ABSTRACT
Insects account for the greatest amount of biodiversity in forests with over 1,400 species reportedly eaten as human food, but are the least studied of all fauna. Studies have shown that they may contribute significantly to livelihoods in both rural and urban areas. This study was carried out to assess the consumer acceptability and nutrient content of Cirina forda larva-enriched vegetable soups. Dry C. forda (CF) larva was purchased from a market in Burkina Faso and refrigerated at -4°C. Four vegetable soup samples (plain vegetable soup; Egusi soup; Vegetable soup+CF; and Egusi soup+CF) were prepared traditionally. Dry CF larva and the four vegetable samples were analysed using standard AOAC methods, while acceptability of the soup samples was carried out using 9-point Hedonic scale. Moisture content of CF was 3.98 g while that of soups ranged from 59.78 to 77.14 g /100 g. C. forda larva contained 54.38 g protein and 16.81 g fat which were rich in essential amino acids and unsaturated fatty acids respectively; and high in macro-minerals. Nutrient content of vegetable soups enriched with CF larva were significantly higher (p<0.05), and more acceptable than un-enriched ones; with Egusi soup+CF larva being the most acceptable. C. forda larva is rich in both macro and micronutrients and generally acceptable to consumers. C. forda larva consumption should be popularized as means of improving dietary diversity, nutrient intake and overall health of humans.
Key words: Cirina forda larva, edible insects, enriched vegetable soups, consumer acceptability, nutrient content.
Protein-energy malnutrition is still an important public health issue in the developing countries of the world, especially in Africa, with its attendant problem of morbidity, mortality, stunted growth, and impaired neurobehavioral development in children (Iombor et al., 2017). The common sources of animal protein such as meat and eggs are becoming more and more costly and out of reach of the common man (Headey et al., 2018;
Ebenebe et al., 2020), hence, alternative cheap sources of protein of high biological value need to be explored in order to meet human body protein requirements. Insects remain a vital and preferred food and essential source of protein, fat, minerals and vitamins in many developing countries and various cultures throughout the world (Durst and Shono, 2010).
Research studies have documented that some edible insects have nutritional value that is comparable with that of meat and fish (Braide et al., 2010); and are often a welcome source of protein in the absence of meat from vertebrates (Sponheimer et al., 2005). Edible insects are important dietary components in many cultures where they contribute significantly to protein, fats, and micronutrient intake of consumers (Akinnawo and Ketiku, 2000; Anvo et al., 2016); and are not used as emergency food to ward off starvation, but included as normal part of the diet whenever available (Adeoye et al., 2014).
Insects have played important part in the history of human nutrition in Africa, Australia, Asia and the Americas (Jongema, 2015; Dossey et al., 2016; Koko and Mariod, 2020).
Several studies have shown that edible insects contain appreciable amount of proteins of good quality and high digestibility, with beneficial amino acid and fatty acid profile comparable to conventional livestock and fish (Braide et al., 2010; Igwe et al., 2011; Iombor et al., 2017). In some African countries such as Malawi, Zambia and Tanzania, malnutrition in children have been fought through flour made out of dried caterpillars. Pregnant and nursing women as well as anaemic patients also eat caterpillar species high in protein, calcium and iron (Igbabul et al., 2014), hence, the potential of insects needs to be considered in malnutrition alleviation strategies in Nigeria.
Insects of nutritional importance include (but not limited to) grasshoppers, caterpillars, beetle grubs and (sometimes) adults, winged termites, Cirina forda larva, and a variety of aquatic insects (Adeoye et al., 2014). Among the edible insect species, C. forda (Westwood) larva has been reported to have the potential to provide substantial amount of protein, minerals and polyunsaturated fatty acids to the diets which are usually deficient in animal protein (Akinnawo and Ketiku, 2000; Adepoju and Daboh, 2013). The larva of this insect is widely consumed in the Northern, Central and South-Western parts of Nigeria (Ogunleye, 2006). The insect larva is processed into dry form and consumed as a delicacy served as snack or as an essential ingredient in vegetable soups along with carbohydrate food in Southern part of Nigeria and many homes in Africa (Omotoso, 2006). Animal sourced foods (ASFs) are often costly and remain out of reach for many low-income households (Headey et al., 2018). Due to changes in environmental factors, dietary changes are urgently required (Springmann et al., 2018); and since insects form a part of traditional food system with high levels of energy, quality protein with good amino and fatty acid profile, and variety of essential minerals (Igwe et al., 2011), they should be considered as important food source.
Our previous study on consumption pattern of C. forda larva as important source of nutrients confirmed the findings of Omotoso (2006), and revealed that the most preferred mode of consumption of the insect larva is as an additive to vegetable soups (Daboh and Adepoju, 2020). This study was therefore carried out to assess the consumer acceptability and nutrient content of C. forda larva-enriched vegetable soups.
Sample collection and preparation
Dry C. forda (CF) larva sample was purchased from a local market in Burkina Faso and kept under refrigeration at –4°C. Fresh Amaranthus hybridus vegetable, ‘Egusi’ (melon seed), pepper, palm oil, onion, maggi cubes and salt used for the study were purchased from Bodija market in Ibadan, Nigeria.
A portion of the dry C. forda larvae was ground and labelled as Sample A.
Soup preparation
The vegetable soups were prepared traditionally (by engaging the service of a local food vendor) at the Dietetic kitchen of the Department of Human Nutrition and Dietetics, University of Ibadan, Ibadan, Nigeria. The vegetable leaves were rinsed with distilled water to eliminate soil and pebbles and then sliced. A total of 350 g C. forda larvae was weighed, rinsed and soaked in hot water to soften a little and was divided into two portions of 175 g each.
Sample B: Efo riro soup
About 250 ml of palm oil was added to the cooking pot placed on a heater to warm. Ground pepper (10 g) and onions (4 g) were added and fried for 5 min, followed by addition of 4.5 g of salt and two bouillon cubes. Water (250 ml) was then added, stirred together and allowed to simmer for 20 min. Then, 25 g of sliced vegetable leaf was added and allowed to cook for 5 min (Adepoju and Ugochukwu, 2019).
Sample C: Efo riro + C. forda larvae enriched soup
About 250 ml of palm oil was added to the cooking pot placed on a heater to warm. Ground pepper (10 g) and onions (4 g) were added and fried for 5 min, followed by addition of 4.5 g of salt, two bouillon cubes, and 175 g of C. forda larvae. Water (250 ml) was then added, stirred together and allowed to simmer for 20 min. Then, 25 g of sliced vegetable leaf was added and allowed to cook for 5 min.
Sample D: Egusi soup
About 250 ml of palm oil was added to the cooking pot placed on a heater to warm. Ground pepper (10 g) and onions (4 g) were added and fried for 5 min, followed by addition of 4.5 g of salt and two bouillon cubes. Water (250 ml) was added, stirred together and allowed to simmer for 20 min. Then, 15 g of ground Egusi and 25 g of sliced vegetable leaf were added and allowed to cook for 5 min.
Sample E: Egusi + C. forda larvae enriched soup
About 250 ml of palm oil was added to the cooking pot and allowed to warm on a gas cooker. Ground pepper (10 g) and onions (4 g) were added and fried for 5 min, followed by addition of 4.5 g of salt, two bouillon cubes, and 175 g of C. forda larvae. Water (250 ml) was then added, stirred together and allowed to simmer for 20 min. Then, 15 g of ground Egusi and 25 g of sliced vegetable leaf were added and allowed to cook for 5 min.
Chemical analyses
The dry CF larva sample and the four soups were analysed for their nutrient content using the standard methods of Association of Official Analytical Chemists (AOAC, 2005).
Proximate composition determination
The moisture content of the samples was determined by hot-air oven method (Plus 11 Sanyo Gallenkamp PLC, UK) at 105°C for 4 h. The crude protein was determined using micro-Kjeldahl method (Method No. 978.04); crude lipid was determined by Soxhlet extraction method (Method No. 930.09). The ash content was determined through incineration in muffle furnace at 550°C for 4 h (Method No. 930.05). Total carbohydrate content was obtained by difference. Gross energy of the samples was determined using ballistic bomb calorimeter.
Mineral content determination
Potassium and sodium content of the samples were determined by digesting the ash of the samples with perchloric and nitric acids, and then taking the readings on Jenway digital flame photometer/spectronic20 (AOAC, 2005: [975.11]). Phosphorus was determined by vanado-molybdate colorimetric method (AOAC, 2005: [975.16]). Calcium, magnesium, iron, zinc, manganese, and copper were determined by atomic absorption spectrophotometric method (Buck Scientific, Norwalk, UK) and compared with absorption of standards of these minerals (AOAC, 2005: [975.23].
Vitamin content determination
Vitamin A determination
Vitamin A was determined through ultraviolet absorption measurement at 328 nm after extraction with chloroform (AOAC Method 960.5 & 974.29, 2005). Calibration curve of vitamin A acetate was made and sample vitamin A concentration estimated as microgram (μg) of vitamin A acetate.
Thiamine (vitamin B1) determination
Thiamine content of the samples was determined by weighing 1 g of sample into 100 ml volumetric flask with addition of 50 ml of 0.1 M H2SO4 and boiled in a boiling water bath with frequent shaking for 30 min. Five milliliter (5 ml) of 2.5 M sodium acetate solution was added and flask set in cold water to cool contents below 50°C. The
flask was stoppered and kept at 45-50°C for 2 h and thereafter made up to 100 ml. The mixture was filtered through a No. 42 Whatman filter paper, discarding the first 10 ml. Ten milliliters (10 ml) was pipetted from remaining filtrate into a 50 ml volumetric flask, and 5 ml of acid potassium chloride solution was added with thorough shaking. Standard thiamine solutions were prepared and treated same way. The absorbance of the samples as well as that of standards was read on a fluorescent UV Spectrophotometer (Cecil A20 Model, USA) at a wavelength of 285 nm.
Riboflavin (vitamin B2) determination
One gram (1 g) of each sample was weighed into a 250 ml volumetric flask; 5 ml of 1 M HCl was added, followed by the addition of 5 ml of dichloroethene. The mixture was shaken and 90 ml of de-ionized water was added. The whole mixture was thoroughly shaken and was heated on a steam bath for 30 min to extract all the riboflavin. The mixture was then cooled and made up to volume with de-ionized water. It was then filtered, discarding the first 20 ml of the aliquot. Two milliliters (2 ml) of the filtrate obtained was pipetted into another 250 ml volumetric flask and made up to mark with de-ionized water. Samples were read on the fluorescent spectrophotometer at 460 nm. Standard solutions of riboflavin were prepared and readings taken at 460 nm. The sample riboflavin was obtained through calculation.
Niacin (vitamin B3) determination
Five grams (5 g) of sample was extracted with 100 ml of distilled water and 5 ml of this solution was drawn into 100 ml volumetric flask and made up to the mark with distilled water. Standard solutions of niacin were prepared and absorbance of sample and standard solutions was measured at of 385 nm on a spectrophotometer, and niacin concentration of the sample estimated.
Pyridoxine (vitamin B6) determination
The vitamin B6 content of the samples was determined by extracting 1 g of sample with 0.5 g of ammonium chloride, 45 ml of chloroform and 5 ml of absolute ethanol. The mixture was thoroughly mixed in a separating funnel by shaking for 30 min, and 5 ml of distilled water added. The chloroform layer containing the pyridoxine was filtered into a 100 ml volumetric flask and made up to the mark with chloroform. Standard solutions of 0-10 ppm of vitamin B6 were prepared and treated in a similar way as samples; and their absorbance measured on Cecil 505E spectrophotometer at 415 nm. The amount of vitamin B6 in the sample was then calculated.
Cyanocobalamin (vitamin B12) determination
Cyanocobalamin content of the samples was determined by extracting 1 g of sample with distilled water with shaking for 45 min, followed by filtering the mixture. The first 20 ml of the filtrate was rejected, and another 20 ml filtrate collected. To the collected filtrate, 5 ml of 1% sodium dithionite solution was added. Standard cyanocobalamin solutions (0-10 μg/ml) were prepared, and absorbance of sample as well as standards was read on spectronic21D spectrophotometer at 445 nm. Amount of sample cyanocobalamin was then estimated through calculation.
Ascorbic acid determination
Ascorbic acid in the samples was determined by titrating the aqueous extract of each sample with solution of 2, 6 – dichlorophenol-indophenol dye to a faint pink end point.
Anti-nutrients determination
Oxalate content of the samples was determined by extraction of the samples with water for about 3 h and standard solutions of oxalic acid prepared and read on spectrophotometer (Spectronic20) at 420 nm, and amount of oxalate estimated.
Phytate was determined by titration with ferric chloride solution (Sudarmadji and Markakis, 1977), while trypsin inhibitory activity was determined on casein and comparing the absorbance with that of trypsin standard solutions read at 280 nm (Makkar and Becker, 1996). The tannin content of the samples was determined by extracting the samples with a mixture of acetone and acetic acid for 5 h, measuring their absorbance and comparing the absorbance of the sample extracts with the absorbance of standard solutions of tannic acid at 500 nm on spectronic20 (Griffiths and Jones, 1977). Saponin was also determined by comparing the absorbance of the sample extracts with that of the standard at 380 nm (Makkar and Becker, 1996). All determinations were carried out in triplicate.
Sensory evaluation of vegetable soups
The vegetable soups acceptability study was carried out at the Department of Human Nutrition and Dietetics sensory evaluation laboratory, University of Ibadan, Nigeria. The soup samples were assessed for their acceptability using 30 untrained panelists drawn within the University community, who had eaten or known C. forda larva before and were willing to participate. The samples were rated on a 9-point hedonic scale in which the degree to which a product is relished was expressed as: like extremely (9), like very much (8), like moderately (7), like slightly (6), neither like nor dislike (5), dislike slightly (4), dislike moderately (3), dislike very much (2), dislike extremely (1). The panelists were required to observe the sample, taste and score based on colour, taste, aroma, texture and overall acceptability. The panelists were provided with water to rinse their mouths in between sample evaluation, and were instructed to rinse their mouth with water before tasting another sample.
Statistical analysis
The data obtained from the chemical analyses were expressed as mean and standard deviation of triplicate determinations and subjected to independent t-test using Statistical Package for Social Sciences (SPSS) version 20 (SPSS Inc., USA); and the data obtained from sensory evaluation presented as means of 30 panelists’ assessment which was analysed using one – way ANOVA. The level of significance was set at p < 0.05.
Proximate composition of C. forda and vegetable soups
The proximate composition of C. forda larva and the four vegetable samples is shown in Table 1. The dry C. forda sample (sample A) was high in crude protein (54%) and gross energy content (492.05 Kcal), high in fat, ash, and dietary fibre, moderate in carbohydrate, but low in moisture content (3.98%).
Vegetable soup (Efo riro) (sample B) was high in moisture (77.14%) and ash (1.35%) contents, high in dietary fibre (11.62%), moderate in crude protein (12.4%), but low in carbohydrate content (4.53%). Addition of ground melon seed (Egusi) to the vegetable soup (sample D) lowered the moisture content (73.04%) and increased the crude protein (14.88%), dietary fibre (12.89%), ash (1.42%), carbohydrate (5.364%), and gross energy content (153.05 Kcal.) of the vegetable soup significantly (p<0.05). Likewise, addition of C. forda larva to the vegetable soup (sample B) and Egusi soup (sample D) led to significant increase in most parameters with significant reduction in the moisture content of enriched vegetable soup samples (samples C and E) (p<0.05).
Mineral content of C. forda and vegetable soups
The C. forda larva contained substantial amount of sodium, potassium, calcium, magnesium and phosphorus, with potassium being the most abundant, followed by sodium, magnesium, calcium and phosphorus. It is also high in iron content compared with other animal sources. However, the larva was low in zinc (1.39 mg 100g-1) and copper (0.33 mg 100g-1), and low in manganese (0.001 mg100g-1) (sample A) (Table 2). Addition of Egusi to vegetable soup (sample B) significantly increased its mineral content (sample D), (p<0.05). Addition of CF larva to the vegetable and Egusi soups (samples B and D) increased the sodium, potassium, iron zinc and copper significantly (p<0.05), while it decreased the calcium, magnesium and phosphorus content significantly (p<0.05).
The vegetable soup (sample B) was rich in magnesium, phosphorus, and calcium, and high in potassium and sodium content. Addition of Egusi to the vegetable (sample D) increased the nutrient content of the product significantly. Also, addition of the insect larva to both vegetable (sample B) and Egusi (sample D) soups significantly increased the sodium, potassium, iron, zinc and copper of the larva-enriched vegetable soups (Samples C and E) (p<0.05).
In Table 3, the vitamin composition of C. forda larva revealed that it contains substantial amounts of vitamins, especially vitamins B12 and C. The larva was low in water soluble vitamins (sample A). The vegetable soup (sample B) was low in vitamins A (0.87), B1 (0.04), B2 (0.09), and B3 (0.71 mg 100 g-1), but high in vitamins B6 (2.11), B12 (17.01), C (15.73) and E (3.01 mg 100 g-1). Addition of ground melon to the vegetable soup significantly improved all the vitamin content of the product (sample D) except vitamin B12 (p<0.05). Enriching both vegetable and Egusi soups (samples B and D) with CF significantly increased the vitamin content of the products (p<0.05) except vitamin B12 (samples C and E).
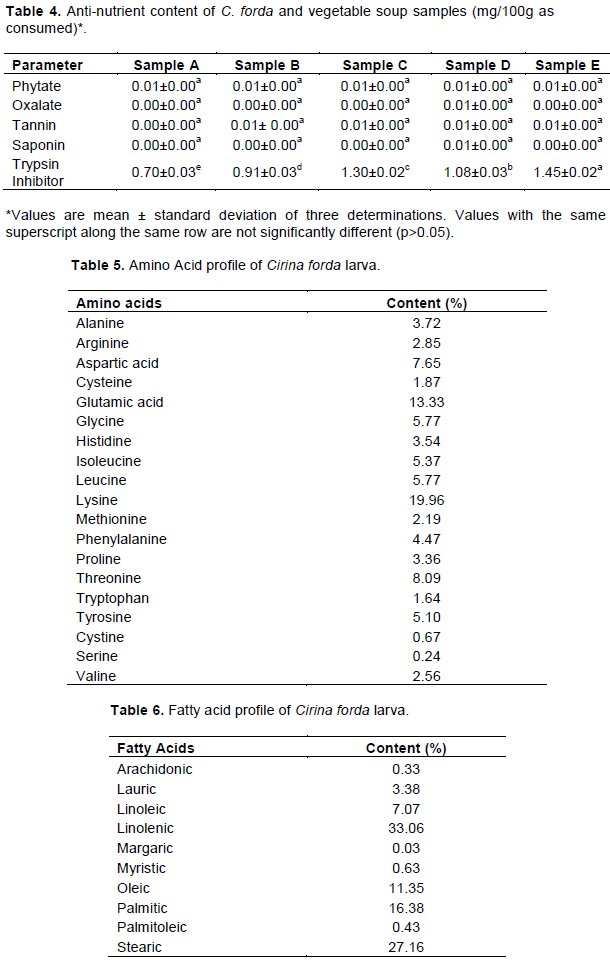
Anti-nutrients level of C. forda larva and vegetable soups
The phytate and trypsin inhibitor content of C. forda larva were negligible, while oxalate, tannin, and saponin were not detectable (Table 4). However, there was a significant difference (p<0.05) in the trypsin inhibitor content of the samples (p<0.05), with the larva – enriched vegetable samples having higher values. Enriched vegetable with Egusi had the highest value of trypsin inhibitors. Addition of C. forda larva to enrich the vegetable soups did not increase their antinutrient content, as their values were negligible except for trypsin inhibitor. The level of trypsin inhibitors in the enriched vegetable soups (samples C and E) was very low, and cannot cause any significant reduction in the bioavailability of protein in the soups.
Amino acid and fatty acid profiles of C. forda larva
The CF larva has good amount of essential amino acids such as lysine, threonine, phenylalanine, leucine, isoleucine, valine, tryptophan and cysteine as well as dispensable amino acids such as glutamic acid, histidine, arginine, aspartic acid and glycine among others, hence, its protein content is of high quality (Table 5).
The C. forda larva is rich in linolenic acid (poly-unsaturated), and high in oleic acid (monounsaturated), stearic and palmitic acids among others (Table 6). Linolenic, stearic, palmitic, and oleic acids were very much abundant in fat of the larva. Unsaturated fatty acids constitute 52.27% of the total fatty acids in the larva and 12.15% of this was monounsaturated fatty acid (MUFA).
Sensory evaluation of vegetable soups
In Table 7a, all the soups were acceptable to the panelists, as none of them was rejected or scored below average. Overall, the control vegetable soup (sample B) was the least accepted for colour, taste, texture, aroma and overall acceptability. The two C. forda larva-enriched vegetable soups (samples C and E) were more relished compared with the plain vegetable soup (sample B) and vegetable + Egusi soup (sample D). The vegetable + Egusi + CF larva soup (sample E) was the most acceptable for colour, taste, aroma and general acceptability; while vegetable soup + CF larva (sample C) was the most accepted for texture.
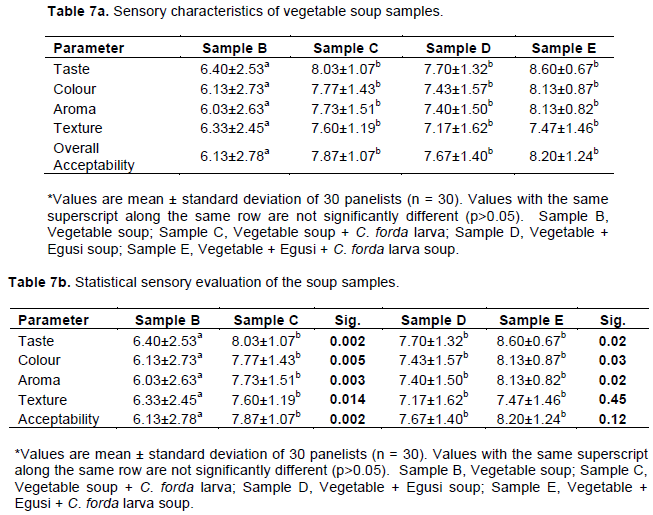
Significant differences existed between plain vegetable soup (sample B) and vegetable soup enriched with CF larva (sample C) in all the parameters assessed, the CF larva-enriched vegetable soup scoring higher (p<0.05) (Table 7b). Egusi soup enriched with CF larva soup (sample E) significantly scored higher than Egusi soup (sample D) in taste, colour and aroma, (p<0.05); while there was no significant difference in their texture and overall acceptability (p>0.05). There was no significant difference in overall acceptability of CF larva – enriched vegetable soups and Egusi vegetable soup (samples C, D, and E) (p>0.05). The Egusi soup enriched with CF larva (sample E) scored highest in all parameters assessed.
Proximate composition of C. forda and vegetable soups
In Table 1, the dry C. forda (CF) larva was low in moisture content (3.98%). The low moisture content obtained for the CF larva is in line with the reports of Banjo et al. (2006) and Osasona and Olaofe (2010). The low moisture content is suggestive of its high keeping quality (that is long shelf life), and supportive of the assertion of respondents in our consumption survey carried out earlier that the insect larva can be kept for a long period of time (Daboh and Adepoju, 2020). The low moisture content will prevent microbial contamination.
The insect larva contained high amount of protein. The value obtained for the larva protein falls within the range of values reported by Igbabul et al. (2014), similar to 55.41% obtained by Yapo et al. (2017) but lower than 63% reported by Anvo et al. (2016) for Cirina butyrospermi. The variation observed in the biochemical composition of the insect larva may be due to the host tree because the amount of protein varies between insect species and within the same species depending on the nutritional quality of the leaves of the host tree (Banjo et al., 2006; Yapo et al., 2017), the difference in the geographical location where the CF larva was obtained, as well as method of harvesting/processing of the insect larva (Ekpo, 2011; Womeni et al., 2012; Adepoju, 2013).
The crude fat content of the insect larva obtained in this study is within the range of values quoted for insects (10 – 30%) (Durst et al., 2010). The value is higher than the values reported by Osasona and Olaofe (2010) and Igbabul et al. (2014) and 15% reported by Anvo et al. (2016), but lower than the 22.21% reported by Ogunleye (2006). The difference in the crude fat content of C. forda in this study compared with other studies could be due to the difference in the geographical location where the samples were obtained and also, the method of processing (Ekpo, 2011; Womeni et al., 2012; Adepoju, 2013). The moderate fat content of the C. forda larva is beneficial because it may reduce its rate of susceptibility to rancidity.
However, the C. forda larva contained a moderate amount of dietary fibre. Dietary fibre helps to regulate the digestive system, aid bowel health and weight management (Rolfe et al., 2009). The value of ash of C. forda larva sample was less than the range of values (2.91 – 3.97%) reported by Igbabul et al. (2014) but comparable to that of Akinnawo and Ketiku (2000), Omotoso (2006) and Osasona and Olaofe (2010). The carbohydrate content of the C. forda larva was higher than the range reported by Igbabul et al. (2014). The gross energy content of C. forda sample was very high. This may be due to the fact that at larval stage, the insect feeds much to derive and store the nutrient and energy needed for growth and development during the pupal stage to the adult stage (Chen et al., 2009). This finding is in line with the report of FAO (2004) that reported high energy content in feeding caterpillar flours. The high energy content could also be as a result of its high protein, fat and carbohydrate content. The gross energy content in this study is very similar to those of 492.31 kcal. reported by Yapo et al. (2017) for Cirina butyrospermi.
The moisture content of vegetable soup was very high (sample B). The vegetable soup was moderately high in protein content compared with other plant protein (Adepoju and Ugochukwu, 2019). This amount of protein is believed to have been contributed partly by other ingredients used in the preparation of the vegetable soup. The soup was low in fat and carbohydrate content despite the addition of palm oil. Leafy vegetables are generally poor source of lipids and carbohydrates (Adepoju and Ugochukwu, 2019). However, the soup had moderate value of ash and gross energy, and high in dietary fibre. Vegetables are generally good source of dietary fibres. The increase in the dietary fibre of the soups is very beneficial, as dietary fibre promotes a healthy bowel function, helps to control blood sugar level, lowers blood cholesterol levels, and helps in weight management (Rolfe et al., 2009). The significant increase in the ash content of the C. forda larva-enriched soups is an indication of their higher mineral content compared with plain and Egusi vegetable soups.
Mineral composition of C. forda larva and vegetable soups
The sodium content of the larva is within the range reported for other edible insects (Ajai et al., 2013). The recommended daily dietary allowance of sodium is 2400 mg per day (FAO, 2010). Sodium assists in maintaining the proper acid-balance and in controlling osmotic pressure that develops between the blood and cells due to ionic concentration differences (Paiko et al., 2014).
Potassium was the most abundant of all the minerals in C. forda larva. This corroborated the findings of Osasona and Olaofe (2010) who reported potassium to be the most abundant mineral in the flour of CF. The high potassium content is highly beneficial because a high intake of potassium has been reported to protect against increasing blood pressure and other cardiovascular risk (Insel et al., 2007), and it is essential for the functioning of the brain and nerve (Iombor et al., 2017). The potassium-sodium ratio (K:Na) has frequently been used as a diagnostic tool to identify adrenal insufficiency (Iombor et al., 2017). The potassium to sodium ratio in this study is 2.1:1, hence, the larva could be incorporated into diets for the management of hypertension as high potassium intake has been found to lower blood pressure by antagonizing the effect of sodium (Iombor et al., 2017).
The calcium content of CF larva was found to be high. The calcium value for the larva in this study was higher than those reported by Akinnawo and Ketiku (2000), Omotoso (2006) and Osasona and Olaofe (2010). The observed difference could be due to the differences in the source of the larva coupled with methods of processing. Calcium is an integral component of the skeleton, about 99% of total body calcium is found in bones and teeth, where it plays a structural role (Insel et al., 2007; Yapo et al., 2017). Calcium is important in the development and maintenance of bones and teeth, blood clotting, nerve impulse transmission, muscle contraction and cell metabolism (Heaney, 2006; Insel et al., 2007).
The magnesium content of C. forda larva was higher than those reported by Akinnawo and Ketiku (2000), Omotoso (2006) and Osasona and Olaofe (2010). Magnesium is a co-factor participating in more than 300 enzyme reactions, making it an essential element for the synthesis of carbohydrates, lipids, nucleic acids and proteins, as well as for other actions in different organs of the cardiovascular and neuromuscular systems (Chen and Feng, 2002; EFSA, 2015). The phosphorus content of C. forda larva in this study is higher than the value reported by Paiko et al. (2014) but lower than the 215.54 mg/100 g reported by Omotoso (2006). Phosphorus like calcium is also involved in calcification of bones and teeth. It plays a vital part in the oxidation of nutrients in the form of phosphate groups in ATP (Paiko et al., 2014).
C. forda larva contained substantial amount of heme iron. Iron plays an important role as a heme molecule in red blood cells, as it permits oxygen transport (WHO, 2006). It can serve as an antioxidant and can prevent cardiomyopathy and growth retardation (Paiko et al., 2014). Since C. forda larva contained substantial amount of iron, its inclusion in human diets will be beneficial, and as blood building element in anaemic conditions (Rolfe et al., 2009).
Micro-minerals such as copper, zinc, and manganese are also contained in substantial amount in C. forda larva. The copper content of C. forda larva is similar to the value reported by Ande (2003). Zinc is important because its deficiency can lead to growth retardation, delayed sexual and bone maturation, skin lesions, diarrhoea, alopecia, impaired appetite, increased susceptibility to infection mediated via defects in the immune system (FAO/WHO, 2001).
Vitamin content of Cirina forda larva and vegetable soups
Generally, insects have been reported to contain varying degree of both water-soluble and fat-soluble vitamins. C. forda larva was found to contain substantial amounts of B-vitamins, vitamins C and E. Among these, vitamin C was the most abundant. Vitamin C helps in maintaining blood vessels flexibility and improves circulation in the arteries (Alamu et al., 2013). One of the most important benefits derivable from vitamins A, C and E is their role as antioxidants, oxygen free radical scavengers, while that of the B-vitamins is their role as co-enzymes in several enzyme systems of the body (Insel et al., 2007; Alamu et al., 2013). Addition of Egusi and the CF larva led to significant increase (p<0.05) in the vitamin content of the products (samples C, D and E).
Anti-nutrients level of C. forda larva and vegetable soups
The absence of oxalates, tannins and saponins and negligible phytate content in C. forda is believed to be due to full conversion of the leaf consumed by the larva during the process of its entering the soil before being harvested, as its name implies in Yoruba language (Kanni wole – meaning the larva has entered the soil) (Osasona and Olaofe, 2010). The phytate, oxalate, tannin, and saponin content of C. forda larva in this study was much lower than those reported by Alamu et al. (2013) and Omotoso and Adesola (2018).
The difference in the antinutrients content of the larva in this study and past studies might have resulted from the differences in the source of the insect larva. Anti-nutrients are generally known to reduce the bioavailability of nutrients in the body. High phytate level in human nutrition decreased the availability of some minerals such as calcium, magnesium and iron by formation of insoluble compounds with the minerals (Anuonye et al., 2012), while tannins reduced protein bioavailability when bound to protein by inducing a decrease in solubility and functionality of the protein. Tannins are capable of lowering available protein by antagonistic competition, and can therefore elicit protein deficiency syndrome (Ekop, 2004).
Amino acid profile of C. forda larva
C. forda larva contained a wide array of both essential and non-essential amino acids (Table 5). The quality of a food protein depends largely on its amino acid component. C. forda larva was rich in essential amino acids. The most predominant amino acids of the larva are lysine, glutamic acid, threonine, aspartic acid, leucine, glycine, isoleucine, tyrosine and phenylalanine. This is similar to the work of Yapo et al. (2017) who also reported most of these amino acids to be the most abundant in C. butyrospermii. Amino acids are important components for healing and protein synthesis processes; any deficiency in these essential amino acids will hinder the recovery process (Zuraini et al., 2006). Glycine together with other essential amino acids such as alanine, arginine and phenylalanine form a polypeptide that promotes growth and tissue healing (Witte et al., 2002; Adeoti et al., 2013). Also, glycine is involved in the transmission of impulses in the nervous system, and alanine strengthens the immune system and prevents buildup of toxic substances in the body (Paiko et al., 2014). The high content of essential amino acid in CF larva is very beneficial, as this implied that its protein is of a high biological value. Addition of CF larva to enrich the vegetables (samples C and E) significantly increased their protein content (p<0.05).
Fatty acid profile of C. forda larva
The larva of CF contained a high amount of polyunsaturated fatty acid (PUFA), (33.05% linolenic acid and 7.07% linoleic acid). The ratio of polyunsaturated to saturated fatty acids (P/S) has been used widely to indicate the cholesterol lowering potential of a food (Akinnawo and Ketiku, 2000). Akinnawo and Ketiku (2000) reported that a P/S ratio of 0.2 has been associated with high cholesterol level with high risk of coronary heart disorders. The P/S ratio of Cirina forda larva in this study is 0.8, and a ratio as high as 0.8 has been reported to be associated with desirable levels of cholesterol and reduction of coronary heart disease [Akinnawo and Ketiku, (2000)]. Thus, regular consumption of Cirina forda larva could help in intake of healthy fat that can prevent onset of cardiovascular diseases.
Sensory evaluation of vegetable soups
Although, no significant difference was observed in the scoring of sensory attributes of samples C, D and E (p>0.05), the enriched samples (Samples C and E) had the highest sensory scores. The exoskeleton of insects has a great influence on the texture. Insects are crunchy and the sounds accompanying their eating resemble the sounds of crackers or pretzels (KouÅ™imská and Adámková, 2016). This may explain why CF-enriched vegetable soups were more preferred and scored higher than either plain or Egusi vegetable soups. The result of sensory evaluation obtained in which the vegetable soups rated higher in all the parameters is similar to the report of Adejoju and Ugochukwu (2019) in which the vegetable sauce was rated higher than ordinary vegetable soup.
C. forda larva was very rich in protein, high in fat, and essential minerals, and very low in anti-nutrients. The amino acid composition of the larva showed that it contained all the essential amino acids needed for human growth in good proportion, hence, the protein content can be considered as a complete protein of high biological value, more so, it is of animal origin. Fat content of the insect larva contained higher amount of unsaturated fatty acid compared with the saturated fatty acid component, showing that it can serve as a good source of healthy fat that is fit for human consumption for promotion of good health.
The insect larva and the enriched vegetable soups contained negligible level of antinutrients which cannot pose any threat to nutrient bioavailability from the larva and the vegetable soups, hence, it is believed that the consumption of the insect larva, either as snack or in vegetable soups or sauces is very safe and will promote quality nutrient intake by consumers.
The soups enriched with C. forda larva were more acceptable than the plain vegetable or vegetable with Egusi soups. C. forda larva-enriched Egusi soup was the most acceptable soup. Likewise, in terms of nutrient content it was the most nutrient-dense of the soups. Inclusion of C. forda larva in vegetable soups improved both its palatability and nutrient content; therefore, its inclusion in soups and sauces should be encouraged. Also, popularizing the consumption of this insect larva should be encouraged as a means of increasing dietary diversity of the population of the people where the insect larva is readily available. This will help to promote the intake of quality protein and essential minerals among the populace of the host community, and assist in combating protein and micronutrient malnutrition; thereby improving the general health of the people. Also, its consumption could serve as a means of conserving the host tree from going into extinction due to human activities.
The authors have not declared any conflict of interests.
REFERENCES
|
Adeoti OA, Elutilo OO, Babalola JO, Jimoh KO, Azeez LA, Rafiu KA (2013). Proximate, mineral, amino acid and fatty acid compositions of Maize Tuwo-Cirina forda flour blends. Greener Journal of Biological Sciences 3(4):165-171.
Crossref
|
|
|
|
Adeoye OT, Oyelowo OJ, Adebisi-Fagbohungbe TA, Akinyemi OD (2014). Eco-diversity of edible insects of Nigeria and its impact on food security. Journal of Biology and Life Science 5(2):175.
Crossref
|
|
|
|
|
Adepoju OT (2013). Geographic and yearly variation in nutrient content of Shea butter (Butyrospermum paradoxum gaertn f) fruit pulp. International Journal of Agriscience 3(5):406-413.
|
|
|
|
|
Adepoju OT, Daboh OO (2013). Nutrient composition of Cirina forda (West wood) enriched complementary foods. Annals of Nutrition & Metabolism 63(1& 2):139-144.
Crossref
|
|
|
|
|
Adepoju OT, Ugochukwu IC (2019). Improving vegetable diversity and micronutrient intake of Nigerians through consumption of lesser known silk cotton (Ceiba pentandra) leaf. International Journal of Nutrition 4(1):19- 30.
Crossref
|
|
|
|
|
Ajai AI, Bankole M, Jacob JO, Audu UA (2013). Determination of some essential minerals in selected edible insects. African Journal of Pure and Applied Chemistry 7(5):194-197.
|
|
|
|
|
Akinnawo OO, Ketuki AO (2000). Chemical composition and fatty acid profile of edible larva of Cirina forda (West wood). African Journal of Biomedical Research 3:93-96.
|
|
|
|
|
Alamu OT, Amao AO, Nwokedi CI, Oke OA, Lawa IO (2013). Diversity and nutritional status of edible insects in Nigeria: A review. International Journal of Biodiversity and Conservation 5(4):215-222.
|
|
|
|
|
Ande AT (2003). Comparative studies of the mineral composition of processed larvae and prepupae of Cirina forda westwood (Lepidoptera: saturniidae). Science Focus 4:11-13.
|
|
|
|
|
Anuonye JC, Jigam AA, Ndaceko GM (2012). Effects of extrusion-cooking on the nutrient and anti-nutrient composition of pigeon pea and unripe plantain blends. Journal of Applied Pharmaceutical Science 2:158-162.
|
|
|
|
|
Anvo MPM, Toguyeni, A, Otchoumou AK, Zoungrana-kabore CY, Kouamelan EP (2016). Nutritional qualities of edible caterpillars (Cirina butyrospermi) in South western of Burkina Faso. International Journal of Innovative and Applied Studies 18(2):639-645.
|
|
|
|
|
Association of Official Analytical Chemists (AOAC) (2005). Association of Official Analytical Chemists official methods of analysis, 16th ed. Arlington V. A. pp. 806-842.
|
|
|
|
|
Banjo AD, Lawal OA, Songonuga EA (2006). The nutritional value of fourteen edible insect species in Southwestern Nigeria. African Journal of Biotechnology 5(3):298-301.
|
|
|
|
|
Braide W, Sokari TG, Hart AD (2010) Nutrition quality of an edible caterpillar of Lepidopteran (Bunea alcinoe). Advances in Science and Technology 4:49-53.
|
|
|
|
|
Chen X, Feng Y (2002). Review on nutritive value of edible insects. Chinese Science and Technology 12:54-59.
|
|
|
|
|
Chen X, Feng Y, Chen Z (2009). Common edible insects and their utilization in China. Entomological Research 39(5):299-303.
Crossref
|
|
|
|
|
Daboh OO, Adepoju, OT (2020). Knowledge and consumption pattern of Cirina forda (Westwood) larva in two local government areas of Oyo State, Nigeria. Nigerian Journal of Nutritional Sciences (In Press).
|
|
|
|
|
Dossey A, Morales-Ramos J, Guadalupe R (2016). Insects as sustainable food ingredients: production, processing and food applications, 1st edn. Academic Press, Cambridge, MA, USA.
|
|
|
|
|
Durst PB, Shono K (2010). Edible forest insects: exploring new horizons and traditional practices. Proceedings of a Workshop on Asia-Pacific resources and their potential for development, 19-21 February 2008, Chiang Mai, Thailand. Bangkok: FAO pp. 1-4.
|
|
|
|
|
Durst PB, Johnson DV, Leslie RL, Shono K (2010). Forest insects as food: humans bite back, proceedings of a workshop on Asia-Pacific resources and their potential for development. FAO, Regional Office for Asia and the Pacific, Bangkok pp. 1-64.
|
|
|
|
|
Ebenebe CI, Ibitoye OS, Amobi IM, Okpoko VO (2020). African edible insect consumption market. In: African edible insects as alternative source of food, oil, protein and bioactive components. Mariod A. A. (ed.) Springer Nature, Switzerland AG 2020:35-36.
Crossref
|
|
|
|
|
European Food Safety Authority (EFSA) (2015). Valeurs nutritionnelles de référence pour le magnésium et le phosphore. Consulté le 22 Février 2015.
View
|
|
|
|
|
Ekop AS (2004). Effects of processing on the chemical composition of Maize. 24th Annual Conference of Nigerian Society of Biochemistry and Molecular Biology. University of Calabar. Nov 24 -27, 2004.
|
|
|
|
|
Ekpo KE (2011). Effect of processing on the protein quality of four popular insects consumed in Southern Nigeria. Archives of Applied Science Research 3:307-326.
|
|
|
|
|
Food and Agricultural Organization (FAO) (2010). Review of food composition data on edible insects: Food and Agricultural Organization/ The International Network of Food Data Systems (INFOODS).
|
|
|
|
|
Food and Agricultural Organization (FAO) (2004). Les insectes comestibles, important source de protéines en Afrique central. 8 Novembre Rome (Italie) P 45.
|
|
|
|
|
Food and Agriculture Organization / World Health Organization FAO/WHO (2001). Human vitamin and mineral requirements. Food and Nutrition Division, FAO, Rome pp. 25-26.
|
|
|
|
|
Griffiths DW, Jones DIH (1977). Cellulase inhibition by tannins in the testa of field beans (Vicia faba). Journal of the Science of Food and Agriculture 28(911): 938-989.
Crossref
|
|
|
|
|
Headey D, Hirvonen K, Hoddinott J (2018). Animal sourced foods and child stunting. American Journal of Agricultural Economics 100(5):1302-1319.
Crossref
|
|
|
|
|
Heaney RP (2006). Bone biology in health and disease. In. Shils ME, Shike M, Ross A, Caballero B, Cousins R (eds.): Modern nutrition in health and disease, 10th ed. Philadelphia, PA, Lippincott Williams and Wilkins. pp.10.
|
|
|
|
|
Igbabul BD, Chia AC, Ufot I (2014). Nutritional and microbial quality of dried larva of Cirina forda. International Journal of Nutrition and Food Sciences 3(6): 602-606.
Crossref
|
|
|
|
|
Igwe CU, Ujowundu CO, Nwaogu LA, Okwu GN (2011). Chemical analysis of an edible African termite, Macrotermesnigeriensis; a potential antidote to food security problem. Biochemistry and Analytical Biochemistry 1:105.
|
|
|
|
|
Insel P, Turner RE, Ross D (2007). Nutrition, 3rd edn., Jones and Barlett Publishers Inc. USA pp. 185, 186, 424, 472-474, 477.
|
|
|
|
|
Iombor TT, Abiya MU, Gbeyonron FN (2017). Quality assessment and consumer acceptability of fermented pearl millet porridge flours fortified with Cirina forda flour. Nigerian Journal of Pure and applied Sciences 9(2):30-41.
|
|
|
|
|
Jongema Y (2015). World list of edible insects. Laboratory of Entomology, Wageningen University, Wageningen.
|
|
|
|
|
Koko MYF, Mariod AA (2020). Sensory Quality of Edible Insects. In: African edible insects as alternative source of food, oil, protein and bioactive components. Mariod AA (ed.), Springer Nature Switzerland AG 2020 pp. 115- 117.
Crossref
|
|
|
|
|
KouÅ™imská L, Adámková A. (2016). Nutritional and sensory quality of edible insects. Nutrition and Food Science Journal 4:22-26.
Crossref
|
|
|
|
|
Makkar HPS, Becker K (1996). Nutritional value and anti-nutritional components of whole and ethanol extracted Moringa oleifera leaves. Animal Feed Science Technology 63:211-238.
Crossref
|
|
|
|
|
Ogunleye RF (2006). Biochemical implications of the consumption of Zonocerus variegatus, (Orthoptera: Pyrgomorphidae) and Cirina forda Westwood (Lepidoptera: Saturnidae). Journal of Food, Agriculture and Environment 4(3&4):23-25.
|
|
|
|
|
Omotoso OT (2006). Nutritional quality, functional properties and anti-nutrient composition of the larva of Cirina forda (Westwood) (Lepidoptera: Saturniidae). Journal of Zhejiang University 7(1):51-55.
Crossref
|
|
|
|
|
Omotoso OT, Adesola AA (2018). Comparative studies of the nutritional composition of some insect orders. International Journal of Entomology and Nematology Research 2(1):1-9.
|
|
|
|
|
Osasona O, Olaofe O (2010). Nutritional and functional properties of Cirina forda larva from Ado- Ekiti, Nigeria. African Journal of Food Science 4(12):775-777.
|
|
|
|
|
Paiko YB, Jacob JO, Salihu SO, Dauda BEN, Suleiman MAT, Akanya HO.(2014). Fatty acid and amino acid profile of emperor moth caterpillar (Cirina forda) in Paikoro Local Government Area of Niger State, Nigeria. American Journal of Biochemistry 4(2): 29-34 .
|
|
|
|
|
Rolfes SR, Pinna K, Whitney E (2009). Understanding normal and clinical nutrition. Eighth Edn Wadsworth Cengage Learning pp. 122-123.
|
|
|
|
|
Sponheimer M, de Ruiter D, Lee-Thorp J, Späth A (2005). Sr/Ca and early hominin diets revisited: new data from modern and fossil tooth enamel. Journal of Human Evolution 48:147-156.
Crossref
|
|
|
|
|
Springmann M, Clark M, Mason-D'Croz D, Wiebe K, Bodirsky BL, Lassaletta L, de Vries W, Vermeulen SJ, Herrero M, Carlson KM, Jonell M, Troell M, DeClerck F, Gordon LJ, Zurayk R, Scarborough P, Rayner M, Loken B, Fanzo J, Godfray HCJ, Tilman D, Rockström J, Willett W (2018). Options for keeping the food system within environmental limits. Nature 662:519-525.
Crossref
|
|
|
|
|
Sudarmadji S, Markakis P (1977). The phytate and phytase of soybean Tempeh. Journal of Science of Food and Agriculture 28(4):381-383.
Crossref
|
|
|
|
|
Witte MB, Thorton FJ, Tantry U, Barbel A (2002). L-arginine supplementation enhances diabetic wound healing: Involvement of the nitric oxide synthase and arginase pathways. Metabolism 51(10):1269-1273.
Crossref
|
|
|
|
|
Womeni HM, Tiencheu B, Linder M, Nabayo EMC, Tenyang N, Mbiapo FT, Villeneuve P, Fanni J, Parmentier M (2012). Nutritional value and effect of cooking, drying and storage process on some functional properties of Rhynchophorus phoenicis. International Journal of Life science and Pharma Research 2:203-219.
|
|
|
|
|
World Health Organization (WHO) (2006). Biological half-time of Cadmium in the Blood of workers after cessation of exposure. Scandinavia Journal of work Environmental Health 1983(9):327-331.
Crossref
|
|
|
|
|
Yapo ML, Amara MF, Tuo Y (2017). Nutritional value of shea carterpillar (Cirina butyspermii Vuillet) sold at the market of Korhogo (Cote d'Ivoire). International Journal of Agronomy and Agricultural Research 10(5):35-44.
|
|
|
|
|
Zuraini A, Somchit MN, Solihah MH, Goh YM, Arifah AK, Zakaria MS, Somchit N, Rajion MA, Zakaria ZA and MatJais AM (2006). Fatty acid and amino acid compositions of three local Malaysian Channa fish spp. Food Chemistry 4(97):674-678.
Crossref
|
|