ABSTRACT
Some rhizospheric fungi of Solanum lycopersicum were isolated from Cairo governorate and identified in Taxonomy Department, Ain-Shams University, Cairo City, Egypt. These isolated fungi were cultivated on soil extract medium enriched with 2% (w/v) glucose and 5% (w/v) yeast extract. The cytological effects of the metabolites of these fungi on the seeds of S. lycopersicum were examined after three different intervals (6, 12 and 24 h). These cytological effects include three parameters; chromosomal aberrations, nucleic acids content, protein banding patterns. Moreover, ochratoxin A was determined in the fungal filtrate of the most effective isolate using HPLC technique.
Key words: Rhizospere, fungi, Solanum lycopersicum, mitotic index, chromosomal aberrations, nucleic acids, protein patterns.
The rhizosphere environment is composed of the interacting area of the plant, the soil immediately adjacent to the root and the organisms related to the roots. The rhizosphere is the region in which the roots must compete with the attacking roots ofadjacentplant species for space, water and mineral supplements and with soilbornemicroorganisms (Ryan et al., 2001).
Local changes within the rhizosphere can include the growth and development of neighboring plant species and microorganisms. Wu et al. (2006) found that the presence of plant growth-promoting rhizobacteria, including nitrogen-fixing bacteria as well as phosphate and potassium solubilizers enhanced plant growth and protected the plant from metal toxicity.
The role played by the fungal metabolites on the host plants was investigated by many workers. Some researchersaffirmed the positive role of common dandelion roots in the aggregation of organic carbon in rhizosphericarea (Kobierski et al., 2018). On the other hand, some of these fungal metabolites have a cytotoxic effect to plants.
Mycotoxins are secondary metabolites that are produced
from different fungal strains; these compoundsare responsible for diseases in animals. Their known toxic syndromes are known as “Mycotoxicoses”.
Similarly, Anninou et al. (2014) found that that certain mycotoxins of Aspergillus and Penicillium spp. are toxic and causes important genotoxic efficacy at rare concentrations. Moreover, De Ruyck et al. (2015) stated that some mycotoxins as ochratoxin A (OTA) and deoxynivalenol (DON) are reported to interfere with mammalian cellular processes including DNA replication and protein synthesis. In this regard, Limbeck et al. (2018) supports a direct link between inhibition of HATs and associated loss of histone acetylation as the key molecular initiating event and repression of gene expression as a prominent cellular response to OTA.
The major goal of this work is to study the cytological changes occurring in Solanum lycopersicum seeds which results from their pre-treatment with the fungal filtrate of some rhizospheric fungi. These cytological changes include chromosomal aberrations, mitotic activity, protein patterns and nucleic acids content.
Experimental fungi
The rhizospheric fungi were isolated from Cairo governorate and identified in Taxonomy Department, Ain-Shams University, Cairo City, Egypt. These fungi are Aspergillus ustus,Aspergillus ochraceous, Cladosporium sp., Trichoderma sp., Fusarium mersmoides, and Ulocladium sp.
Media used
Czapek,sDox agar medium, Soil extract medium (1 kg garden soil with 1 L tap water were mixed and autoclaved, and the extracted filtrate was enriched with 2% (w/v) glucose and 5% (w/v) yeast extract).
Chemicals used
Chemicals for cytology: Carnoy's fixative solution (1 part of glacial acetic acid to 3 parts of absolute ethyl alcohol), the hydrolyzing solution (1 N - HC1),and Leucobasicfuchsin.
Chemicals for electrophoresis: Acetonitrile fine grade HPLC, Methanol HPLC, chloroforme, Trifluroacetic acid (TFA), Anhydrous sodium sulfate,Zinc sulfate, hexane.
Chemicals for nucleic acids determination: Trichloroacetic acid (TCA) 5% (w/v), Methanol: chloroform (1: 2 v/v), 1N-KOH, 6N-HCl, Orcinol freshly prepared, Diphenylamine (DPA), and Acid reagent
Isolation and inoculation
Experimental fungi were isolated from the root area and the root surface of the tomato (Solanum lycopersicum) in Cairo governorate. Isolation from the root surface was done by making sections in the roots after surface sterilizing by 5% aqueous sodium hypochlorite solution for 3 min. These sections were plated into Petri dishes containing CzepekDox medium. Isolation from the soil surrounding the roots was done by plate dilution method. 1 ml of spore suspension of each of the isolated fungi were inoculated on 250-ml Erlenmeyer flasks containing 100 ml of sterilized soil extract medium enriched with 2% (w/v) glucose and 5% (w/v) yeast extract.
Cytological preparation
For cytological analyses, seeds of the studied plant were soaked in 10 ml fungal filtrate (fungal filtrate) of the experimental fungi for three different intervals 6, 12 and 24 h and then germinated in Petri dishes lined with several layers of filter paper moisture with water. Roots of the seedlings were cut off and fixed immediately in Carnoy fixative for 24 h. Control treatment (water and media) was also done. The Feulgen squash technique was used for the cytological preparation. The prepared slides were examined and photographed microscopically using Carl Zeiss photomicroscope III at a magnification of x2000.
Mitotic indices (M. I.) and mitotic stage indices (M. S. I.) of the roots of treated and untreated seedlings were calculated.
Polyacrylamide gel electrophoresis
In the present study, the seedlings proteins were analyzed in acrylamide slab gels using discontinuous polyacrylamide gel electrophoresis method as described by Laemmli (1970).
Quantitative estimation of nucleic acids
RNA and DNA were determined according to Morse and Carter (1949). Breifly, 0.5 g air dried plant tissue was ground with 5% (w/v) ice cooled trichloroacetic acid (TCA) and centrifuged. The precipitate was washed three times with 2ml volumes of 5% (w/v) TCA. The initial supernatant and the washings were combined to form fraction (A) which contains ice-cooled soluble compound.The residue after fraction (A) was extracted three times with 5 ml of methanol: chloroform mixture (1:2) for complete delipidation. The dilapidated tissue was solubilized in 2.0 ml of 1N-KOH at 37°C for 16-20 h and the solubilized material was then reprecipitated with 0.4 ml of 6N-HCL and centrifuged. The residual precipitate is the protein and DNA fractions while the supernatant contains RNA.
Extraction of mycotoxins
The tested fungi was grown on Czapek,s Dox agar medium for 14 days at 28°C. The fungal filtrate of the tested fungi was harvested by filtration through Whatman filter paper (No.4); thereafter the filtrate was extracted with chloroform by mixing equal volume for each and shaking in separating funnel and filtered. Chloroform extract was dried by addition of anhydrous sodium sulfate and evaporated to dryness under nitrogen. The residue was transferred to vial and evaporated off using a steam of nitrogen at temperature below 60°C according to Munimbazi and Bullerman (1998). The dry film was used for toxin determinations by high performance liquid chromatography (HPLC) using different mycotoxins control samples.
Statistical analysis
All experiments were performed in triplicates and the mean values were presented as ± standard(SD).
Soil management has become a crucial matter in order to prevent and/or reduce dangers that are being caused to agricultural sustainability in terms of plant development, yield and health. Widespread varieties of microorganisms including bacteria, archaea, fungi and other microbes are found inhabiting the rhizosphere with various interactions and with the plant host (Odelade and Babalola, 2019).
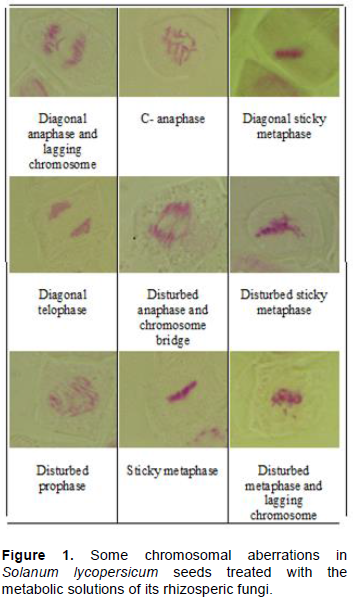
Chromosomal aberrations
All treatments caused different types of abnormalities especially, in seeds treated by the fungal filtrate of A. ochraceus. This can be attributed to mycotoxin production by this strain. High amounts of abnormalities were recorded at metaphase and ana-telophase compared to prophase (Figure 1). The most common abnormalities, observed mostly at all mitotic phases are disturbance then stickiness. Other abnormalities resulting from mitotic disturbance include the diagonal mitotic phases, disturbed configurations, and chromosome lagging. These abnormalities are generally regarded to be the result of the disturbance of the spindle apparatus. All cells in plants are driven by two interactive and yet independent metabolic activities of two cycles; the cell cycle and the growth cycle. Interference with cell cycle affect cell division and several chemicals are known to inhibit cell division by preventing the progression of cells from G2 phase to mitosis or from G1 to DNA synthesis phase (S-phase). In this regard, Badr et al. (1995) noted similar effects with mixture of aflatoxins on Vicia faba root meristems and suggested that bridges may also result from chromosomal stickness while micronuclei may result from lagging chromosome and chromosome fracturing as induced by aflatoxins. Similarly, Agar and Alpsoy (2005) found that all concentrations of AFG1 increased the frequencies of abnormalities such as C-mitosis, chromosomal stickiness, anaphase and telophase bridges. Also, Zheng et al. (2018) reported that ZEA can cause cell death through inducing cell cycle arrest, oxidative stress, DNA damage, mitochondrial damage, and apoptosis.
Electrophoretic pattern of seedlings protein
It was clear from Figure 2(a, b and c) that the highest number of protein bands (17) was observed in the seedlings treated with the fungal filtrate of A. ochraceus and Cladosporium while the lowest number of protein bands (14) was observed in the seedlings treated with the fungal filtrate of Trichoderma sp. At 12 h, the highest number of protein bands (20) was observed in the seedlings treated with the mixture of the fungal filtrate of the experimental fungi while the lowest number of protein bands (12) was observed in the seedlings treated with the fungal filtrate of A. ochraceus. At 24 h, the highest number of protein bands (18) was observed in the seedlings treated with the fungal filtrate of Trichoderma sp while the lowest number of protein bands (12) was observed in the seedlings treated with the fungal filtrate of Cladosporium. Moreover, it was found that these rhizospheric fungi caused the appearance of new bands and disappearance of others.Also, fromthe reproductive toxicity and teratogenic, nephrotoxic, hepatotoxic and embryotoxic effects of citrinin, it was stated that mycotoxins can interfere in the cascade of cell machinery and thus affect cellular function.
This does not provide an interpretation for the cause of the induced alterations in the protein electrophoretic profile, but may be considered as a valid support for the use of this approach to identify mutants and to indicate the mutagenic potential of genotoxic agents. On this account, KÅ‘szegi and Poór (2016) found that ochratoxin A (OTA) was thought to have an important role in inhibition of protein synthesis and energy production, induction of oxidative stress, DNA adduct formation, as well as apoptosis/necrosis and cell cycle arrest.
Changes in nucleic acids content
In the present work, it has been found that the rhizospheric fungi caused variable effect on the nucleic acids content of the tested plant. It was found that the most effective fungus which caused obvious decrease in DNA and RNA content is A. ochraceus while Trichoderma sp. showed obvious increase in the DNA content and A. ustus caused obvious increase in RNA content of the seedlings as the time of treatments prolonged (Tables 1 and 2).
Similarly, Mally et al. (2005) found that ochratoxin A &B causes DNA-strand breaks in liver, kidney, and spleen of animals. Oxidative DNA damage as a mechanism of OTA-dependent DNA damage is consistent with the absence of lipophilic DNA adducts as assessed by 32P-postlabeling analysis. These data suggest that OTA may cause genetic damage in tissues independent of direct covalent binding to DNA.
With reference to mycotoxin production, it was found by many authors that these toxins inhibit cell division and reduce the levels of DNA and RNA. Ochratoxin A was determined in the fungal filtrates of A. ochraceus using HPLC technique andamounted to26.96 µg/l (Figure 3). In this regard, Ochratoxin A was reported to inhibit the biosynthesis of DNA and proteins (Braunberg et al., 1992). Similarly, Yang et al. (2014) found that OTA-induced DNA damage in HEK 293 cells and could induce cell cycle arrest at the S phase in HEK 293 cells. Also, it was reported by Babayan et al. (2019) that ochratoxin A (OTA), produced by a number of Aspergillus and Penicillium fungal species, may cause some cellular impairment.
The authors have not declared any conflict of interests.
REFERENCES
|
Agar G, Alpsoy L(2005). Antagonistic effect of selenium against aflatoxin G1 toxicity induced chromosomal aberrations and metabolic activities of two crop plants. Botanical Bulletin-Academia Sinica Taipe i46(4):301-305.
|
|
|
|
Anninou N, Chatzaki E, Papachristou F, Pitiakoudis M,Simopoulos C (2014). Mycotoxins' activity at toxic and sub-toxic concentrations: Differential cytotoxic and genotoxic effects of single and combined administration of sterigmatocystin, ochratoxina andcitrinin on the hepatocellular cancer cell line Hep3B. International Journal of Environmental Research and Public Health11 (2):1855-1872.
Crossref
|
|
|
|
|
Babayan N, Tadevosyan G, Khondkaryan L, Grigoryan R., Sarkisyan N, Haroutiounian R, Stopper H (2019).Ochratoxin A induces global DNA hypomethylation and oxidative stress in neuronal cells in vitro. Mycotoxin Research 36:73-81.
Crossref
|
|
|
|
|
Badr A, Sheha AS, Kheiralla ZH, El-Shazly HH (1995). Mutagenic potential of aflatoxin produced by Aspergillusparasiticus and its effect on growth and yield of Viciafaba. Congress Proceedings 2:99-114.
|
|
|
|
|
Braunberg RC, Gantt O, Barton C, Friedman L (1992). In vitro effects of the nephrotoxins ochratoxin A and citrinin upon biochemical function of protein kidney. Archives of Environmental Contamination and Toxicology 22:464-470.
Crossref
|
|
|
|
|
De RuyckK, De Boevre M, Huybrechts I, De Saeger S (2015). Dietary mycotoxins, co-exposure, and carcinogenesis in humans: Short review. Mutation Research/Reviews in Mutation Research 766:32-41.
Crossref
|
|
|
|
|
Kobierski M, Kondratowicz-Maciejewska K, Banach-Szott M, Wojewódzki P, PeñasCastejón JM (2018). Humic substances and aggregate stability in rhizospheric and non-rhizosphericsoil. Journal of Soils and Sediments18(8):2777-2789.
Crossref
|
|
|
|
|
KÅ‘szegi T, Poór M (2016). Ochratoxin A: Molecular interactions, mechanisms of toxicity and prevention at the molecular level. Toxins 8(4):111-111.
Crossref
|
|
|
|
|
Laemmli UK (1970). Cleavage of structural proteins during assembly of head bacteriophage T4. Nature 227:680-685.
Crossref
|
|
|
|
|
Limbeck E, Limbeck E, Vanselow JT, Vanselow JT, Hofmann J, Hofmann J, Mally A (2018). Linking site-specific loss of histone acetylation to repression of gene expression by the mycotoxinochratoxin A. Archives of Toxicology 92(2):995-1014.
Crossref
|
|
|
|
|
Mally A, Pepe G, Ravoori S, Fiore M, Gupta RC, Dekant W, Mosesso P(2005). Ochratoxin A causes DNA damage and cytogenetic effects but no DNA adducts in rats. Chemical Research in Toxicology 18(8):1253-1261.
Crossref
|
|
|
|
|
Morse ML, Carter CF (1949). The synthesis of nucleic acid in cultures of Escerchia coli strains B and B/R. Journal of Bacteriology 58:317-326.
Crossref
|
|
|
|
|
Munimbazi C, Bullerman LB (1998). Isolation and partial characterization of antifungal metabolites of Bacillus pumilus. Journal of Applied Microbiology84:959-969.
Crossref
|
|
|
|
|
Odelade K, Babalola O (2019). Bacteria, fungi and archaea domains in rhizospheric soil and their effects in enhancing agricultural productivity. International Journal of Environmental Research and Public Health16 (20):3873.
Crossref
|
|
|
|
|
Ryan P, Delhaize E, Jones D (2001). Function and mechanism of organic anion exudation from plant roots. Annual Review of Plant Physiology and Plant Molecular Biology 52:527.
Crossref
|
|
|
|
|
Wu SC, Cheung KC, Luo YM,Wong MH (2006). Effects of inoculation of plant growth-promoting rhizobacteria on metal uptake by Brassica jncea. Environmental Pollution140 (1):124-135.
Crossref
|
|
|
|
|
Yang Q, He X, Li X, Xu W, Luo Y, Yang X, Wang Y, Li Y, Huang K (2014). DNA damage and S phase arrest induced by ochratoxinA in human embryonic kidney cells (HEK 293). Mutation Research 765:22-31.
Crossref
|
|
|
|
|
Zheng W, Wang B, Li X, Wang T, Zou H, Gu J, Yuan Y, Liu X, Bai J, Bian J, Liu Z (2018). Zearalenone promotes cell proliferation or causes cell death? Toxins 10(5):184.
Crossref
|
|