Full Length Research Paper
ABSTRACT
The increasing epidemic of methicillin-resistant Staphylococcus aureus (MRSA), one of the most important hospital and community pathogens, has led to a demand for new agents to treat the infection. Natural products may be used to reduce this problem with low side effects. The objective of this study was to determine the antibacterial effect of fermented extracts of Angelica koreana and Arnebia euchroma by Lactobacillus spp. against S. aureus, which were tested by disk diffusion test. Extracts of A. koreana and A. euchroma showed a clear zone of 15.5 ± 1.1 and 15.9 ± 2.2 mm, respectively. Fermented extracts by Lactobacillus sp. showed more improved antibacterial activity against S. aureus than the extracts.
Key words: MRSA; Lactobacillus spp.; fermentation; disk diffusion test; plant extract.
INTRODUCTION
Staphylococcus aureus is a major pathogen causing nosocomial infections. The emergence of antibiotic-resistant strains of S. aureus that caused infections among hospitalized patients is a severe problem worldwide (Li and Webster, 2018).
For example, the rate of hospital-acquired MRSA reached 50.4% in China and MRSA is causative of almost 44% of cases and over 20% of excess mortality among healthcare-acquired infections in Europe (Guo et al., 2020). Treatment options for MRSA infection are currently limited because most MRSA strains are resistant to widely used antibiotics such as lactams, macrolides, aminoglycosides, and fluoroquinolones (Schentag et al., 1998; Tacconelli et al., 2008; Kaur and Chate, 2015). Therefore, it is necessary to find alternative treatments to prevent and control MRSA infections.
Angelica koreana, also named Ostericum koreanum, has traditionally been used in oriental Korean medicine to treat the common cold, and to reduce rheumatic pains or headaches. This plant has been reported for various biological activities including anti-tumor, anti-microbial, antioxidant, and anti-inflammatory effects (Kang et al., 2009; Shin, 2005; Park et al., 2007, 2008).
Arnebia euchroma Royle is a well-known traditional herb used for various skin diseases in Iranian tribal medicine (Ashkani-Esfahani et al., 2012). Shikonin derivatives isolated from the roots of A. euchroma have been reported for antimicrobial, anti-inflammatory and anti-tumor activities and thus to be considered as important compounds for potential medicinal use (Kim et al., 2001).
The bioconversion process could enhance the biological activities of medicinal plants and herbs. A previous study showed that lactic fermented herbal teas have more composition in phenolic, flavonoid compounds (Ibrahim et al., 2014). Another study also showed that the antioxidant and antibacterial activities of medicinal plants fermented by fungi are increased compared to non-fermented control (Dong et al., 2015). Lactobacillus-fermented Artemisia princeps has been used as a functional component to increase the growth performance, meat lipid stability, and intestinal health of chickens (Kim et al., 2012).
This study aimed to evaluate the antibacterial effects of A. koreana and A. euchroma extracts(AK and AE, respectively) after fermentation by Lactobacillus spp. against S. aureus using the disk diffusion and biofilm formation method.
MATERIALS AND METHODS
Raw materials
The roots of A. koreana and A. euchroma Royle were commercially purchased (Barumhanyak, Korea). 1 kg each of dried plant roots was immersed in 5-10 volumes of 70-80% ethanol. They were extracted by maceration for overnight with constant shaking at 24 °C 3 times. The obtained extracts were filtered with Whatman 2 filter paper to discard impurities. The filtered extract was concentrated under reduced pressure in a rotary concentrator and dried to obtain a solid content of the extract.
Bacterial strains and culture
S. aureus (CCARM3505, MRSA and CCARM3506, QRSA), Lactobacillus acidophilus (KACC12419, AD), Lactobacillus acidifarinae (KACC16342, AF) and Lactobacillus acidipiscis (KCTC12394, AP) were purchased from CCARM (The Canadian Centre for Agri-Food Research in Health and Medicine) and KCTC (Korean Collection for Type Cultures). All strains were kept in 20% glycerol at -70°C. S. aureus was cultured in tryptic soy broth (TSB, BD Difco, Franklin Lakes, USA) containing tryptone 17 g, soytone 3 g, glucose 2.5 g, sodium chloride 5 g, and dipotassium phosphate 2.5 g at 37°C for 24 h. Lactobacillus spp. were cultured in MRS containing Lactobacilli MRS Broth 55 g/L (BD Difco, Franklin Lakes, USA) at 37 °C for 24 hours under anaerobic condition (Bae et al., 2019).
Fermentation of A. koreana and A. euchroma with Lactobacillus spp.
Extracts of A. koreana and A. euchroma were inoculated to be 1% of the total volume at 107 CFU/mL of Lactobacillus spp. Fermentation was carried out in 14 mL round tube for 24-96 h at 37°C under anaerobic condition. Filtration of the supernatant was done using a nominal 0.22 µm filter (ProLabs, Korea) to remove residual cells (Hashemi et al., 2017). It was repeated three or more times to produce fermentations.
Antimicrobial susceptibility testing
Bacterial suspensions with a turbidity equivalent to a McFarland standard of 0.5 were swabbed evenly onto TSA plates with a sterile cotton swab for the disk diffusion method. Antibiotic disks containing ampicillin, plant extracts, and fermented extracts were placed on TSA plates. The plates were incubated at 37 °C for 24 h and then the inhibition zone diameters, including the diameter of the disk, were measured (Dušková and Karpíšková, 2013). The test was repeated three or more times and all data are the average ± STDEV.
Biofilm formation assay
A slightly modified biofilm formation assay was used (Hobby et al., 2012; O'Toole, 2011). The 6-well plate was incubated with extracts at 37°C for 24 h; the wells were gently washed twice with 200 µL of phosphate-buffered saline (PBS) to remove all planktonic cells. After aspiration of planktonic cells, adherent biofilms were fixed with absolute ethanol before staining with 200 µL 0.2% crystal violet solution (Thermo Fisher Scientific, USA) for 5 min. Following aspiration of the stain, wells were washed three times with PBS and air-dried. A quantitative assessment of biofilm formation was then taken by adding 33% acetic acid and incubating for 10 min. The absorbance of eluate was calculated from optical density (OD570) values measured using a microplate reader (BioTek Instruments, Korea). The assay was repeated three or more times and all data are the average ± STDEV.
Cell viability assay
A slightly modified MTT assay was used to test the cell viability of HaCaT cells (Park et al., 2021). Briefly, HaCaT cells in Dulbecco’s modified eagle’s medium (DMEM) at a density of 104 cells are cultured in a 96-well plate for 24 h. Serum-free medium containing extracts or fermented extracts was added to the wells. After 24 h of incubation, MTT (3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide, Sigma) was added, followed by incubating for 3 h at 37°C. The solution was then discarded, and cells were suspended in 100 µL of DMSO (Dimethyl Sulfoxide, Junsei, Japan). Absorbance was calculated from optical density (OD540) values measured using a microplate reader (BioTek Instruments, Korea). The assay was repeated three or more times and all data are the average ± STDEV.
Ames mutagenicity assay
The Ames mutagenicity assays were performed, according to the method of manufacturer recommendation (Xenometrix, Switzerland) (Flückiger-Isler and Kamber, 2012). Both tester strains, Salmonella Typhimurium TA98 and Escherichia coli WP2 uvrA, were grown in growth medium overnight at 37°C. The test cultures were exposed to the indicated concentrations of extracts for 90 min in liquid minimal exposure media in a 24-well plate. After each well of the 24-well plates was added with 2.6 ml of indicator medium, 100 µL aliquots of culture were then dispensed into a 96-well plate. The 96-well plates were incubated at 37°C for 48 h. The number of positive (yellow) wells out of 48 wells per replicate was compared to the number of revertants from the negative control.
RESULTS AND DISCUSSION
Antibacterial test
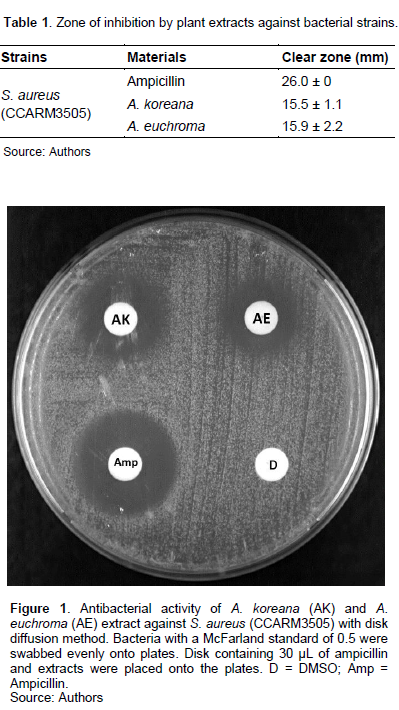
The extracts of AK and AE showed clear zone sizes of 15.5 ± 1.1 and 15.9 ± 2.2 mm against S. aureus, respectively (Table 1 and Figure 1). Lactic acid bacteria (LAB), particularly those belonging to beneficial and non-pathogenic bacteria have traditionally been used in the food industry. Recent studies have shown their preventive effect against infection. For example, the incidence of infections and acute diarrhoea in children is reduced (Gleeson et al., 2011; Sur et al., 2011). In addition, LAB is widely used as functional foods, and LAB fermentation products and supernatant are also useful as cosmetic ingredients.
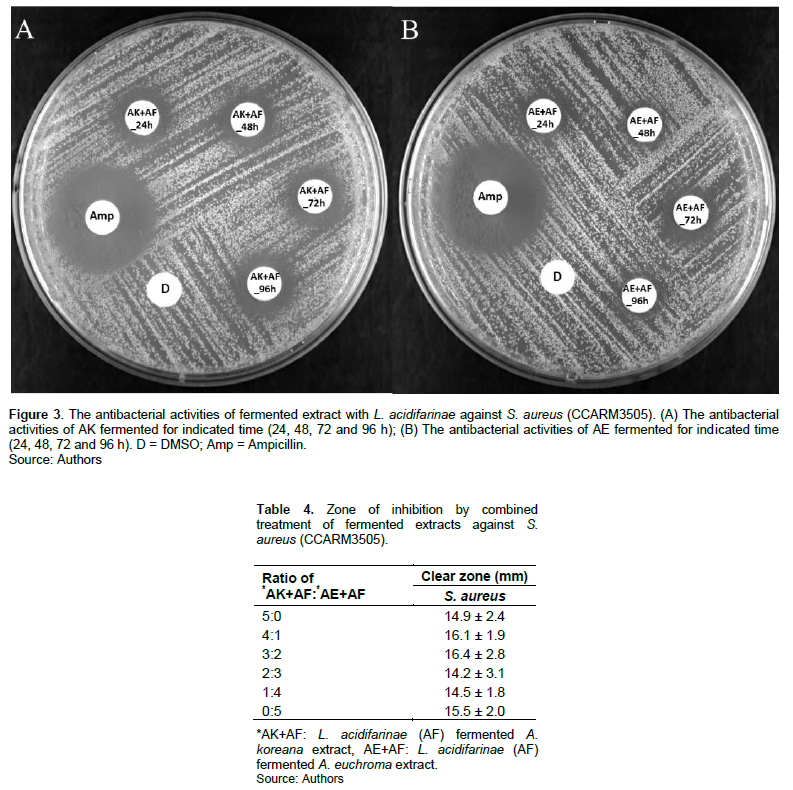
Streptococcus spp., Lactobacillus spp., and Lactococcus spp. are mainly applied for fermentation. Various substrates such as soybeans, fruit, and plants are used for culture (Izawa and Sone, 2014). With three Lactobacillus spp., AK and AE extracts were separately fermented for 72 h. Fermentation with AF showed a larger clear zone size of 19.2 ± 2.3 mm compared with non-fermented AK extract. In the case of AE extract, fermentation with AF also showed a prominent clear zone size of 16.6 ± 1.5 mm (Table 2 and Figure 2). To test how long time of fermentation is most effective, AK and AE extracts were fermented with AF for indicated times. Both extracts showed clear zone sizes of 17.8 ± 2.6 and 16.4 ± 1.9 mm, respectively, when fermented for 72 hours (Table 3 and Figure 3).
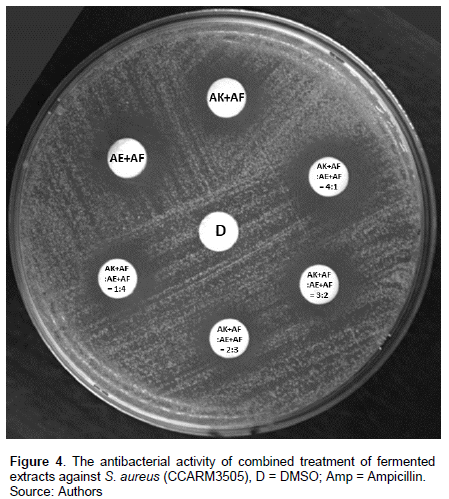
Various combinations of the two fermented extracts were tested for synergistic effect. The largest clear zone size was expressed as 16.4 ± 2.8 mm by AK+AF: AE+AF=3:2 combinations (Table 4 and Figure 4). AK and AE extracts showed antibacterial activity against S. aureus but to make the extract showed higher activity, the fermentation by LAB was applied.
.png)
Fermentation with LAB is used in various fields, and in this study, three types of LAB were tested. Among the three LAB, the largest clear zone was observed in the fermented extract with AF, and the greatest clear zone was observed with 72 h fermentation, which was selected as the best fermentation condition. AF was first identified in 2005 (Vancanneyt et al., 2005), but research about AF has not been largely conducted. Therefore, the increased antibacterial activity by fermentation with AF found in this study appeared to be meaningful.
Biofilm formation inhibition
Surface adhesion of bacteria is an essential step and is necessary for bacteria to infect host in their environment. The role of biofilm is to attach to abiotic surfaces, epitheliums, and interfaces in multicellular organisms (Berne et al., 2015).
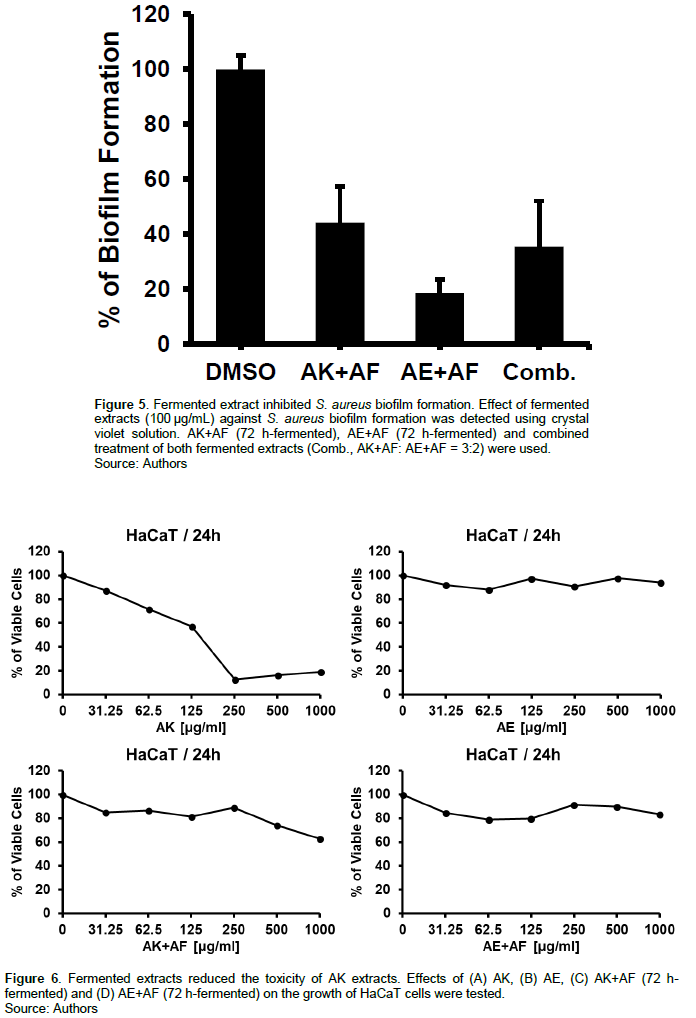
Biofilm serves to promote bacteria survival by preventing antibiotic activity and host immune responses, so it is considered as a good target for the biofilm forming pathogen (Schulze et al., 2021). To examine the potential anti-biofilm formation effect of the fermented extracts, S. aureus was cultured in the presence of the fermented extracts and biofilm formation was detected using crystal violet biofilm assay. Each fermented extract and combination of both fermented extracts dramatically decreased S. aureus biofilm formation by over 50%. The most prominent anti-biofilm formation activity was observed with AE extract fermented by AF. 100 µg/mL of AK and AE extracts fermented with AF inhibited biofilm formation by 44.19 ± 13.06 and 18.57 ± 4.81 compared with untreated bacteria, respectively. Combined treatment of both fermented extracts showed 35.55 ± 6.53% inhibition of S. aureus biofilm formation (Figure 5). These results suggest that AF-fermented AK and AE extracts are good candidates to control the biofilm forming pathogenic S. aureus (Ames et al., 1973).
Cell viability
Although AK extract, AE extract, and fermented extracts exhibit antibacterial effects against S. aureus, it is important to test toxicity with normal mammalian cell lines including HaCaT (Human keratinocyte cell line). AK extract showed cell viability lower than 20% at 250 μg/mL treatment (Figure 6A) but AK extract fermented with AF for 72 h showed reduced toxicity with higher than 80 and 60% of cell viability at 250 and 1000 μg/mL treatment, respectively (Figure 6C). AE extract and fermented AE extract did not influence the cell’s viability (Figure 6B, 6D).
Ames test
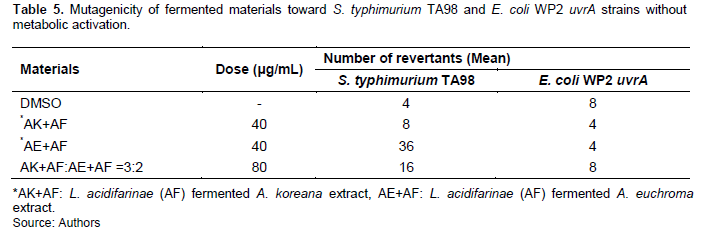
The Ames test was repeated three times without S9 metabolic activation. Fermented extract and combined treatment did not show any mutagenic activity in TA98 and WP2 uvrA strains without metabolic activation (Table 5). None of the investigated fermentations showed any potential mutagenic effects.
CONCLUSION
This manuscript showed that A. koreana and A. euchroma extracts exhibited the antibacterial effect against S. aureus. Fermented extracts by L. acidifarinae showed improved antibacterial effect and anti-biofilm formation activity against S. aureus with reduced animal cell toxicity compared with non-fermented extract.
FUNDING
This research was funded by the TIPA, Korea Technology and Information Promotion Agency for SMEs (#1425153495).
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Ames BN, Durston WE, Yamasaki E, Lee FD (1973). Carcinogens are mutagens: a simple test system combining liver homogenates for activation and bacteria for detection. Proceedings of the National Academy of Sciences 70(8):2281-2285. |
|
|
Ashkani-Esfahani S, Imanieh MH, Khoshneviszadeh M, Meshksar A, Noorafshan A, Geramizadeh B, Ebrahimi S, Handjani F, Tanideh N (2012). The healing effect of Arnebia euchroma in second degree burn wounds in rat as an animal model. Iranian Red Crescent Medical Journal 14(2):70-74. |
|
|
Bae WY, Kim HY, Kim KT, Paik HD (2019). Inhibitory effects of Inula britannica extract fermented by Lactobacillus plantarum KCCM 11613P on coagulase activity and growth of Staphylococcus aureus including methicillin-resistant strains. Journal of Food Biochemistry 43(4):e12785. |
|
|
Berne C, Ducret A, Hardy GG, Brun YV (2015). Adhesins involved in attachment to abiotic surfaces by Gram?negative bacteria. Microbial Biofilms 163-199. |
|
|
Dong JW, Cai L, Xiong J, Chen XH, Wang WY, Shen N, Liu BL, Ding ZT (2015). Improving the antioxidant and antibacterial activities of fermented Bletilla striata with Fusarium avenaceum and Fusarium oxysporum. Process Biochemistry 50(1):8-13. |
|
|
Dušková M, Karpíšková R (2013). Antimicrobial resistance of lactobacilli isolated from food. Czech Journal of Food Sciences 31(1):27-32. |
|
|
Flückiger-Isler S, Kamber M (2012). Direct comparison of the Ames microplate format (MPF) test in liquid medium with the standard Ames pre-incubation assay on agar plates by use of equivocal to weakly positive test compounds. Mutation Research 747(1):36-45. |
|
|
Gleeson M, Bishop NC, Oliveira M, Tauler P (2011). Daily probiotic's (Lactobacillus casei Shirota) reduction of infection incidence in athletes. International Journal of Sport Nutrition and Exercise Metabolism 21(1):55-64. |
|
|
Guo Y, Song G, Sun M, Wang J, Wang Y (2020). Prevalence and therapies of antibiotic-resistance in Staphylococcus aureus. Frontiers in Cellular and Infection Microbiology 10:107. |
|
|
Hashemi SMB, Khaneghah AM, Barba FJ, Nemati Z, Shokofti SS, Alizadeh F (2017). Fermented sweet lemon juice (Citrus limetta) using Lactobacillus plantarum LS5: Chemical composition, antioxidant and antibacterial activities. Journal of Functional Foods 38:409-414. |
|
|
Hobby GH, Quave CL, Nelson K, Compadre CM, Beenken KE, Smeltzer MS (2012). Quercus cerris extracts limit Staphylococcus aureus biofilm formation. Journal of Ethnopharmacology 144(3):812-815. |
|
|
Ibrahim NA, Mustafa S, Ismail A (2014). Effect of lactic fermentation on the antioxidant capacity of Malaysian herbal teas. International Food Research Journal 21(4):1483-1488. |
|
|
Izawa N, Sone T (2014). Cosmetic ingredients fermented by lactic acid bacteria. In Microbial production, Tokyo, Springer, pp. 233-242. |
|
|
Kang TJ, Lee SY, Singh RP, Agarwal R, Yim DS (2009). Anti-tumor activity of oxypeucedanin from Ostericum koreanum against human prostate carcinoma DU145 cells. Acta Oncologica 48(6):895-900. |
|
|
Kaur DC, Chate SS (2015). Study of antibiotic resistance pattern in methicillin resistant Staphylococcus aureus with special reference to newer antibiotic. Journal of Global Infectious Diseases 7(2):78-84. |
|
|
Kim CH, Kim GB, Chang MB, Bae GS, Paik IK, Kil DY (2012). Effect of dietary supplementation of Lactobacillus-fermented Artemisia princeps on growth performance, meat lipid peroxidation, and intestinal microflora in Hy-line Brown male chickens. Poultry Science 91(11):2845-2851. |
|
|
Kim SH, Kang IC, Yoon TJ, Park YM, Kang KS, Song GY, Ahn BZ. (2001). Antitumor activities of a newly synthesized shikonin derivative, 2-hyim-DMNQ-S-33. Cancer Letters 172(2):171-175. |
|
|
Li B, Webster TJ (2018). Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. Journal Orthopaedic Research 36(1):22-32. |
|
|
O'Toole GA (2011). Microtiter dish biofilm formation assay. Journal of Visualized Experiments 47:e2437. |
|
|
Park HJ, Bae GS, Kim DY, Seo SW, Park KB, Kim BJ, Song JM, Lee KY, Na C, Shin BC, Park SJ (2008). Inhibitory effect of extract from Ostericum koreanum on LPS-induced proinflammatory cytokines production in RAW264. 7 cells. The Korea Journal of Herbology 23(3):127-134. |
|
|
Park S, Kim J, Shin YK, Kim KY (2021). Antimicrobial activity of 4-hydroxyderricin, sophoraflavanone G, acetylshikonin, and kurarinone against the bee pathogenic bacteria Paenibacillus larvae and Melissococcus plutonius. Journal of Apicultural Research 60(1):118-122. |
|
|
Park YJ, Kim HJ, Lee SJ, Choi HY, Jin C, Lee YS (2007). A new chromone, 11-hydroxy-sec-O-glucosylhamaudol from Ostericum koreanum. Chemical and Pharmaceutical Bulletin 55(7):1065-1066. |
|
|
Schentag JJ, Hyatt JM, Carr JR, Paladino JA, Birmingham MC, Zimmer GS, Cumbo TJ (1998). Genesis of methicillin-resistant Staphylococcus aureus (MRSA), how treatment of MRSA infections has selected for vancomycin-resistant Enterococcus faecium, and the importance of antibiotic management and infection control. Reviews of Infectious Diseases 26(5):1204-1214. |
|
|
Schulze A, Mitterer F, Pombo JP, Schild S (2021). Biofilms by bacterial human pathogens: Clinical relevance - development, composition and regulation - therapeutical strategies. Microbial Cell 8(2):28-56. |
|
|
Shin S (2005). In vitro effects of essential oils from Ostericum koreanum against antibiotic-resistant Salmonella spp. Archives of Pharmacal Research 28(7):765-769. |
|
|
Sur D, Manna B, Niyogi SK, Ramamurthy T, Palit A, Nomoto K, Takahashi T, Shima T, Tsuji H, Kurakawa T, Takeda Y (2011). Role of probiotic in preventing acute diarrhoea in children: a community-based, randomized, double-blind placebo-controlled field trial in an urban slum. Epidemiology and Infection 139(6):919-926. |
|
|
Tacconelli E, De Angelis G, Cataldo MA, Pozzi E, Cauda R (2008). Does antibiotic exposure increase the risk of methicillin-resistant Staphylococcus aureus (MRSA) isolation? A systematic review and meta-analysis. Journal of Antimicrobial Chemotherapy 61(1):26-38. |
|
|
Vancanneyt M, Neysens P, De Wachter M, Engelbeen K, Snauwaert C, Cleenwerck I, Van der Meulen R, Hoste B, Tsakalidou E, De Vuyst L, Swings J (2005). Lactobacillus acidifarinae sp. nov. and Lactobacillus zymae sp. nov., from wheat sourdoughs. International Journal of Systematic and Evolutionary Microbiology 55(2):615-620. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0