to these, the effect of seed priming on the chickpea seed yield and its components is evidenced first in better and faster seedling establishment, earlier flowering and earlier maturity that allow some escape from terminal drought and heat stress (Musa et al., 2001). The significant increase in the number of plant at harvest and number of seeds per plant and as a result of seed treatments may be contributor of an increment of seed yield. Similar findings were observed by Sharma et al. (1993) who indicated higher yield due to early floral initiation, more flowers, and seeds plant-1 in salicylic acid primed soybean seed. The improvement in the stand establishment due to priming can increase drought tolerance, reduce pest damage and increase seed yield of chickpea (Harris et al., 1999; Musa et al., 1999; Harris et al., 2000). The increase in yield of primed seed plots may be due to the fact that primed seed emerge faster and more uniformly and seedlings grow more vigorously, leading to a wide range of phenological and yield related benefits (Harris et al., 2000). These results are also in conformity with Arif et al. (2007) who reported that seed priming increase seed yield of chickpea. Arif et al. (2008) findings also confirmed that seed priming with water and osmoticum for 6 h improved seed yield of soybean.
Main effect due to variety on Seed yield kg ha-1 is presented in Table 3. There were significant differences among the varieties with respect to seed yield in which highest seed yield was obtained for variety Natoli followed by Arerti. However, seed yield of varieties Arerti and Habru was at par. On the contrary, variety DZ-10-4 which produced the lowest seed yield was at par with DZ-10-11 and Akaki.
Main effect due to seed priming treatment was not significantly different for biomass yield kg ha-1. In other ways, enhancement in any of the above traits as a result of seed treatments did not translate into biomass yield kg ha-1. This result is in contrary with the finding of the Ghassemi-Golezani et al. (2010) who obtained satisfactory result for plant biomass as a result of hydropriming of pinto bean (Phaseolus vulgaris L.). Arif et al. (2007) who reported that seed priming increase biological yield of chickpea. Interaction effects between two factors were not effective with respect to plant biomass yield. However, variety showed significant difference for biomass yield kg ha-1at p<0.01 (Table 4). Out of all the varieties, Natoli variety accumulated the highest above ground biomass yield. Even though the lowest biomass yield was obtained from DZ-10-11, it had similar biomass yield with all the varieties except Natoli (P<0.05) (Table 3).
Harvest index and hundred seed weight
Harvest index (%) was affected by seed priming (p<0.01) but hundred seed weight (g) was not affected by priming treatments (p>0.05). Interaction of priming treatment × variety for these traits were not significant (p>0.05). Harvest index for water priming was significantly higher by 12% than that of osmopriming and control, but osmopriming was not significantly different from the control (Table 3).This result disagreed with the findings of Ghassemi-Golezani et al. (2010) who could not obtain satisfactory results for harvest index up on hydropriming of pinto bean (Phaseolus vulgaris L.) but he had found similar results with the present findings with respect to hundred seed weight. Earlier findings which are against the current findings were also reported by Musa et al. (1999) and Arif et al. (2007) who reported that thousand seed weight was increased in chickpea. The main effect due to variety was significantly affected both traits. Mean harvest index for Arerti was significantly higher than any other varieties followed by Natoli. In contrast, DZ-10-4 produced the lowest harvest index among all the varieties .Habru, DZ-10- 11 and Akaki almost produced similar mean harvest index among themselves (Table 3). Mean number of 100 seed weight for the varieties could be arranged in order of increasing from the lowest to the highest harvest index as followed as: DZ-10-4, DZ-10-11, Akaki, Arerti, Natoli and Habru. From this we concluded that 100 seed weight was found to be affected by seed size in which smaller seeded varieties produced lower 100 seed weight and larger seeded varieties produced higher 100 seed weight (Table 3).
Pods per plant, seeds per plant and seeds per pod
These three traits were affected by both seed priming (p<0.01) and variety (p<0.01). Interaction of seed priming x variety for all the three traits were not significant (p>0.05). Osmo seed priming produced higher seeds per plant as well as seeds per pod by 28 and 20%, respectively as compared to unprimed. But there was no significant difference between water priming and control for both seeds per plant and seeds per pod (Table 3).These results endorsed the findings of Pongkao and Yothasiri (1995) that correlated increase in yield with increase in number of seeds per pod by soaking in water. Manigopa et al. (2007) findings revealed that seed priming increased number of seeds per plant in chickpea. Furthermore, these findings are also similar with Ros et al. (2000) who reported that soaking the seeds in KH2PO4 and NaH2PO4 improved the yield attributes namely seeds per plant and/or seeds per pod. But these results were not as per with the findings of Ghassemi-Golezani et al. (2010) who could not obtained satisfactory results for seeds per plant as well as seeds per pod up on hydropriming of pinto bean (P. vulgaris L). Number of pods per plant was not affected by seed treatments (p>0.05). However, seed primed in 0.5% KH2PO4 solution produced maximum pods plant-1 followed by seed primed in distilled water. Minimum pods plant-1 were produce from untreated seed. Similar results were reported by Arif et al. (2007) who mentioned that chickpea seed priming with 0.05 or 0.075% ZnSO4 and water priming did not affect pods plant-1 of chickpea. These results are also similar with earlier studies where chickpea seeds treated overnight with 0.5% KH2PO4 and water could not be effective for improvement of pods per plant over the unprimed. Sarwar et al. (2006) and Ghassemi-Golezani et al. (2010) also did not obtain significant results for pods per plant due to hydropriming of pinto bean (P. vulgaris L.). It was also endorsed with the findings of Arjunan and Srinivasan (1989) who reported that seed treatment with 0.5% CaCl2 did not influence significantly matured pods per plant as compared to control in TMV-7 groundnut variety. However, he reported that number of pods per plant could be appreciably increased through pre-sowing groundnut seed treated with 2% KH2PO4. Narayanaswamy (2003) also recorded higher number of graded pods per plant when groundnut seed was treated with 1% KH2PO4. Likewise, number of seeds per plant and number of pods per plant also affected significantly by the variety (p<0.01). Mean number of pods per plant and seeds per plant of DZ-10-4 and DZ-10-11 were significantly higher than any other variety though these were at par for both traits. Habru, Akaki and Natoli for pods per plant and Arerti, Akaki, and Natoli for seeds per plant had showed similar effect. Among all varieties, Habru produced the least number of seeds per plant (Table 3). In contrast, DZ-10-4 produced the largest number of seeds per pod among the varieties followed by statistically similar DZ-10-11 and Akaki. Arerti, Habru and Natoli also produced the same number of seed(s) per pod. In addition, Natoli had the same number of seed(s) per pod with Akaki (Table 3).
Primary and secondary branches per plant
Analysis of variance revealed that number of primary and secondary branches per plant were only affected by variety (p<0.01). Significant interactions were not observed for seed priming × variety on these traits (p>0.05). Mean number of primary branches per plant for DZ-10-4, Habru, DZ-10-11 and Natoli varieties as well as mean number of secondary branches per plant for DZ-10-4, Arerti, DZ-10-11 and Akaki were not different significantly. Arerti and Akaki furnished lesser number of primary branches per plant. Arerti variety had also the same number of primary branches per plant with that of Natoli variety. In contrary, lesser and similar numbers of secondary branches per plant were recorded for Habru and Natoli varieties. In addition, number of secondary branches per plant for DZ-10-4, Akaki and Natoli varieties were not differed significantly (Table 3). Different responses among different varieties for the considered traits may be due to genetical differences in varieties which agreed with the findings of Armin et al. (2010) who found different responses among the watermelon
varieties due to priming treatments
Numbers of primary and secondary branches per plant were not significantly affected by seed priming. This finding is in agreeable with the findings of Paula et al. (1996) who recorded that mustard seed treated with 1% of KH2PO4 did not show significant difference for number of branches per plant. However, Manigopa et al. (2007) reported that seed priming brought considerable increase in lateral branches (primary and /or secondary) per plant in chickpea.
Correlations between yield and yield associated parameters
Correlation analysis between growth parameters, yield and yield related traits, were given in Table 4. Correlation analysis between yield and yield components revealed that stand count at emergence (r=0.49, p=0.01), stand count at harvest (r=0.52, p=0.01), biomass yield (r=0.83, p=0.01), harvest index (r=0.57, p=0.01) and hundred seeds weight (r=0.48, p=0.01) were positively and significantly associated with seed yield (Table 4). Moreover, negative and significant association of number of seeds per pod (r = -0.35) with seed yield was obtained (Table 4).
From these correlation results, it could be concluded that stand count at emergence and stand count at harvest were found to be important yield components that may improve the yield potential of chickpea due to good stand establishment as a result of hydro priming.
Farmers and researchers have recognized that poor crop establishment is one of the major bottlenecks for crop production. This is particularly a problem for post rainy crops like chickpea mainly grown in such a sub-optimal environment. Therefore, seed priming (pre-sowing soaking) has been offered as a solution to this problem that will maximize the probability of obtaining a good stand of healthy and vigorous plants. From the present investigation, hydro priming had substantially increased seed yield by 15% over the control. Therefore, hydropriming is viable and sound technology that could increase economic benefit of chickpea especially for resource poor farmers in the marginal areas and small holder chickpea producers in Ethiopia.
The author have not declared any conflict of interests.
The author would like to express our deepest thanks to Debre Zeit Agricultural Research Center for financially and materials supports during the research work. His profound thanks is extended to his younger sister Mrs. Gete Sori for unreserved financial and encouragement throughout the study.
REFERENCES
|
Arif M, Waqas, Nawab MK, Shahi M (2007). Effect of seed priming in Zn solutions on chickpea and wheat. Afr. Crop Sci. Conf. Proc. 8:237-240.
|
|
|
|
Arif M, Jan MT, Marwat KB and Khan MA (2008). Seed priming improves emergence and yield of soybean. Pak. J. Bot. 40(3):1169-1177.
|
|
|
|
Arjunan A, Srinivasan PS (1989). Pre-sowing seed hardening for drought tolerance in groundnut (Arachis hypogaea L.). Madras Agric. J. 76(9):523-526.
|
|
|
|
Armin M, Asgharipour M, Razavi-Omrani M (2010). Effect of seed priming on the germination and seedling growth of watermelon (Citrullus lanatus). Adv. Environ. Biol. 4(3):501-505.
|
|
|
|
Basu RN (1994). An appraisal of research on wet and dry physiological seed treatments and their application with special reference to tropical and subtropical countries. Seed Sci. Technol. 22:107-126.
|
|
|
|
Basu S (1999). Effect of season and pre-sowing treatments on crop growth, flowering and seed quality of parents of hybrids. Ph.D. Thesis, Indian Agric. Res., Institute, New Delhi.
|
|
|
|
Bray CM, Davison PA, Ashraf M, Taylor RM (1989). Biochemical changes during osmopriming of leek seeds. Ann. Bot. 63:185-193.
|
|
|
|
Clark LJ, Whalley WR, Ellis JJ, Dent K, Rowse HR, Finch-Savage WE, Gatsai T, Jasi L, Kaseke NE, Murugu FS, Riches CR, Chiduza C (2001). On-farm seed farming in maize: A physiological Evaluation. Seven Eastern and southern Africa Regional Maize conference 11th-15th Feb.2001.pp. 268-273.
|
|
|
|
Finch-Savage WE, Dent KC, Clark LJ (2004). Soak conditions and temperature following sowing influence the response of maize (Zea mays L.) seeds to on-farm priming (pre-sowing seed soak). Field Crops Res. 90:361-374.
Crossref
|
|
|
|
Geletu B (1994). Forty years of Research Experience. Debere Zeit Agricultural Research Center (1955-1994). Debere Zeit, Ethiopia.
|
|
|
|
Geletu B, Yadeta A (2002). Evaluation of Ethiopian chickpea landraces for tolerance to drought. Genet. Resour. Crop Eval. 49:557-564.
Crossref
|
|
|
|
Genève RL (2008). Vigor testing in small- seeded horticulture crops. Acta Hortic. 782:77-82.
Crossref
|
|
|
|
Ghana SG, William FS (2003). Seed priming winter wheat for germination, emergence and yield. Crop Sci. 43:2135-2141.
Crossref
|
|
|
|
Ghassemi-Golezani K, Chadordooz-Jeddi A, Nasrullahzadeh S, Moghaddam M (2010). Influence of hydro-priming duration on field performance of pinto bean (Phaseolus vulgaris L.) variety. Afr. J. Agric. Res. 5(9):893-897.
|
|
|
|
Harris D (1996). The effects of manure, genotype, seed priming, depth and date of sowing on the emergence and early growth of Sorghum bicolor (L.) Moench in semi-arid Botswana. Soil Tillage Res. 40:73-88.
|
|
|
|
Harris D, Joshi A, Khan PA, Gothkar P, Sodhi, PS (1999). On farm seed priming in semiarid agriculture: development and evaluation in maize, rice and chickpea in India using Participatory methods. Exp. Agric. 35:15-29.
Crossref
|
|
|
|
Harris D, Tripathi RS, Joshi A (2000). On farm seed priming to improve crop establishment and yield in direct-seeded rice, in IRRI: International Workshop on Dry-seeded Rice Technology; held in Bangkok, 25-28 January 2000. The International Rice Research Institute, Manila, The Philippines, P. 164.
|
|
|
|
Harris D, Raghuwanshi RB, Gangwar JS, Singh SC, Joshi KD, Rashid A, Hollington PA (2001). Participatory evaluation by farmers of on-farm seed priming in wheat in India, Nepal, and Pakistan. Exp. Agric. 37:403-415.
Crossref
|
|
|
|
Harris D (2004). On farming seed priming reduces risk and increase yield in tropical crops. New directions for adverse planet: Proceedings of the 4th International Crop Science Congress. Brisbane, Australia, 26 Sep-1 Oct, 2004.
|
|
|
|
ICRISAT (2004). Area, production and productivity of Chickpea (Cicer arietinum L.). Patancheru, Hyderabad India.
|
|
|
|
ICRISAT (2006). Chickpea.
View
|
|
|
|
Jyotsna V, Srivastava AK (1998). Physiological basis of salt stress resistance in pigeon pea (Cajanus Cajan L.)-II pre-sowing seed soaking treatment in regulating early seedling metabolism during seed germination. Plant Physiol. Biochem. 25:89-94.
|
|
|
|
Karivaratharaju TV, Ramakrishnan V (1985). Seed hardening studies in two varieties of ragi (Eleusine coracana Gaertn). Indian J. Plant Physiol. 28:243-248.
|
|
|
|
Khan A, Khalil AK, Khan AZ, Marwat KB, Afzal A (2008). The role of seed priming in semi-arid area for Mung bean phenology and yield. Pak. J. Bot. 40(6):2471-2480.
|
|
|
|
Menale K, Shiferaw B, Asfaw S, Abate T, Muricho G, Ferede S, Eshete M, Assefa K (2009). Current situation and future outlooks of the chickpea sub-sector in Ethiopia. ICRISAT and EIAR.
View
|
|
|
|
Manigopa C, Ghosh JD, Virk DS, Prasad SC (2007). Effect of seed priming on germination, growth and yield of chickpea variety. J. Arid Legumes 4(1):56-58.
|
|
|
|
MoARD (2008). Ministry of Agriculture and Rural Development. Crop Variety Development Department: Crop Variety Register, 11. Addis Ababa, Ethiopia.
|
|
|
|
Muehlbauer FT, Abebe T (1997). Chickpea (Cicer arietinum L). New Crop Fact sheet. Ethiopia.
|
|
|
|
Murungu FS, Madanzi T (2010). Seed priming, genotype and sowing date effects on emergence, growth and yield of wheat in a tropical low altitude area of Zimbabwe. Afr. J. Agric. Res. 5(17):2341-2349.
|
|
|
|
Musa AG, Johannes C, Kumar J, Harris D (1999). Response of chickpea to seed priming in the highland of Barind Tract of Bangladesh. Int. Chickpea and Pigeon pea Newsl. 6:20-22.
|
|
|
|
Musa AG, Harris D, Johansen J, Kumar J (2001). Shorter duration chickpea to replace fallow after aman rice: the role of on farm seed priming in the high Barind Tract of Bangladesh. Exp. Agric. 37(4):430-435.
Crossref
|
|
|
|
Narayanareddy AB (2008). Effect of invigoration on seed quality, field performance and storability in sunflower hybrid kbsh-1. An M.Sc. Thesis presented to University of Agricultural Sciences, Dharwad-580 005.
|
|
|
|
Narayanaswamy S (2003). Effect of pre-sowing seed treatments for invigoration and better crop establishment in groundnut (Arachis hypogaea L.). National Workshop on Groundnut Seed Technol. pp. 143-144.
|
|
|
|
Paula M, Perezâ€Otaola M, Darder M, Torres M, Frutos G, Martimezâ€Honduvilla CJ (1996). Function of the ascorbateâ€glutathione cycle in aged sunflower seeds. Physiol. Planta. 96(4):543-550.
Crossref
|
|
|
|
Pongkao SA, Yothasiri A (1995). Effect of Juvenile leaf removal on symbiotic N.fixation and yield of mungbean (Vigna radiata L.). Kasetsart J. Nat. Sci. 29(2):246-251.
|
|
|
|
Poehlman JM, Sleper DA (1995). Breeding field crops.3rded.Iowa state of university press, Iowa 50014.USA.
|
|
|
|
Ros C, Bell RW, White PF (2000). Phosphorus seed coating and soaking for improving seedling growth of Oryza sativa (rice) cv. IR-66. Seed Sci. Technol. 28:391-401.
|
|
|
|
Sarwar R, Yousaf S, Jamil FF (2006). Induction of salt tolerance in chickpea by using simple and safe chemicals. Pak. J. Bot. 38(2):325-329.
|
|
|
|
Saxena MC (1987). Agronomy of chickpea. In: Sexana MC, Singh KB (Eds.). The chickpea: C.A.B International. Wallingford, UK.
|
|
|
|
SAS (2001).SAS Institute Inc. Statistical Analysis Systems, version 9.1.Cary, North Carolina, USA.
|
|
|
|
Sharma R, Kwon EH, Ganeshan KP (1993). Response of soybean (Glycine max (L). Merril to seed priming with salicylic acid. Indian J. Ecol. 20:27-29.
|
|
|
|
Singh KB (1987). Chickpea breeding. In: Saxena MC, Singh KB (Eds.). The chickpea. C.A.B. International, Wallingford, UK. pp. 127-162.
|
|
|
|
Wikipedia (2012). the free encyclopedia.
View
|