ABSTRACT
Being a handmade product, the rennet cheese is prepared following regional and family traditions. In some places, the milk is obtained under sanitary conditions disabled and therefore has a high number of micro-organisms such as Escherichia coli. This study aimed to verify the survival of E. coli in rennet cheese made from experimentally contaminated milk, after storage for 21 days under refrigeration, at 4.0°C. According to the proposed treatment, each liter of pasteurized milk used to prepare the rennet cheese was inoculated with 1.0 ml concentrations of 103, 106 and 109 CFU/ml of E. coli ATCC 25922. After storage of rennet cheese for 21 days in refrigeration, it was observed that the pH varied with the concentration of E. coli present in the sample. A reduction of three log cycles E. coli stored in the cheese was observed as compared to initial concentration added to the milk. The need to maintain the cold chain was emphasized during their storage, because if there is E. coli in low counts, they can remain viable in the cheese made from contaminated milk.
Key words: Rennet cheese, milk, food microbiology, Escherichia coli.
Rennet cheese is a typical Brazilian product that was developed from raw or pasteurized milk in the Northeast for over 150 years. This cheese has a high commercial value, due to the simple technology applied during manufacture, high performance and good acceptance by consumers. This is the semi-rigid type cheese, with medium to high humidity, after the coagulation of milk using rennet of animal origin or other coagulating enzymes. Once prepared must be marketed as far as seven days of storage at 10°C (Mangueira et al., 2002; Cavalcante et al., 2007).
When the milk has hygienic-sanitary deficient conditions, microbial contamination can occur (Santana et al., 2008). The characteristics of the raw material used for the production of rennet cheese associated with the lack of standardization of the production method, make the rennet cheese a favorable food growth of pathogenic micro-organisms (Nassu et al., 2001; Oliveira et al., 2010). Thus, consumers are exposed to foodborne disease, caused by bacteria such as Salmonella species, Clostridium botulinum, Campylobacter species and Escherichia coli (Tondo et al., 2010), especially when consuming rennet cheese type that were produced from unpasteurized milk, without meeting the requirements of good manufacturing practices.
The rennet cheese has nutritional characteristics which serve as substrate for the growth of microorganisms, with 48% fat and approximately 23 to 25% protein (Perry, 2004). Among them, E. coli can be spread to the milk during milking inadequate and also by the use of cows with subclinical mastitis and possible contamination by the manipulators, which represent one of the main causes of the high content of pathogenic bacteria in milk and its derivatives (Pigatto et al., 2009). The E. coli is part of intestinal microbiota tract of man and animal being able to multiply between 7.0 to 46°C. Some strains have virulence factors which make them capable of causing intestinal and extra-intestinal diseases (Farrokh et al., 2013).
This work aimed to verify the survival of E. coli in manufactured rennet cheese and kept under refrigeration from experimentally contaminated milk after 21 days storage under refrigeration.
Inoculation of E. coli ATCC 25922
The manufacturing process of rennet cheese 16 samples was carried out in the Food Microbiology Laboratory of Pharmacy Course of the Federal University of Piauí – UFPI in the Brazil. To prepare a piece of cheese with 200 g, 2 L of pasteurized milk purchased in the local market was used. Thus, 32 L of milk was used to prepare all parts necessary. To test the absence of E. coli in the packaging pasteurized milks of a 1.0 ml aliquot was aseptically transferred to 84 wells plates, then the package was sealed and stored refrigerated (4.0°C) during the incubation period in the plates bacteriological incubator for 24 h.
As the proposed treatment in each liter of pasteurized milk used to prepare the rennet cheese, 1.0 ml was inoculated with 106, 109 and 1012 UFC/ml of E. coli ATCC 25922. Concentrations were prepared according to the scale as 0.5 McFarland (Bier, 1981) scale tested spectrophotometer model SP-22 BIOSPECTRO, with absorbency of 625 nm. The strain used was courtesy by the Hospital Infection Research Laboratory of the Oswaldo Cruz Foundation (FIOCRUZ - Brazil, RJ).
Manufacture of rennet cheese
After inoculation, as the treatments, the milk was transferred in a stainless to rectangular shapes capable to of taking 2.8 L, sterilized in an autoclave, for heating the milk at 38°C. Then, each liter of milk was added at intervals of 2 min 20 ml yogurt, 0.5 ml of calcium chloride solution to 50% and 1.5 ml of chymosin microbial dissolved in 20 ml of distilled water.
After 45 min of the addition of constituents, the curd was cut and the resulting mass remained at rest for 5 min. After the break, there was homogenizing during heating at 55°C for 25 min. After this time, the removal of 50% of the serum was made and 14 g per liter of sodium chloride was added. The resulting slurry was transferred to a container of Polyvinyl Chloride (PVC), for holding the pressing for 8 h. After this time, it was held to take it out of the container, when the cheese was properly packaged in plastic bag and stored under refrigeration at 4.7°C during the trial period of 21 days.
pH measurement
The electrometrical determination of hydrogen potential pH, measurement of water activity (Aw), moisture content and content of sodium chloride was performed according with the standards of the Institute Adolfo Lutz (2010). 10 g of the sample was macerated and 100 ml of distilled water was added. After homogenization, the digital pH meter electrode MOD: 210P MS (previously calibrated with pH buffer 7.0 and 4.0) was introduced into the homogenized sample.
Measurement of water activity (Aw)
The reading of the water activity was held in meter device water activity, DECAGON brand PAWKIT model, calibrated with distilled water. Thus, 1.0 g of the sample was transferred to the metal container which was attached to the unit.
Moisture content
Loss method was used for desiccation by direct drying in an oven at 105°C, based on water removal by heating. 5.0 g of each sample which was transferred to drying oven at 105°C for about 4 h was used. Once cooling, the sample was weighed again. Getting the weight of the wet sample to the weight of the dry sample, the sample humidity percentage was calculated by the following formula:
U% = 100 × N / P
where U% = humidity or volatiles at 105°C Percent m/m, N = final sample weight in grams, and P = initial weight of sample.
Content of sodium chloride
The determination of chlorides was performed by volumetry and titration with silver nitrate as indicator with potassium chromate. 5 g of each sample was weighed, then the sample was maintained at 105°C for 4 h to obtain constant weight. After drying, they were incinerated at 550°C for 2 h. 30 ml of hot water was added to the ash, transferred to a volumetric flask of 100 ml, to complete the balloon volume. An aliquot of 10 ml was removed which was added two drops of potassium chromate solution at 10%, and then was titled with a silver nitrate solution of 0.1 M until the appearance of a brick-red color. With the volume of silver nitrate, there need to change the coloring, by applying the following formula:
V × f × 0.584 / P = Chlorides, Sodium Chloride %m/m Where, V = Nº ml of silver nitrate solution of 0.1 M spent titration, f = silver nitrate solution of 0.1 M factor, and P = n° g sample in the aliquot used for titration.
Counting of E. coli
The enumeration of E. coli was performed on the day in which the piece of cheese was produced, and after 7, 14 and 21 days of storage under refrigeration. To carry out the enumeration of E. coli, the counting method on plates for Coliform/Escherichia coli was used, therefore, transferred a portion of 25 g of rennet cheese sample bottle with peptone water 225 ml (0.1%) to prepare dilution 10-1 UFC/ml, which was used to carry the other dilutions up 10-9 UFC/ml. Next, inoculation was done using special plates method (SimPlate), according to the manufacturer recommendation. Then, the plates were incubated at 35°C for 24 h. After this time, the total count of the plate in colony forming units per gram (UFC/g) ultraviolet light of 365 nm in Transside light L-PIX was used.
Statistical analysis
The survival of E. coli was checked in rennet cheese made from contaminated milk experimentally used it completely randomized design in a split plot, in which treatment (plot) is the concentration of E. coli ATCC 25922 (0, 103, 106 and 109 UFC/ml) and the sub-plot is the refrigeration storage time (0, 7, 14 and 21 days), performed with four replications (processing).
In the exploratory study data, it was found out that there was no variance homogeneity. So the nonparametric Kruskal-Wallis test to variables humidity and contamination was used. To hydrogen potential, water activity and content of sodium, analysis of variance and regression was used, and the sodium content was transformed to log 10 (X + 0.5) in accordance with the procedures of SAS version 9.0.
After storage, for 21 days under refrigeration, the rennet cheese type manufactured from milk contaminated experimentally observed that the pH varied with concentration of E. coli present in the sample (Table 1). The lowest values ​​were observed in concentration 9.00 UFC/g in log10. E. coli is capable of producing acids (lactic, acetic, formic and succinic acids) from lactose (Mogensen et al., 2003; Konneman et al., 2010; Machado et al., 2011), even when kept under refrigeration, they were able to keep the limited metabolism to maintain food (Forsythe, 2002). Thus, E. coli in the samples may have been responsible for most major acidification of the rennet cheese at a concentration 9.00 UFC/g in log10 compared with other concentrations. This fact may be due to the remaining amount of E. coli sufficient to maintain its metabolism such that the pH of the samples decreased.
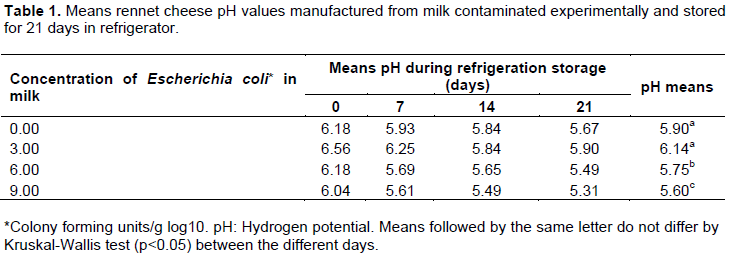
Although, all concentrations have been prepared at the same time under the same conditions for processing and storage of the rennet cheese samples which showed significantly different humidity values ​​(P<0.05), for treatments 6.00 and 9.00 UFC/g having the highest percentages (Table 2). In the metabolism of E. coli of lactose degradation, the formation of galactose and glucose took place and then the final product of glycolysis are acids, carbon dioxide and water (Koneman et al., 2010). In large quantities, such as the cheeses of concentrations from 6.00 to 9.00 UFC/g, E. coli can degrade part of the glucose present in the cheese and probably increase the humidity in small amounts that do not come to interfere with the water activity.
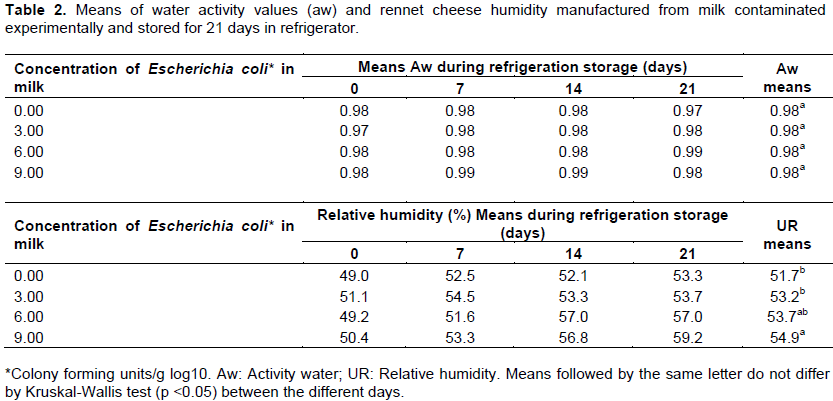
In the experiment, the sodium content in the cheese parts ranged from 2.13 to 3.19% and was similar at all concentrations of E. coli used for the treatments (Table 3). In rennet cheese production process, salt is added to the curd formed, so that the serum dissolved sodium chloride. During the salting of the cheese, the osmotic pressure difference between the brine and the mass causes part of the moisture to be released, dragging soroproteíns, lactic acid and dissolved minerals, while the NaCl is absorbed (Perry, 2004). In low acid environment (pH 5.5 to 7.0), sodium chloride concentrations up to 6.0% have a stimulatory effect on the growth of E. coli (Jordan and Davies, 2001). In these experimental conditions, the sodium chloride content found in the samples could stimulate the growth of E. coli, if the cheeses were not stored under refrigeration.
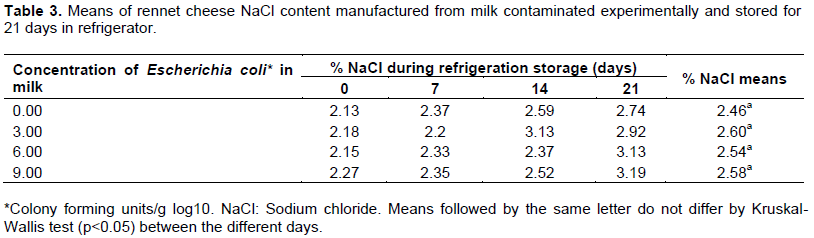
E. coli tolerate low pH values ​​and temperatures. When exposed to low acidic environments develop tolerance expressing adaptive response regulator genes, which favors the survival of the bacteria in fermented foods, even if stored under refrigeration (Castanie-Cornet et al., 1999; Vernozy-Rozand et al., 2005). Generally, E. coli multiplies well between 10 and 46°C; some strains can be multiplied at 6.5°C and survive weeks at -18°C (Grzadkowska and Griffiths, 2001). The time-temperature relationship for each micro-organism is specific and dependent on the middle of the features in which it appears. In most nutritious means, more favorable conditions for reproduction, such as foods high in fat, there is a tendency of resistance to thermal inactivation increase (Farrokh et al., 2013; Fogolari et al., 2012). Although commercial validity of rennet cheese is approximately seven days; the amount of E. coli of the produced rennet cheese was constant in all treatments, even after 21 days of storage time in refrigeration probably, the viability of the strains was enhanced by low acid conditions and nutrient composition of the cheese prepared with whole milk.
E. coli is thermo sensitive (Usajewicz and Nalepa, 2006; Poonnoy et al., 2014), at 49.5°C begins the process of thermal inactivation and after 5 min at 55°C the destruction of 90% occurs (D(55) value) with a reduction of one log cycle, in the same period of 58.6°C reduction from two cycles occurs (Z(58.6) value) of this bacterium (Romero et al., 2011). A decrease of three log cycles of Escherichia coli was observed in cheeses stored compared to the initial concentration added to milk (Table 4). During the processing of rennet cheeses, the dough was baked at 55°C for 25 min, so the temperature used is sufficient to reduce 3.00 UFC/g log10 E. coli in final product at resourced concentrations.
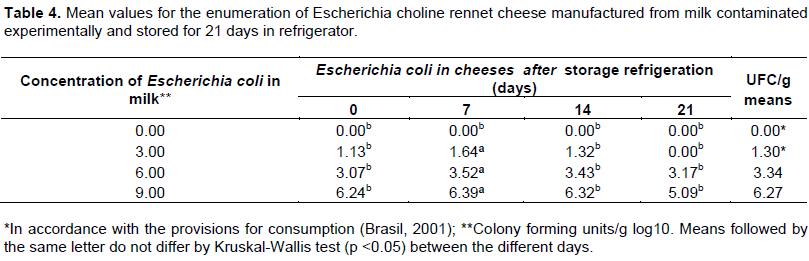
Although, prepared with experimentally contaminated milk, a reduction in the count of colonies in E. coli was observed in the process of preparation. For concentration of initial contamination of 3.00 cfu/g, this reduction allows the cheese within the bacteriological standards recommended by current legislation (Brasil, 2001) for E. coli. Differently, in concentrations 6.00 and 9.00 UFC/g in log10, there was a reduction of 3.00 UFC/g for cheese after processing for all the earlier mentioned permitted laws, even leading to a reduction of the initial counting. The use of milk, which is possible is not recommended to detect E. coli, even with low counts of E. coli to be used in the preparation of cheeses such as curd, even if baked for 25 min at 55°C, which is prepared in productive environment controlled by good agricultural practices (GAP) with the implementation of the analysis of critical control points (ACCP) in the process operational. Due to the fact that the rennet cheese is a nutritious food with sodium compatible of microbial development, there is need to keep the cold chain for their storage, because E. coli for low levels, they may remain viable in cheese manufactured from contaminated milk, even after storage for 21 days under refrigeration and is therefore able to multiply up to unacceptable levels.
The authors have not declared any conflict of interests.
REFERENCES
|
Bier O (1981). Bacteriologia e imunologia: em suas aplicações à medicina e à higiene, Melhoramentos: São Paulo,
|
|
|
|
Castanie-Cornet M-P, Penfound TA, Smith D, Elliott JF, Foster AW (1999). Control of Acid Resistance in Escherichia coli. J. Bacteriol. 181(11):3525-3535.
|
|
|
|
|
Cavalcante JFM, Andrade NJ, Furtado MM, Ferreira CLLF, Pinto CLO, Elard E (2007). Processamento do queijo de coalho regional empregando leite pasteurizado e cultura lática endógena. Ciênc. Tecnol. Aliment 27:205-214.
Crossref
|
|
|
|
|
Farrokh C, Jordan K, Auvray F, Glass K, Oppegaard H, Raynaud S, Thevenot D, Condron C, DeReu K, Govaris A, Heggum K, Heyndrickx M, Hummerjohann J, Lindsay D, Miszczycha S, Moussiegt S, Verstraete K, Cerf O (2013). Review of Shiga-toxin-producing Escherichia coli (STEC) and her significance in dairy production. Int. J. Food Microbiol. 162(2):190-212.
Crossref
|
|
|
|
|
Fogolari O, Reis CZ, Philippi LS (2012). Determining kinetic parameters for thermal inactivation of Escherichia coli in sewage sludge. Eng. Sanit. Ambient. 17(3):255-262.
Crossref
|
|
|
|
|
Forsythe SJ (2003). Microbiologia da Segurança Alimentar. 2. Ed. Porto Alegre: [s.n.], 607p.
|
|
|
|
|
Grzadkowska D, Griffiths MW (2001). Cryotolerance of Escherichia coli O157:H7 in laboratory media and food. J. Food Sci. 66(8):1169-1173.
Crossref
|
|
|
|
|
Jordan KN, Davies KW (2001). Sodium chloride enhances recovery and growth of acid-stressed E. coli O157:H7. Lett. Appl. Microbiol. 32(5):312-315.
Crossref
|
|
|
|
|
Koneman E, Allen S, Janda W, Win Jr W, Procop G, Schreckenberger P, Woods G (2010). Diagnóstico Microbiológico - Texto e Atlas Colorido. 6. Ed. São Paulo: 1465p.
|
|
|
|
|
Machado TF, Borges MF, Oliveira FEM, Sousa CT (2011). Isolamento e Identificação de Patógenos em Queijo de coalho. Boletim de pesquisa e desenvolvimento/Embrapa Agroindústria Tropical. Fortaleza: 16p.
|
|
|
|
|
Mangueira TFB, Travassos AER, Moreira RT (2002). Teste de aceitabilidade sensorial de queijo de coalho com baixo teor de gordura e enriquecimento com ferro. B.CEPPA 20(2):279-290.
|
|
|
|
|
Mogensen G, Salminen S, O'brien (2003). Food microorganisms - health benefits, safety evaluation and strains with documented history of use in foods. Bull. Int. Dairy Fed. 377:4-9.
|
|
|
|
|
Nassu RT, Araújo RS, Borges MF, Lima JR, Macêdo BA, Lima MHP, Bastos MSR (2001). Diagnóstico das condições de processamento de produtos regionais derivados do leite no Estado do Ceará. Boletim de Pesquisa e Desenvolvimento1/Embrapa Agroindústria Tropical. Fortaleza: 28p.
|
|
|
|
|
Oliveira ABA, Paula CMD, Capalonga R, Cardoso MRI, Tondo EC (2010). Foodborne diseases, main etiologic agents and general aspects: a review. Rev HCPA 30(3):279-285.
|
|
|
|
|
Perry KSP (2004). Cheese: chemical, biochemical and microbiological aspects. Quim. Nova 27(2):293-300.
Crossref
|
|
|
|
|
Pigatto CP, Schocken-Iturrino RP, Fadel-Pichetch CMT, Chioda TP, Vittori J, Marin JM (2009). Viabilidade de Escherichia coli produtora de toxina shiga (STEC) não-O157 em queijo tipo minas frescal. Ciência Anim. Bras. 10(2):663-668.
|
|
|
|
|
Poonnoy P, Klayroung S, Tanongkankit Y (2014). Time and temperature on E. coli survival during hot water treatment of spoons. Food and Appl. Biosci. J. 2(2):135-142.
|
|
|
|
|
Romero E, López-Malo A, Palou E (2011). Escherichia coli de tipo patógeno en alimentos y modelación de suinactivaciónal aplicar diversos factores de conservación. Temas selectos de ingeniaría de alimentos 5:28-39.
|
|
|
|
|
São Paulo - Brasil (2010). Instituto Adolfo Lutz. Métodos físico-químicos para análise de alimentos: normas analíticas do Insitituto Adolfo Lutz. 5. ed. Brasília: Agência Nacional de Vigilância Sanitária; 2010. 1018p.
|
|
|
|
|
Santana RF, Santos DM, Martinez ACC, Lima AS (2008). Qualidade microbiológica de queijo-coalho comercializado em Aracaju, SE. Arq. Bras. Med. Vet. Zootec. 60(6):1517-1522.
Crossref
|
|
|
|
|
Usajewicz I, Nalepa B (2006). Survival of Escherichia coli O157:H7 in milk exposed to high temperatures and high pressure. Biotechnol. 44(1):33-39.
|
|
|
|
|
Vernozy-Rozand C, Mazuy-Cruchaudet C, Bavai C,Montet MP, Bonin V, Dernburg A, Richard Y (2005). Growth and survival of Escherichia coli O157:H7 during the manufacture and ripening of raw goat milk lactic cheeses. Int. J. Food Microbiol. 105(1):83-88.
Crossref
|
|



