ABSTRACT
Cowpea can obtain N through biological nitrogen fixation (BNF) through symbiosis with rhizobacteria. However, nodulation and BNF are influenced by edaphoclimatic factors that may bring about benefits or damages to the process, and the availability of nutrients is among the main factors that affect BNF and phosphorus (P). Thus, the present study aimed to determine the effectiveness of doses and sources of soluble P on nodulation, accumulation of nutrients, N and P absorption and use efficiency in cowpea plants inoculated with or without rhizobacteria. The experiment was conducted in a greenhouse at the Federal Institute of Education, Science and Technology, Rondônia, Colorado do Oeste-RO Campus, Brazil. The experimental design was randomized blocks in a 5 x 2 x 2 factorial arrangement, corresponding to five P rates (0, 20, 40, 80 and 160 kg ha-1 of P2O5), two soluble sources of P2O5 [single superphosphate (SSP) and thermophosphate], absence and presence of inoculation, with four replication. The findings of this study show that inoculation with Bradyrhizobium sp. promotes increment in the dry matter production and increases N and P absorption efficiency in cowpea plants. The single superphosphate led to higher N and P absorption efficiency, production of shoot dry matter and production of nodules, when compared with thermophosphate. Inoculation with Bradyrhizobium sp. associated with SSP fertilization promotes higher P absorption efficiency in cowpea plants. Therefore, increase in P rates promoted increments in P concentrations in cowpea leaves.
Key words: Vigna unguiculata (L.), Bradyrhizobium, phosphorus, fertilization, nutrition.
Cowpea [Vigna unguiculata (L.) Walp], also known as “feijão-de-corda”, constitutes one of the main leguminous crops cultivated in Brazil, predominantly in the north and northeast regions. The crop is used for food purposes as the main source of protein for low-income populations (Freire et al., 2005). It is estimated that 369.5 thousand hectares of land are used for cowpea cultivation but, mean yield of approximately 207 kg ha-1 can be achieved in Brazil (Conab, 2016). The participation of the cowpea crop in the North region in terms of, cultivated area and production is still low, in spite of the fact that, its yield is virtually equal to the national mean. Among the various factors that contribute to the low mean yield of this crop is, management of soil fertility, particularly, insufficient supply of nitrogen (N).
Nitrogen is the nutrient required in largest amount by the bean crop and mineral fertilization is the main form of N supply to plants. However, the application of mineral N in tropical soils usually has low recovery efficiency by plants, normally lower than 50%, and may be limited to 5 to 10% in certain situations in sandy soils, due to the great losses through leaching and volatilization (Duque et al., 1985).The cowpea crop, through the symbiosis of bacteria of the genus Bradyrhizobium, can obtain N through the process of biological N fixation (BNF), and is one of the forms to increase the yield of leguminous plants; thus, avoiding costs with soluble N fertilizers (Franco et al., 2002).
BNF is shown to be efficient in cowpea and if well nodulated, can achieve high yield levels (Rumjanek et al., 2005). Guimarães et al. (2015) reported that cowpea responded significantly to inoculation, with increase in height of plants, number of pods, grain number, number of nodules, length of pods, dry mass of nodules, grain dry mass and dry mass of aerial part which is equal to or greater than the nitrogen fertilization. However, nodulation and BNF are influenced by edaphoclimatic factors that can bring benefits or damages to the process.
The availability of nutrients is among the main factors that affect BNF and phosphorus (P) is among the main nutrients that influence this process. Although, P is extracted in smaller amounts when compared with other macronutrients, it is important for the establishment of nodulation, because it increases the number of root hairs and promotes more sites of infection for N2-fixing bacteria (Nkaa et al., 2014). The efficiency of N2 fixation is dependent on the availability of P due to its participation in the symbiotic process (Burity et al., 2000).
Among the existing sources of soluble P, single superphosphate (SSP), triple superphosphate and thermophosphate are considerably used and high doses are applied in highly weathered soils, due to the process of adsorption to clay minerals and iron and aluminum oxides. SSP has the advantage of adding S to the soil and, consequently, meeting the requirements of the plants regarding this element, while thermophosphate adds Ca and Mg to the soil. However, due to the high costs of N fertilizers which resource rural poor farmers cannot afford and coupled with losses of N in the soil, contributing to environmental pollution, it becomes necessary to search for techniques that can maximize its use efficiency.
The use of atmospheric N through BNF, performed by diazotrophic bacteria in symbiosis with various leguminous species, such as cowpea, can be an important alternative in the total or partial substitution of N fertilizers (Bonilla and Bolanos, 2009). Although, the application of N2-fixing bacteria in cowpea is important, studies on its behavior in the Amazon region of Rondônia are scarce. Thus, due to the lack of conclusive results, further studies are necessary to evaluate the efficiency of diazotrophic bacteria and P sources and doses in this region. Therefore, the study aimed to determine the effective rates and sources of soluble P to enhance nodulation, accumulation of nutrients and N and P absorption and use efficiency in cowpea plants inoculated with or without rhizobacteria from soils of the North region of Brazil.
The experiment was carried out in a greenhouse at the Federal Institute of Education, Science and Technology, Rondônia, Colorado do Oeste-RO Campus, Brazil, at the geographic coordinates of 13° 06' S and 60° 29' W, with mean altitude of 407 m. The climate of the region, according to Köppen’s classification, is Awa, tropical hot and humid with two well defined seasons. The soil sample used in the study was classified as Red Yellow Argisol with very clayey texture (EMBRAPA, 2013), collected in the layer of 0 to 20 cm. The chemical analysis of the soil before the experiment resulted in the following values: organic matter (OM): 10.00 g dm-3; pH (CaCl2): 5.30; P: 1.10 mg dm-3; K: 0.14 cmolc dm-3; Ca: 5.56 cmolc dm-3; Mg: 1.15 cmolc dm-3; Al: 0.0 cmmolc dm-3; H+Al: 2.25 cmolc dm-3; SB: 6.90 cmolc dm-3; cation-exchange capacity (CEC): 9.10 cmolc dm-3 and base saturation: 75.30%.
Granulometric analysis showed 199 g kg-1 of sand, 166 g kg-1 of silt and 635 g kg-1 of clay. The experimental design was randomized blocks in a 5 x 2 x 2 factorial scheme, corresponding to five doses of P, two soluble sources of P2O5 and absence and presence of inoculation, with four replicates. P doses were 0, 20, 40, 80 and 160 kg ha-1 of P2O5, applied at planting. The utilized sources of P2O5 were SSP (21% P2O5) and thermophosphate (MG Yoorin) in powder (19% P2O5).
Based on the results of the soil chemical analysis, basal fertilization was performed to guarantee the establishment of the crop. The 60 kg ha-1 of K2O was applied in the form of potassium chloride. P and K doses were converted to mg kg-1 using values of soil density.
The micronutrients were applied according to crop demand, in the form of a solution, using deionized water and salts (p.a.), according to Epstein and Bloom (2006). The experimental units consisted of plastic pots with capacity of 10 dm-3 filled with air-dried soil and passed through a 4 mm-mesh sieve. The moisture content in the pots was controlled daily by weighing in order to maintain the soil with 60% of field capacity. Irrigation was performed with distilled water.The seeds of cowpea, cultivar ‘BRS Tumucumaque’, were inoculated with the commercial inoculant ‘TotalNitro Feijão Caupi’ (concentration of 109 cells g-1) of the strain Bradyrhizobium sp. (Semia 6462 and Semia 6463), produced by the company, Total Biotechnology.
The utilized dose was 150 mL of liquid inoculant for every 5 kg of seeds. For increase in the diazotrophic bacteria population introduced in the plant and, and consequently, increase in the beneficial effects on the host plant, humic acids were applied together with the inoculation, which were extracted and provided by the Laboratory of Biotechnology of the University of Norte Fluminense – UENF, established in the municipality of Campos dos Goytacazes-RJ, and isolated from vermicompost (Canellas et al., 2005). The material was previously dissolved in water at 50 mg L-1. The humic substance was directly applied on the seeds, inside plastic bags with a volumetric pipette. After application, the plastic bags were closed and agitated vigorously for 2 min for a homogeneous distribution of the product on the seeds. The seeds were placed to germinate directly in the pots and thinning was performed five days after emergence (DAE), leaving only one plant in each experimental unit.
Evaluations were performed at 45 DAE, which corresponds to the phenological stage of full flowering of the crop. Plant height was measured from the base of the plant to the apical meristem, using a graduated ruler, while stem diameter was determined using a digital caliper, at the height of 2 cm from the base of the plant. Plants were later collected and divided into roots and shoots. After that, all the collected plant materials were washed in running water and deionized water, respectively. Root length was determined with a graduated ruler and root volume through the volumetric cylinder method, in which the roots were submerged in a graduated cylinder with a known volume of distilled water and root volume was determined by the difference between the initial and final volumes of the cylinder.
During the period, the nodules were removed, counted and dried in an oven at 65°C for 72 h, for later determination of their mass. After drying the plant material, the dry matter was weighed and ground in a Wiley-type mill, and the samples were subjected to sulfuric and nitric-perchloric digestion, for the determination of N and P contents in the different plant parts (roots and shoots), according to the methodology described by EMBRAPA (2009).The absorption efficiency index, the ratio between the total content of nutrient in the plant and root dry matter, was calculated according to Swiader et al. (1994), while N and P use indices, ratio between the total dry matter produced and the total accumulation of nutrient in the plant were calculated according to Siddiqi and Glass (1981). The data were subjected to normality test (Shapiro Wilk) and analysis of variance using the computational program for statistical analysis SISVAR. The effects of inoculation, for each source of the nutrient, were evaluated by Tukey test at 0.05 probability level. For the variable with statistical significance as a function of P doses, regression analysis was used for the Student’s t-test.
There were significant effects (p≤0.05) of the interaction of inoculation (I) and P sources (P) on the P content in the shoots, total P in the plant and P absorption efficiency. The other results had no significant effect of interaction and are independently presented for each source, dose and inoculation (Tables 1 and 2). Inoculation with rhizobacteria in the presence of humic acids influenced plant height, shoot dry matter, root dry matter, N content in the roots, P content in the roots, total N in the plant, N absorption efficiency and P absorption efficiency (Table 3). Plants inoculated with Bradyrhizobium sp. (150 mL of liquid inoculant for every 5 kg of seeds) showed increase in plant height, shoot dry matter production and root dry matter production in the order: 20.44, 12.55 and 23.74%.



This occurred because, normally, plants accumulate biomass until reproduction stage and, from this stage on, senescence begins, with consequent gradual decrease in biomass. Corroborating the results found in the present study, Zilli et al. (2009, 2011) cultivated cowpea under field conditions and in greenhouse, respectively, and observed higher results of shoot dry matter in treatments inoculated with strains recommended for the cowpea crop. In a similar study, Araújo et al. (2010) evaluating cowpea plants inoculated with Bradyrhizobium strain BR3262 in red latosol, observed higher values of root dry matter in inoculated treatments, when compared with the control (without inoculant or fertilization) and the treatment fertilized with N, P and K. Frigo (2013) reported that the production of root dry matter in the treatment inoculated with the strainBR 3267 was approximately 3% higher than that in the fertilized treatment.
The distribution of dry matter in the plant is a variable that allows discussion on a little-studied process, which is the translocation of photoassimilates, and which often facilitates the understanding of the response of plants in terms of yield (Benincasa, 2003). Gualter et al. (2011) pointed out that N-fixing bacteria can significantly contribute to great supply of N to the plant and, consequently, with increase in plant dry matter. The number of nodules in the inoculated treatment was approximately 161, showing an increase of 7.32% in relation to the control performed at 45 DAE. These values for number of nodules demonstrate a satisfactory nodulation for the utilization of cowpea cultivar. Xavier et al. (2007) reported a significant increase in the number of nodules up to 50 DAE, but the number reduced after 60 DAE, while the dry mass of the nodules and specific mass of nodules significantly increased up to 70 DAE. This is explained by the fact that the older nodules began to undergo senescence and then decomposition, resulting in the decrease of the number of nodules after 56 days.
The N content of the roots, P content of the roots, total N of the plant, N absorption efficiency and P absorption efficiency increased by approximately 38.82, 33.92, 12.01, 40.29 and 37.46%, respectively, with the inoculation of Bradyrhizobium sp. in relation to the non-inoculated control as a result of the beneficial effects of rhizobacteria on N and P assimilation by cowpea plants (Table 3). The accumulated amounts of N and P tended to follow the production of dry matter. Hence, it is proved that the population of rhizobium inoculated in the cowpea was able to perform symbiosis and provide the N necessary for the initial development of the crop, and that the inoculant was effective in the process of biological fixation, promoted higher N and P fixation by the roots, when compared with the control treatment (without P or inoculation), and presented itself as a viable alternative for N fertilization in the crop. Corroborating the observed results, Brito et al. (2011) reported that BNF provided the largest part of the N accumulated in cowpea plants.
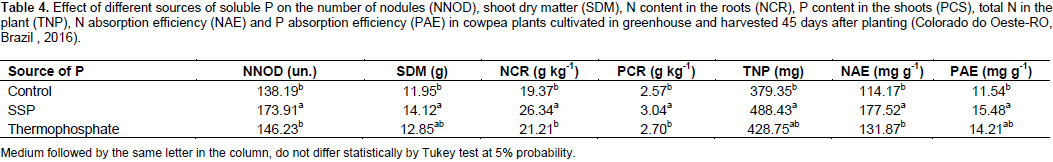
The effect of the different sources of soluble P was significant (p<0.05) on the number of nodules, shoot dry matter, N content in the roots, P content in the shoots, total N in the plant, N absorption efficiency and P absorption efficiency in cowpea plants (Table 4). The single superphosphate (SSP) showed significant difference in relation to the control and the thermophosphate. With regards to the number of nodules, the source SSP contributed to the nodulation which is satisfactory and these results are similar to those obtained by Xavier et al. (2008) and Gualter et al. (2011) in cowpea. These results demonstrated that, besides promoting higher P absorption, the application of SSP can also promote a better use of this element by the plant.
The effect of interaction between inoculation and P sources on P content of the shoots, total P in the plant and P absorption efficiency is presented in Table 5. It is observed that the available SSP in the presence of inoculation with Bradyrhizobium sp. promoted increase in P content of the shoots, total P in the plant and P absorption efficiency in cowpea plants. These increases were approximately 21.87, 18.77 and 52.07%, respectively, and there was no significant difference for the source thermophosphate in the presence or absence of inoculation. The source, SSP was also superior to the thermophosphate, because it is more efficient in increasing the P contents of the plant and its absorption efficiency.

The cowpea crop has the characteristic of low P requirement, but it has demonstrated higher and frequent response when cultivated in soil with good availability of the nutrient. In the present study, the P content in the soil was equal to 1.10 mg dm-3, a content considered as very low, which justifies the absence of response to most variables as a function of the increasing doses of P in the soil. Only leaf P contents and the total P in the plant were significantly (p<0.05) influenced by the P doses (Figure 1). This occurred due to the greater availability of this nutrient. The highest contents of P in cowpea leaves were obtained with the application of 160 kg ha-1of P2O5, a rate which is more than that recommended for maintenance fertilization of cowpea in soils of Rondônia. These contents are within the required range considered as adequate for cowpea, which is from 2.6 to 5.0 mg kg-1 (Malavolta et al., 1997).

Increases in P contents of cowpea leaves in response to P doses were also observed by Silva et al. (2010) that studied doses and forms of application of P in yellow latosol in the state of Roraima. Besides promoting higher P absorption, the application of P also promotes increases in the total P in the plant and, consequently provided better use of the nutrient by the plant. This increase in leaf P content with the application of phosphate fertilization can lead to future increase in grain production observed when the crop is cultivated until its phenological stage, considering the previous findings on correlation between leaf contents of nutrients and yields of crops. Although, there are some experimental results, the studies on the relationships between N, P and inoculation in cowpea in the presence of humic acids in the Western Amazon are in the initial stage, and there is a lot to be studied in tests conducted both in greenhouse and under field conditions for comparisons, and to further strengthen the scientific basis for a new technological process for agricultural production.
Inoculation with Bradyrhizobium sp. promotes increase in dry matter production and increase in N and P absorption efficiency in cowpea plants. Single superphosphate leads to higher N and P absorption efficiency, production of shoot dry matter and production of nodules, in comparison with thermophosphate. The inoculation with Bradyrhizobium sp. associated with single superphosphate fertilization promotes higher P absorption efficiency in cowpea plants. The increase in P doses promotes increase in the P contents of cowpea leaves, which will contribute to improvement in cowpea productivity in Brazil.
The authors have not declared any conflict of interests.
The authors thank the Federal Institute of Education, Science and Technology, Colorado Unit from the West for granting financial aid and scholarship to the second author and the Total Biotechnology for the biological inoculants.
REFERENCES
|
Araújo ASF, Carneiro RFC, Bezerra AAC, Araújo FF (2010). Coinoculation rhizobia and Bacilus subtilis in cowpea and Leucaena: Effects on nodulation, N2 fixation and plant growth. Ciência Rural Online. 40(1):1-4. |
|
|
|
Benincasa MMP (2003). Análise de crescimento de plantas - noções básicas. 2.ed. Jaboticabal: Funep, 41p. |
|
|
|
Bonilla I, Bolanos SL (2009). Mineral nutrition for legume-rhizobia symbiosis: B, Ca, N, P, S, K, Fe, Mo, Co, and Ni: A review. Organic Farming, Pest Control Remediat. Soil Pollut. 1:253- 274. |
|
|
Burity HA, Lyra MCCP, Souza ES (2000). Effectiveness of inoculation with Rhizobium and fungos micorrízicos arbusculares in thrush seedlings subjected to different levels of phosphorus. Pesq. Agrop. Braz. 35(3):801-807.
Crossref |
|
|
|
Brazilian Agricultural Research Corporation (EMBRAPA) (2013). National Research Center of soils. Brazilian system of soil classification. 3 Ed. rev. ampl.- Brasília, DF: Embrapa Soil, 353p. |
|
|
|
Brazilian Agricultural Research Corporation-EMBRAPA (2009). Manual of chemical analysis of soils, plants and fertilizers. 2. Ed, Brasília, Embrapa Information Technology, 627p. |
|
|
Brito MMP, Muraoka T, Salva EC (2011) Contribution of nitrogen from biological nitrogen fixation, nitrogen fertilizer and soil nitrogen on the growth of the common bean and cowpea. Bragantia 70(1):206-215.
Crossref |
|
|
|
Canellas L P, Zandonari DB, Médici LO, Peres LEP, Olivares FL, Façanha AR (2005). Bioactivity of humic substances – action on development and metabolism of plants. In: Canellas, L.P; Santos; G.A. Humosfera. Preliminary treaty about the chemistry of humic substances. Campos dos Goytacazes – RJ, pp. 224-243. |
|
|
|
Conab (2016). Monitoring the Brazilian grain Harvest 2015/2016: Tenth survey, July/2016, National supply compan. Brasília, Conab, 179p. |
|
|
Duque FF, Neves MCP, Franco AA, Victoria RL, Boddey RM (1985). The response of fieldgrown Phaseolus vulgaris to Rhizobium inoculation and qualification of N2 fixation using 15N. Plant Soil 88(3):333-343.
Crossref |
|
|
|
Epstein E, Bloom AJ (2006). Mineral nutrition of plants: principles and perspectives. London: Editora Plant, 403p. |
|
|
Franco MC, Cassini ST, Oliveira VR, Vieira C, Tsai SM (2002). Nodulation in Andean and Mesoamerican cultivars of dry bean. Pesquisa Agropecuária Brasileira 37(8):1145-50.
Crossref |
|
|
|
Freire Filho FR, Lima JAA, Ribeiro VQ (2005). Cowpea: technological advances. Brasilia: Embrapa Information Technology. 519p. |
|
|
|
Frigo GR (2013). Cowpea submitted to inoculation with Rhizobium and cultivated in the Cerrado Latosol Matogrossense. Rondonópolis: University State of Mato Grosso, 69 f. Dissertation. |
|
|
Gualter RMR, Boddey RM, Rumjanek NG, Freitas ACR, Xavier GR (2011). Agronomic efficiency of rhizobia strains in cowpea cultivated in the Pre-Amazon region, in Maranhão state. Pesquisa Agropecuária Brasileira 46(3):303-308.
Crossref |
|
|
Guimarães SL, Cardinal MS, Bonfim-Silva EM, Polizel AC (2015). Development of cv. BRS Novaera cowpea inoculated with rhizobium recommended for pigeonpea. Científica 43(2):149-155.
Crossref |
|
|
|
Nkaa FA, Nwokeocha OW, Ihuoma O (2014). Effect of phosphorus fertilizer on growth and yield of cowpea (Vigna unguiculata). J. Pharm. Biol. Sci. 9(5):74-82. |
|
|
|
Rumjanek NG, Martins LMV, Xavier GR, Neves MCP (2005). Biological nitrogen fixation. In: Freire Filho FR, Lima JAA, Ribeiro VQ (Eds.) Cowpea: Technological advances. Brasília: Embrapa. pp. 281-335. |
|
|
Siddiqi MY, Glass ADM (1981). Utilization index: A modified approach to the estimation and comparison of nutrient utilization efficiency in plants. J. Plant Nutr. 4(3):289-302.
Crossref |
|
|
Silva AJ, Uchoa SCP, Alves, JMA, Lima ACS, Santos CSV, Oliveira JMF, Melo VF (2010). Response of cowpea (Vigna unguiculata (L.) Walp.) to phosphorus fertilization levels and application forms in Yellow Latosol of Roraima State/Brazil. Acta Amazônica. 40(1):31-36.
Crossref |
|
|
Swiader JM, Ccyan, Freiji FG (1994). Genotypic differences in nitrate uptake and utilization efficiency in pumpkin hybrids. J. Plant Nutr. 17(10):1687-1699.
Crossref |
|
|
|
Xavier TF, Araújo ASF, Santos VB, Campos FL (2007). Ontogeny of nodulation in two cultivars of cowpea. Ciência Rural 37(2):572-575. |
|
|
Xavier TF, Araújo ASF, Santos VB, Campos FL (2008). Inoculation and nitrogen fertilization on nodulation and grain yield of cowpea. Ciência Rural 38(7):2037-2041.
Crossref |
|
|
Zilli JÉ, Marson LC, Marson BF, Rumjanek NG, Xavier GR (2009). Contribution of rhizobia strains to cowpea development and grain yield in Roraima - Brazil. Acta Amazônica 39(4):749-758.
Crossref |
|
|
|
Zilli JÉ, Neto MLS, Júnior IF, Perin L, Melo AR (2011). Response by Cowpea Bradyrhizobium inoculation with strains recommended for soybeans. J. Braz. Sci. Soil. 35(1):739-742. |