ABSTRACT
Prevalence of obesity is on the increase globally with high fat diet (HFD) known to be the main contributing factor. This study was carried out to determine the actions of fermented Parkia biglobosa (Iru) and Sphenostyles stenocarpa (Otili) on the kidney of obese induced albino rats. The rats were grouped into a control group fed with normal rats chow and three different high fat diet groups (HFD1, HFD2, HFD3) mixed with different proportions of P. biglobosa and S. stenocarpa. After feeding ad-libitum for six weeks blood samples were collected to determine albumin, total cholesterol (TC), triglyceride (TG), high density lipoprotein (HDL) serum low density lipoprotein, enzymes (ALT, ALP, and AST) and Kidney histopathology. Results showed that there was a significant reduced body weight (p<0.05) in the treated rats compared with the control animals. Furthermore, the plasma lipid profiles were also improved, with a decrease in total cholesterol (TC), triglycerides (TG), low density lipoprotein (LDL) while boosting the high density lipoprotein (HDL). Similarly, histological examination revealed normal kidney with no negative changes such as dilation in blood vessels, cell infiltration, tubular defects, etc associated with taking high fat diet. In conclusion, supplementing a combination of fermented P. biglobosa (iru) and S. stenocarpa (otili) into diets show promise as a natural and safe anti-obesity agent that can ameliorate renal biochemical and histopathological changes associated with obesity.
Key words: Parkia biglobosa, Sphenostyles stenocarpa, high fat diet, kidney, obesity.
Obesity is one of the leading but avoidable causes of death worldwide (WHO, 2000; Paras et al., 2011).
Currently, about 1 billion adults are overweight and at least 300 million of them are obese. Obesity is responsible for 2-6% of total health care costs in many developed nations (Puska and Stahl, 2010; Greenway and Smith, 2000).
Obesity is itself a diseased state and is also a risk factor for many other diseases. It decreases life expectancy, increases the risk of heart diseases, stroke and gout (Norris et al., 2005; Slanc et al., 2009; Karallas et al., 2009). It is associated with obstructive sleep apnea and reduces the quality of life; it has been linked with increased risk of some surgical and post-surgical complications (Popkin, 2001; De Ferranti and Monzaffarian, 2008). It is equally estimated that, by the year 2030, about 58% of the world population will be obese (Peltonen et al., 2003; Diament et al., 2003; Finucane et al., 2014). Obese is defined by Body mass index (BMI) greater than 30 and further evaluated in terms of fat distribution via the waist–hip ratio and others (Sweeting, 2007; Flegal et al., 2001; Paras et al., 2011).
Preliminary data evidence revealed that eating beans-based diet such as Sphenostylis stenocarpa; known as wild yam beans and Otili in the Yoruba tribe of Nigeria competed favorably with the common edible bean, Phaseolus vulgaris in bioactive compound constituents (FAO, 1988; Ejere et al., 2018). So far, we have been able to have an insight into the organic products that underlies the chemo-preventive activities of this underutilized wild bean (Awoyinka et al., 2016). Parkia biglobosa (African locust bean) although commonly consumed locally as condiment is overlooked as a gem in disease management (Balunas and Kingworm, 2005; Millogo-Kone et al., 2006; Oguntola, 2019). In past decades there have been records on the use of P. biglobosa in traditional medicine-traditional healers in Senegal and in the South Western region of Nigeria use it for the treatment of diabetes mellitus (Dièye et al., 2008). The yellow pulp, containing the seeds is naturally sweet “and can be processed into food as well as seasoning, known as dawadawa among the Hausas in Nigeria and Ashanti tribes of Ghana. The pulp is also used to make beverages (Pieroni, 2005; Olaniyan, 2013).
Recently, many researches have focused on improving health through diet and natural resources, thus, this project would be a contribution to such innovations. The present study aims to rationally investigate the impact of regular intake of P. biglobosa and S. stenocarpa on the kidney of albino rats fed with a very high fat diet. This is intending to establish scientifically, the benefits (if any) of its intake given the importance and widespread popularity of both beans (Awoyinka et al., 2018).
Chemicals
All the chemicals/reagents used in the current research were of analytical grade and were purchased from Sigma Chemical Company (St. Louis, Missouri, USA. Diagnostic kits for enzyme analysis were purchased from Randox Laboratories (USA).
Collection and preparation of materials
Dry wild bean, S. stenocarpa were sourced from the open bushes within Ado Ekiti metropolis while P. biglobosa were bought from a local market and authenticated with a voucher number- (UHAE-1010065). The P. biglobosa were collected with the pods. The seeds were identified and authenticated by the Chief Botanist of the Department of Plant Science, Ekiti State University and deposited in the University Herbarium with Voucher Number (UHAE 2020063).
The S. stenocarpa beans were further sun dried for some hours and later blended into powdered form using a blender. This was stored in a tight container prior to use. For preparation of P. biglobosa, the seeds were selected and rinsed with clean water. Then the seeds were initially boiled for 2 h, followed by de-hulling and washing with cold water. Thereafter, the seeds were boiled again for 45 min and drained using a plastic sieve. For fermentation of the P. biglobosa seeds, starter culture that is, Bacillus subtilis was introduced after draining, the seeds were then spread into a fermenting can and wrapped with cloth for 48 h. Subsequently, the fermented P. biglobosa seeds were dried in an oven at a temperature of 55 to 60°C for 5days and powdered using a blender before storing in a tight container.
Experimental animals
Fifty albino rats with no bias in their sex were obtained from the Animal House, Faculty of Basic Medical Sciences, Ekiti State University Ado Ekiti. They were housed in plastic cages with steel wire lids, at room temperature with adequate access to rat chow and water throughout the experimental period. We got Ethical approval from the Experimental Animal Research Ethics Committee of Ekiti State University, Ado-Ekiti, (ORD/ETHICS/AD/043).The rats were separately grouped into experimental, and control group. They were fed for five weeks with bean and food formation of high fat diet adapted from Monk et al. (2019) while the control group was fed with regular rat chow diet formulated by BioOrganic Feeds (Table 2). Daily food consumption, body weight, behavioral and physiological changes were observed for four weeks as shown in Table 1.
Sacrifice of the animals and collection of tissue
All the animals were anesthetized with chloroform, sacrificed via an aortic cut and immediately dissected. Venous blood was immediately collected from the orbital vein placed in respective tubes before centrifuge to obtain serum. The kidney tissues were collected for biochemical estimation and the remaining portion were fixed using 10% formalin.
Lipid analysis
Preparation of the cholesterol fraction
Blood samples were collected in a test tube with no anticoagulant and allowed to clot at room temperature for half an hour before centrifugation at 2500 × g for 20 min. The serum layer was removed and stored on ice. However, extra care was taken to avoid disturbing the white buffy layer and stored at -80°C prior to performing the assay. Cholesterol level was assessed following Kit manufacturer instruction on the aliquot samples.
Preparation of HDL fractions
Blood was collected in tube containing citrate to avoid hemolyzes and centrifuge at 2000 × g and 4°C for 10 min. The white buffy layer-plasma was gently removed and stored on ice. Dilutions in 1X assay were performed on aliquot samples after storage at -80°C. Later, 200 μl of sample was added to 200 μl of the precipitation Reagent (Randox, USA) and mixed well by vortexing. The mixture was allowed to incubate for 5 - 10 min at room temperature before reading the absorbance at 570 nm.
Preparation of LDL/VLDL fraction
Pellet obtained after removal of the HDL fraction was re-suspended and dissolved in 400 μl of PBS and mixed well to obtain the LDL/VLDL fraction. Assay was carried out immediately by following the instructions of the Kit Manufacturers.
AST, ALT, ALP analysis
Blood samples were maintained at 4°C for 2h then centrifuged at 3000 × g for 20 min at 4°C. The supernatant was stored at -80°C total protein (TP), Alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) were analyzed assiduously following the method of Reitman and Frankel (1957) as described in the commercial Random Kit. Absorbance was determined using an automatic Biochemical Analyzer - Camspec M106 Spectrophotometer
Histopathological investigation
Following an incision in the anterior abdominal wall, the kidney from each group of rats were removed and fixed in 10% buffered formalin by total immersion for 48hrs as described by Alese et al. (2018). Thereafter, using an automatic tissue processor (Leica TP 1020), the organs were dehydrated using ethanol ranging from 50, 70 and 90%, Absolute 1 and 2 for 1 h each and cleared in xylene. Thereafter, the tissues were sectioned with a rotary microtome (Leica RM 2125 RTS) at a thickness of 4 μm, floated on a water bath and picked using glass slides. Various sections were then stained with H & E for the demonstration of general tissue architecture; photomicrographs were examined and taken at various magnifications under an OMAX 40X-2000X microscope.
Statistical analysis
The results are expressed as mean values ±S.E.M of three replicates. Results were considered significant with p<0.05 using statistical Graph Pad- prism software (2003).
Obesity is associated with increased adipose tissue that results from both increased fat cell number and size. It is a lipoprotein disorder with derangement in the levels of triglycerides (Karalis, et al., 2009). Once there is an onset of obesity, patients need to use a synthetic drug to control their body weight, body mass index (BMI), and lipid profile levels (Puska and Stahl, 2010). Recently, there is an increasing popularity for the use of natural products in the management of obesity and other ailments. Weight gain is commonly associated with obesity; this occurs when energy uptake surpasses expenditure in an individual such that the store of energy in body fat is enlarged (Greenway and Smith, 2000). Obesity induction using a high fat diet in an animal model was used in this study as the approach has high relevance of mimicking the usual route of obesity occurrence in humans (Flint, 2011; Inukai, 2013).
In this study, the initial weights of the rats were between 120-125 g but after a week of obesity induction, there was an increase to133-142 g. This increase in body weight may be attributed to a disproportionate increase in organs like kidney and liver as reported by Christiansen et al. (1981) and Thomson et al. (2001) respectively. The consumption of high fat diets led to obesity because it facilitates the development of a positive energy balance, leading to an increase in visceral fat deposition, and thus abdominal obesity (Mercer and Archer, 2005). However, it was observed that feeding the rats on high fat diet with the powder of fermented P. biglobosa and S. stenocarpa might have had a hypolipidemic effect on the rats by significantly reducing their weight from an average of 133 to 115 g.
In the biochemical analysis of the lipid profile (Table 3), the results showed a significant decrease in albumin, triglycerides (TG), total cholesterol (TC) and Low density lipoproteins-cholesterol; (LDL), as well as increase in High density lipoprotein-cholesterol (HDL) for the groups fed with fermented locust beans+otili+fat (Figure 1). Albumin is the protein with the highest concentration with the highest concentration in plasma and it transports many small molecules in the blood (for example, bilirubin, calcium, drugs etc (Duncan et al., 1994). The significant albumin may suggest that the fermented P. biglobosa and S. stenocarpa have ability to inhibit in vivo protein biosynthesis.
Cholesterol is transported via blood by lipoproteins.
HDL (good cholesterol) transports it from tissues to liver and LDL (bad cholesterol) does it in the opposite direction. Therefore decrease in serum LDL cholesterol is an indication of low rate of transportation of cholesterol from liver to tissues and subsequent transformation of triglycerides and cholesterol into bile acid by liver enzymes. Meanwhile, increase in albumin, TG, TC, LDL and decrease in HDL was observed in high fat diet only (HFD). This is because dietary cholesterol raises TC, TG and LDL levels. The intake of cholesterol rich food has been positively related to hypercholestolemia and risk of cardiovascular diseases (Zulet et al., 1999). Thus it can be suggested that the extract of the two plants could have an effect on dietary cholesterol which could result in the level of cholesterol in the blood. This is similar to the work of Jorge et al. (1998) who carried out work on the effect of eggplant juice on plasma lipid levels.
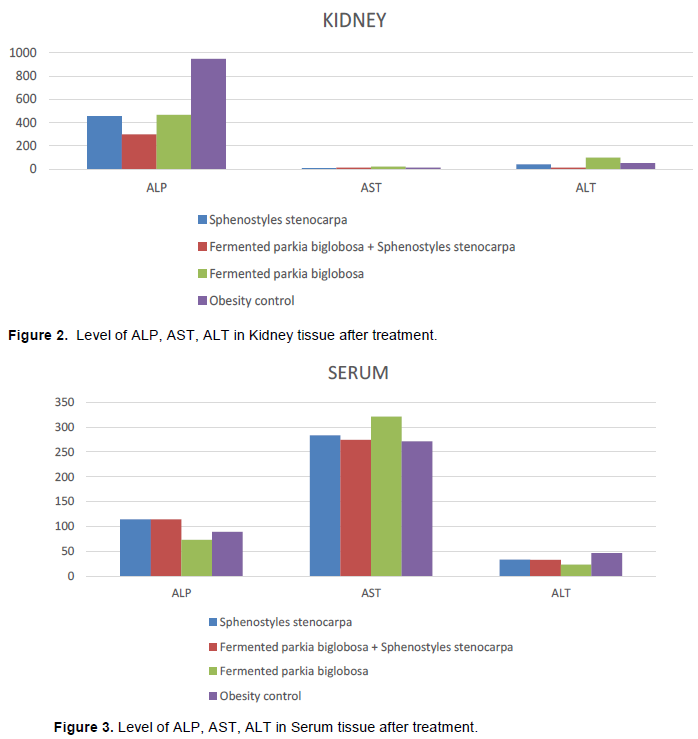
The kidney is an important organ that is responsible for the metabolism, detoxification, storage, and excretion of xenobiotics and their metabolites. It can be damaged by external substances. Intracellular enzymes including AST, ALT and ALP appear in the plasma and are indicative of cellular damage (Evans et al., 2014; Debelo et al., 2015). Hence, their serum levels could be used to assess any situation of organ damage, particularly with the presence of established normal ranges for the detection of organ damage (Oh and Hustead, 2011). As seen in Figure 2, results from this work show that feeding the HFD rats with fermented P. biglobosa and S. stenocarpa significantly reduces the activities of these enzymes in the kidney when compared with the group fed the HFD alone. However, in Figure 3 biochemical analysis of the serum revealed that the activities of the enzymes were increased in the treatment animals when compared with the animals fed with HFD only. Injac et al. (2008) explained that the increase in the serum enzyme levels helps in contributing to increased leakage from damaged and necrotic cells preventing the kidney and other organs from effects of atherosclerosis.
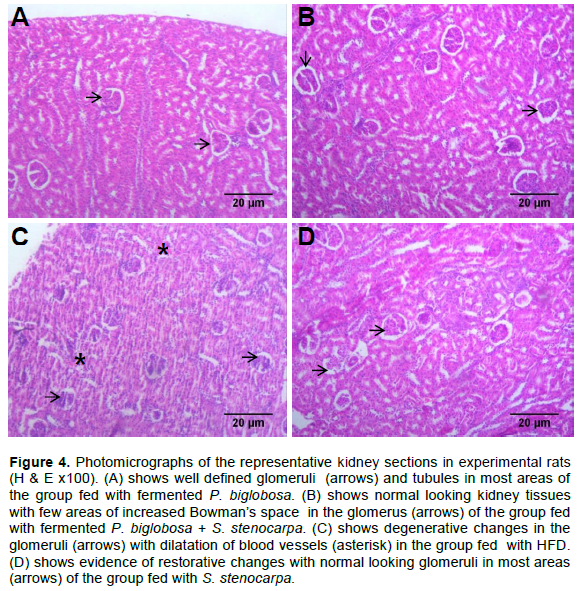
As seen in Figure 4, the photomicrographs show the architecture of the representative kidney sections from the experimental groups. In Figure 4A, the HFD group fed with fermented P. biglobosa shows an essentially normal kidney tissue but with splitting of the glomerulus in few areas. Similarly, as seen in Figure 4B, the HFD group fed with fermented P. biglobosa + S. stenocarpa shows normal kidney tissue with evidence of restorative changes as there is increased size of the Bowman’s space in few areas. The representative kidney of the obesity control group fed with HFD alone revealed dilatation in a few blood vessels with enlargement of Bowman’s space (Figure 4C). Also, evidence of mononuclear cell infiltration is present in the renal cortices. Glomerulosclerosis and necrosis which are features of degenerative changes in the nephrons are also seen. Figure 4D shows essentially normal kidney tissue with restorative changes in the glomerulus in few areas. The evidence of degenerative changes observed in the HFD group in this study is similar to that of Altunkaynak et al. (2008) in a study of the structure of the kidneys of adult Sprague-Dawley rats fed a HFD for 3 months. They concluded that a fatty diet is responsible for the observed obesity and renal abnormalities as a result of histopathological changes such as dilation, tubular defects, inflammation and connective tissue hypertrophy of the kidney. However, the treatments in our study were able to produce ameliorate effects when compared with the HFD group alone. Adeyeye (2013) confirmed the presence of high levels of lecithin and phytosterols in both fermented and unfermented samples of P. biglobosa. Lecithin and phytosterols are bioactive substances that are proven to be effective for the prevetion of obesity as well as lowering cholesterol (Spilburg et al., 2003; Furlan et al., 2013). Ndidi et al., (2014) suggested the potentials of S. stenocarpa seeds in reducing cholesterol levels and preventing disorders.
This study is novel for demonstrating the effectiveness of the combination of fermented P. biglobosa and S. stenocarpa in reducing the risk of obesity. This could be a cost effective approach in the treatment of obesity especially in low income countries such as Nigeria.
The authors have not declared any conflict of interests.
REFERENCES
|
Adeyeye EI (2013). The effect fermentation on the dietary quality of lipids from African losudt bean (Parkia biglobosa) seeds. Elixir Food Science 58:14912-14922.
|
|
|
|
Alese MO, Agbaje MA, Alese OO (2018). Cadmium induced damage in Wistar rats, ameliorative potentials of progesterone. Journal of Trace Element in Medicine and Biology 50:276-282.
Crossref
|
|
|
|
|
Altunkaynak ME, Ozbek E, Altunkaynak BZ, Can I, Unal D, Unal B (2008). The effects of high-fat diet on the renal structure and morphometric parametric of kidneys in rats. Journal of Anatomy 212(6):845-852.
Crossref
|
|
|
|
|
Awoyinka OA, Ileola AO, Imeoria CN, Tijani TD, Oladele FC, Asaolu MF (2016). Comparison of phytochemicals and anti-nutritional factors in some selected wild and edible bean in Nigeria. Food and Nutrition Sciences 7(2):102-111.
|
|
|
|
|
Awoyinka OA, Omodara TR, Oladele FC, Ajayi DD (2018). In-vitro protein digestibility of selected underutilized local wild beans and bio-availability in rats model. Academia Journal of Biotechnology 6(10):258-262.
|
|
|
|
|
Balunas MJ, Kinghorn AD (2005). Drug discovery from medicinal plants. Life Sciences 78(5):431-441.
Crossref
|
|
|
|
|
Christiansen JS, Gammelgaard J, Frandsen M, Parving HH (1981). Increased Kidney Size, Glomerular Filtration Rate and Renal Plasma Flow in Short-Term Insulin-Dependent Diabetics. Diabetologia 20(4):451-456.
Crossref
|
|
|
|
|
De Ferranti S, Mozaffararian D (2018). the perfect storm: Obesity, adipocyte dysfunction, and metabolic consequences. Clinical Chemistry 54(3):945-955.
Crossref
|
|
|
|
|
Debelo N, Afework M, Debella A, Makonnen E, Ergete W Geleta B (2015). Histopathological and biochemical assessment of chronic oral administration of aqueous leaf extract of Tymus serrulatus in mice. Journal of Clinical and Experimental Pathology 5(258):2161-0681.
Crossref
|
|
|
|
|
Dièye AM, Sarr A, Diop SN, Ndiaye M, Sy GY, Diarra M, Rajraji/Gaffary I, Ndiaye/Sy A, Faye B (2008). Medicinal plants and the treatment of diabetes in Senegal: Survey with patients. Fundamental and Clinical Pharmacology 22(2):211-216.
Crossref
|
|
|
|
|
Duncan JR, Prasse KW, Mahaffey EA (1994). Veterinary Laboratory, Medicine (Clinical Pathology). Iowa State University Press: Ames pp. 94-96.
|
|
|
|
|
Ejere VC, Ogbuke EF, Nnamonu EI, Ikele BC, Nweze BC (2018). Evaluation of Anti-Obesity Potentials of Sphenostylis stenocarpa Ethanolic Seed Extract. Annual Research and Review in Biology pp. 1-9.
Crossref
|
|
|
|
|
Evans CC, LePard KJ, Kwak JW, Stancukas MC, Laskowski S, Dougherty J, Moulton L, Glawe A, Wang Y, Leone V (2014). Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLoS ONE 9(9):21-93.
Crossref
|
|
|
|
|
FAO (Food and Agriculture Organization) (1988). Traditional food plants. FAO Food and Nutrition Paper 42(11):593.
|
|
|
|
|
Finucane MM, Sharpton TJ, Laurent TJ, Pollard KS (2014). A taxonomic signature of obesity in the microbiome? Getting to the guts of the matter. PLoS ONE 6(8):46-89.
|
|
|
|
|
Flegal KM, Ogden CL, Wei R, Kuczmarski RL, Johnson CL (2001). Prevalence of overweight in US children: comparison of US growth charts from the Centers for Disease Control and Prevention with other reference values for body mass index. The American Journal of Clinical Nutrition 73:1086-1093.
Crossref
|
|
|
|
|
Flint HJ (2011). Obesity and the gut microbiota. Journal of Clinical Gastroenterology 45:128-132.
Crossref
|
|
|
|
|
Furlan CPB, da Silva Marineli R, Júnior MRM (2013). Conjugated linoleic acid and phytosterols counteract obesity induced by high-fat diet. Food Research International 51(1):429-435.
Crossref
|
|
|
|
|
Greenway FL, Smith SR (2000). The future of obesity research. Nutrition 16(12):976-982.
Crossref
|
|
|
|
|
Graph P (2003). Statistical Software (prism3.0) Graph Pad software Incorporated, 2236 Avenida de la Playa La Jolla, CA92037 USA.
|
|
|
|
|
Injac R, Boskovic M, Perse M, Koprivec-Furlan E, Cerar A, Djordjevic A, Strukelj B (2008). Acute doxorubicin nephrotoxicity in rats with malignant neoplasm can be successfully treated with fullerenol C60 (OH) 24 via suppression of oxidative stress. Pharmacological Reports 60(5):742-749.
Crossref
|
|
|
|
|
Inukai T (2013). Symptomatic obesity-classification, pathogenesis, diagnosis and therapy. Nihon Rinsho 71(2):291-296.
|
|
|
|
|
Jorge PA, Neyra LC, Osaki RM, Almeida E, Bragagnolo N (1998). Effect of eggplant on plasma lipid levels, lipidic peroxidation and reversion of endothelial dysfuntion in experimental hyper-cholesterolemia. Brazilian Archives of Cardiology 70:87-91.
Crossref
|
|
|
|
|
Karalis KP, Giannogonas P, Kodela E, Koutmani Y, Zoumakis M, Teli T (2009). Mechanisms of obesity and related pathology: linking immune responses to metabolic stress. Federation of European Biochemical Societies Journal 37(3):5747-5754.
Crossref
|
|
|
|
|
Mercer JG, Archer ZA (2005). Diet-induced obesity in the Sprague-Dawley rat: dietary manipulations and their effect on hypothalamic neuropeptide energy balance systems. Biochemical Society Transactions 33(5):1068-1072.
Crossref
|
|
|
|
|
Millogo-Kone H, Guissou JP, Nacoulma O, Traore AS (2006). Study of the antibacterial activity of stem bark and leaf extracts of Parkia biglobosa (Jacq) Benth on Staphylococcus aureus. African Journal of Traditional, Complementary and Alternative Medicines 32(2):74-78.
Crossref
|
|
|
|
|
Monk JM, Wenquing W, Dion L, Hannah RW, Amber LH, Danyelle ML, Daniela G, Pauls KP, Lindsay ER, Power KA (2019). Navy bean supplemented high-fat diet improves intestinal health, epithelial barrier integrity and critical aspects of the obese inflammatory phenotype. Journal of Nutritional Biochemistry 70:91-104.
Crossref
|
|
|
|
|
Ndidi US, Ndidi CU, Olagunju A, Muhammad A, Billy FG, Okpe O (2014). Proximate, antinutrients and mineral composition of raw and processed (Boiled and Roasted) Sphenostylis stenocarpa seeds from Southern Kaduna, Northwest Nigeria. International Scholarly Research Notices.
Crossref
|
|
|
|
|
Oguntola S (2019). African locust beans prevent complications of diabetes- Scientists. The Nigerian Tribune: Article on Natural Health.
|
|
|
|
|
Oh RC, Hustead T R (2011). Causes and evaluation of mildly elevated liver transaminase levels. American Family Physician 84(9):1003-1008.
|
|
|
|
|
Olaniyan A (2013). Locust Bean Products. Non-Wood News-No.10.
|
|
|
|
|
Paras G, Sandeep T, Minky M, Arminder SS, Rohit G, Pyare LS (2011). Obesity: An Introduction and Evaluation. Journal of Advanced Pharmacy Education and Research 2:125-137.
|
|
|
|
|
Peltonen M, Lindroos AK, Roberson JS (2003). Musculoskeletal pain in the obese. A comparative with a general population and long-term changes after conventional and surgical obesity treatment. Pain 10(4):549-557.
Crossref
|
|
|
|
|
Pieroni A (2005). Prance, Ghillean; Nesbitt, Mark (eds.). The Cultural History of Plants. Routledge P 30.
|
|
|
|
|
Popkin BM (2001). The nutrition transition and obesity in the developing world. Journal of Nutrition 131:871-873.
Crossref
|
|
|
|
|
Puska P, Stahl T (2010). Health in all policies-the Finnish initiative: background, principles and current issues. Annual Review of Public health 31:315-328.
Crossref
|
|
|
|
|
Reitman S, Frankel SA (1957). Colorimetric method for the determination of serum glutamate-oxaloacetate transaminase and pyruvate transaminase. American Journal of Clinical Pathology 28(1):56-63.
Crossref
|
|
|
|
|
Slanc P, Doljak B, Kreft S, Lunder M, janes D, Strukelj B (2009). Screening of selected food and medicinal plant extracts for pancreatic lipase inhibition. Phytotherapy Research 23(6):874-877.
Crossref
|
|
|
|
|
Spilburg CA, Goldberg AC, McGill JB, Stenson WF, Racette SB, Bateman J, McPherson TB, Ostlund Jr RE (2003). Fat-free foods supplemented with soy stanol-lecithin powder reduce cholesterolabsorption and LDL cholesterol. Journal of the American Dietetic Association 103(5):577-581.
Crossref
|
|
|
|
|
Norris S L, Zhang X, Avenell A, Gregg E, Schmid CH, Lan J (2005). Long-term non-pharmacological weight loss interventions for adults with pre-diabetes. Cochane Database Systemic Review 33(2):224-226.
Crossref
|
|
|
|
|
Diament AL, Fisler JS, Warden CH (2003). Obesity alleles in mice and humans. Obesity Reviews 4(4):249-255.
Crossref
|
|
|
|
|
Sweeting HN (2007). "Measurement and definitions of obesity in childhood and adolescence: A field guide for the uninitiated". Nutritional Journal 6(1):32.
Crossref
|
|
|
|
|
Thomson SC, Aihua D, Dingjiu B, Joseph S, Roland CB, Volker V (2001). Ornithine Decarboxylase, Kidney Size, and the Tubular Hypothesis of Glomerular Hyperfiltration in Experimental Diabetes. Journal of Clinical Investigation 107(2):217-224.
Crossref
|
|
|
|
|
World Health Organization (WHO) (2000). Preventing and Managing the Global Epidemic. World Health Organization. Obesity P 894.
|
|
|
|
|
Zulet MA, Barber A, Garchin H, Hsigueret P, Martnez JA (1999). Alterations in Carbohydrate and Lipid metabolism induced by a diet rich in coconut oil and cholesterol in a rat model. Journal of the American College of Nutrition 18(1):36-42.
Crossref
|
|