ABSTRACT
Acute lung infection induced by Extended Spectrum β-Lactamases (ESBL) producing isolates was determined by measuring inflammatory mediators; malondialdehyde (MDA), myeloperoxidase (MPO) and nitric oxide (NO). The mice were randomly divided into three groups of 20 animals each. All mice were given 104 c.f.u. ml-1 of the test organism intranasally in a volume of 50 µl while holding the mouse in an upright position without any anaesthesia. Group A received an intraperitoneal injection of an antibiotic, imipenem at a dose of 20 mg ml-1/ 25 g body weight which was administered 48 h post infection, Group B received only normal saline orally while group C, control-mice did not receive any treatment. The animals were sacrificed by cervical dislocation; lungs were removed aseptically and examined for various inflammatory mediators. The MDA, NO and MPO estimations in the lung homogenates in each group was measured and compared. Group treated with imipenem recorded lower absorbance values when compared with group treated with normal saline. The different parameters were statistically significant since the P-values were less than 0.05.
Key words: Isolates, malondialdehyde, myeloperoxidase, nitric oxide, inflammation, imipenem.
Animal flesh is considered one of the most nutritive sources of proteins consumed by humans. The meat is rich in proteins with high levels of amino acids and polysaturated fats (Listratet et al., 2016). Its water content also makes it a good substrate for microbial growth. Tissues of most healthy animals are sterile but may get contaminated by microorganisms during slaughter, dressing and cutting through the knives and other equipment used, through the exterior of the animal, the intestinal tract, the air and the handlers (Cook et al., 2017). The extrinsic factors that determine microbial growth are temperature, moisture content and oxygen availability (Gundogan et al., 2011). It has been reported that Gram negative bacteria account for approximately 69% of bacterial food borne diseases (Cook et al., 2017). A number of foods (meat inclusive) have been reported to have high incidence of bacteria (Overdevest et al., 2014; Casella et al., 2015; Belmahdi et al., 2016). There is, however, limited information on the health implications of food borne diseases associated with ESBL producing organisms. Extended Spectrum β-Lactamases (ESBL) are a group of β-Lactamases which have the ability to hydrolyze third generation cephalosporins such as cefotaxime, ceftazidime and monobactams such as azetreonam (Bush, 2008). They are an increasingly important cause of multidrug resistance in Gram-negative bacteria throughout the world and have coevolved with the β-lactam antibiotics ever since they came into clinical use (Bush and Jacoby, 2010; Doi et al., 2013; Adesokan et al., 2015). The spread of ESBL-producing organisms is greater in developing economies due to poor personal and environmental hygiene conditions; extensive self-treatment, use of non-prescription antimicrobials, very low infection control methods and other numerous reservoirs such as; water, soil, animals, pets and food (Chong et al., 2013; Tansarli et al., 2014; Adenipekun et al., 2015). Animal models of infections caused by ESBL producing organisms have served as a guide to test the efficacy of mono and combination therapies in vitro but in vivo evaluation in some cases give surprising contrast to results obtained in vitro (Bali et al., 2016; Overdevest et al., 2014). All the isolates used in this study are associated with the environment; they are ubiquitous in nature and have the ability to survive long stretches of time under highly desiccated conditions on abiotic surfaces. To the authors knowledge, there is no literature on ESBL induced pulmonary damage in Nigeria, hence, this study was aimed at determining oxidative stress and other inflammatory markers induced by ESBL producing organisms and the effect of imipenem- a carbepenem as a treatment option.
Extended Spectrum Beta Lactamase (ESBL) producing isolates of Stenotrophomonas maltiphilia, Acinetobacter baumanii, Pseudomonas monteilli, Achromobacter ruhlandii and Pseudomonas fulva obtained from swab samples of the intestine of animals killed on the spot in the abattoir and from the surfaces of tables where the meat is sold in an abattoir were used for this study.
Induction of acute lung infection
Healthy albino mice of either sex, 6 to 8 weeks old, weighing 20 to 25 g were used. Acute lung infection in mice was induced with test organism, following the modified method by Yadav et al. (2003). A single isolated colony of test organism obtained on a nutrient agar plate was transferred to 50 ml nutrient broth and incubated at 37°C for 18 h. Bacterial cells were harvested by centrifugation at 5000 rpm. for 15 min. The bacterial pellet so obtained was given three washings with sterile PBS (0.1 M, pH 7.2). The final pellet was suspended in a minimum volume of Phosphate Buffer Solution (PBS) (0.1 M, pH 7.2) to get the desired concentration (OD600 = 0.03 Ω 104 c.f.u. ml ̶1). The mice were randomly divided into three groups (A - C) and each group comprised 20 animals. Groups A, B and C were given 104 c.f.u. ml-1 of the test organism intranasally in a volume of 50 µl while holding the mouse in an upright position without any anaesthesia. After infection, animals were sacrificed on different days post-infection after drug administration by cervical dislocation and lungs were removed aseptically and examined for various inflammatory parameters. The three groups were given one of the following treatments:
A. Mice infected received an intraperitoneal injection of an antibiotic, imipenem at a dose of 20 mg ml-1/ 25 g body weight; it was administered 48 h post infection with test organisms.
B. Mice infected with 104 cfu/ml of the test organisms in a volume of 50 µl received only normal saline orally and no standard drug treatment.
C. Control- Mice were infected but received no treatment.
Quantitative bacterial count in lungs
Mice were sacrificed on different days post-infection by cervical dislocation; lungs were removed aseptically and then homogenized in 1 ml normal saline. Serial dilutions of the homogenized lung tissue were made and plated on nutrient agar plates to determine bacterial load. The plates were incubated at 37°C for 24 h. The lung homogenate from each mouse was also processed for the following parameters.
Malondialdehyde (MDA) estimation
The extent of tissue damage in terms of lipid peroxidation was estimated by measuring the amount of MDA by the method of Ohkawa et al. (1979). In brief, 0.2 ml of the lung homogenate was mixed with 4 ml 0.045 M sulphuric acid, 1.5 ml of freshly prepared 0.8% thiobarbituric acid and 0.2 ml 8.1% SDS. This mixture was then kept in a boiling water bath for 1 h. After cooling the mixture under tap water, 5.0 ml butanol: pyridine (15:1) was added and the mixture was shaken vigorously. The contents were centrifuged at 4000 r.p.m. for 10 min, the upper organic layer was taken in a separate tube and its A532 was taken.
Myeloperoxidase (MPO) estimation
Pulmonary neutrophil infiltration was quantified by measuring the MPO activity using a spectrophotometric method as proposed by Greenberger et al. (1995). Briefly, the lungs were removed, weighed to determine the wet weight and then homogenized in 2 ml homogenizing solution containing 50 mM potassium phosphate buffer (pH 6.0) with 0.5% hexadecyltrimethylammonium bromide and 5 mM EDTA. The homogenate was sonicated and centrifuged at 15,000g for 15 min. The supernatant was mixed in a ratio of 1:15 with assay buffer comprising 100 mM potassium phosphate buffer (pH 6.0), 0.167 mg o-dianisidine hydrochloride ml-1 and 0.0005% hydrogen peroxide. MPO activity was assayed by measuring the change in A460 from 0 min to 4 min over intervals of 30 s.
Nitric oxide (NO) estimation
The nitrite level was estimated in the lung homogenate according to the method of Tsai et al. (1997). Lung homogenate (0.1 ml) was mixed with 0.4 ml PBS (0.1 M, pH 7.2) and 2 ml Griess reagent. Then 2 ml trichloroacetic acid was added and the mixture was vortexed and incubated for 20 min. The mixture was then centrifuged at 14000 g for 10 min and the A540 of the supernatant was taken. The nitrite concentration was determined from a standard curve prepared with 100 mM sodium nitrite.
Statistical analysis
Results were analyzed statistically by applying two way ANOVA for comparing various parameters in treated and untreated control rats. Differences were considered statistically significant if P-values were less than 0.05. The statistical software used was SPSS version 20.0.
Null hypothesis
There is no difference in effect between the rats treated with the antibiotic and the rats which received normal saline.
Alternative hypothesis
There is difference in effect between the rats treated with antibiotic and the rats which received normal saline.
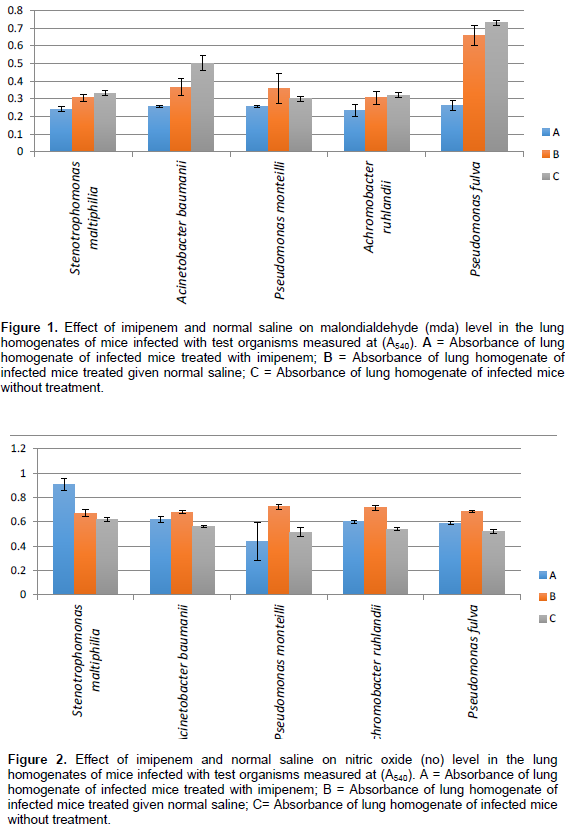
Effect of imipenem and normal saline on malondialdehyde (MDA) and nitric oxide (NO) level
Figure 1 shows the different absorbance values for the MDA levels in the lung homogenate of mice infected with test organisms. Mice infected with Stenotrophomonas maltiphilia showed a reduction in MDA levels when imipenem was administered. Mice infected with the other test organisms also showed a reduction in lung injury as the MDA levels were also reduced when treated with imipenem except for mice infected with Pseudomonas monteilli where treatment with normal saline showed greater efficacy. Lung injury caused by intranasal injection of the mice with the Extended Spectrum Beta Lactamase (ESBL) producing isolates resulted in a significant increase in neutrophil infiltration in the lungs along with increased production of nitric oxide (NO) to cellular injury. Figure 2 shows the treatment response observed. Treatment with imipenem and normal saline had different effects on the mice post infection. The nitric oxide level was highest when the mice were treated with normal saline except in S. maltiphilia and nitric oxide levels remained lowest in the control mice. The effect of time on myeloperoxide (MPO) estimation of lung homogenate of mice infected with different test organisms was determined. Figures 3 to 7 shows the myeloperoxide (MPO) level for each of the isolates.


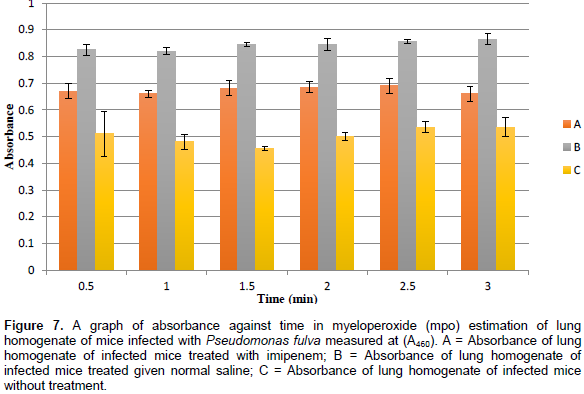
Myeloperoxidase is a phagocytic enzyme found in granulocytes that form halogen ions (OCl-) that are strong oxidizing agents. The effect of time on the myeloperoxidase (MPO) level was determined in the different mice groups for infections caused by the ESBL isolates. There was an increase in MPO level in the mice infected with S. maltiphilia due to lung injury. Treatment with normal saline had no significant effect because an increase in MPO level was observed. Mice group treated with imipenem drastically reduced the MPO level which signifies a reduction in the bactetrial load, that is, inhibition of bacterial proliferation which caused the initial MPO influx to the site of injury. Time had no effect on the MPO level since it remained constant when measured at different time intervals. Infections caused by Acinetobacter baumanni, Achromobacter ruhlandii and Pseudomonas species increased the MPO levels when normal saline was administered post infection as compared to the control group. Treatment with imipenem reduced the MPO level when inflammatory property compared with the results of treatment with normal saline but was higher than the control group.
An attempt was made in this study to confirm the effect of imipenem in an acute lung injury model. On the basis of the results of all the different parameters studied, induction of acute lung inflammation by ESBL producing isolates was observed. Acute inflammation in the lungs is characterized by increased activity of neutrophils (Yamamoto and Pop-Vicas, 2014; Bali et al., 2016). The migration of neutrophils at the site of acute inflammation involves the activity of various inflammatory cytokines and chemokines, and expression of various cell leukocyte and endothelial adhesion molecules (Bansal and Chhiber, 2010; Mehmet et al., 2013). These facts are further confirmed by the production of the inflammatory parameters namely; malondialdehyde (MDA), myeloperoxidase (MPO), nitric oxide (NO) in the cell. Enzyme MPO is also an indirect indicator of neutophil infiltration and its levels measured at different time intervals induced no noticeable change but the lowest production was observed with imipenem treatment. In a similar study by Bansal and Chhiber (2010), mice induced with bacterial lung infection were treated with Augmentin® and curcumin (an immunomodulatory and anti-inflammatory agent) alone and in combination, the results showed that Augmentin® in combination with curcumin reduced the nitric oxide levels better than when Augmentin® was administered alone due to the antiflammatory properties of curcumin but curcumin administered alone had no effect on the lung homogenate.
This suggests that though treatment with imipenem inhibit bacterial proliferation, adjunct therapy with antioxidative and anti-inflammatory properties may also be required (Mun et al., 2013; Betts and Wareham, 2014; Bali et al., 2016). This further confirms other studies on the significance of combined therapy in the treatment of ESBL infections (Iroha et al., 2010; Ikegbunam et al., 2014; Zhu et al., 2015). NO generated during acute infection causes tissue damage by acting as a free radical and by generating more active species such as peroxynitrite which lead to lipid peroxidation of the cell membrane (Ward, 2010) and resulted in acute lung injury (Matthay et al. , 2012; Zhao et al., 2017). The level of cellular injury which might have occurred due to release of reactive oxygen species as a result of lipid peroxidation was also estimated in terms of MDA levels in lung homogenates. Treatment of mice with imipenem alone in the present study also resulted in decreased MDA levels in infected lung tissue thereby reducing lung injury. The results of the present study clearly demonstrate the positive effect of imipenem as reduced acute lung injury associated with pneumonia was observed in treated animals due to decreased production of various inflammatory markers such as NO, MPO and MDA and neutrophil recruitment. The P- values for MDA and NO was .030 and 0.002, respectively.
Pulmonary inflammatory damage caused by induced infection with ESBL producing isolates was susceptible to treatment with imipenem. Although, the observations in vitro and in animal models are not always applicable in clinical practice which may be due to disparate findings and the vast genetic heterogeneity of the organism and because the studies and case series that illustrate the experience with different antibiotics in the treatment of ESBL infections are difficult to interpret and do not have a direct correlation with clinical experiences. But be that as it may, the use of the carbepenems as a monotherapy or in combination suffices for the treatment of infections caused by ESBL isolates after appropriate susceptibility testing.
The authors have not declared any conflict of interests.
REFERENCES
|
Adenipekum EO, Jackson CR, Oluwadun A, Iwalokun BA, Frye JG, Barrett JB, Hiott LM, Woodley TA (2015). Prevalence and antimicrobial resistance in Escherichia coli from food animals in Lagos, Nigeria. Microb. Drug Resist. 21(3):358-365.
Crossref
|
|
|
|
Adesokan HK, Akanbi IO, Akanbi IM, Obaweda RA (2015). Pattern of antimicrobial usage in livestock animals in south-western Nigeria: the need for alternative plans. J. Vet. Res. 82(1):01-06.
|
|
|
|
Bali C, Altintas N, Ozmete O, Gelincik I, Yabanoglu H, Tekin N, Altinsoy B, Turan BC, Aribogan A (2016). Protective Effect of Curcumin on Carbapenem-Resistant Escherichia coli–Induced Lung Injury in Rats. Int. Surg. 101(7):304-312.
Crossref
|
|
|
|
Bansal S, Chhibber S (2010). Curcumin alone and in combination with augmentin protects against pulmonary inflammation and acute lung injury generated during Klebsiella pneumonia B5055-induced lung infection in BALB/c mice. J. Med. Mcb. 59:429-437.
Crossref
|
|
|
|
Belmahdi M, Bakour S, Al Bayssari C, Touati A, Rolain JM (2016). Molecular characterisation of extended-spectrum b-lactamase- and plasmid AmpCproducing Escherichia coli strains isolated from broilers in Algeria. J. Glob. Antimicrob. Resist. 6:108-112.
Crossref
|
|
|
|
Betts JW, Wareham DW (2014). In vitro activity of curcumin in combination with epigallocatechin gallate (EGCG) versus multidrug-resistant Acinetobacter baumannii. BMC Microbiol. 14(1):172.
Crossref
|
|
|
|
Bush K, Jacoby GA (2010). MINIREVIEW Updated functional classification of Beta-Lactamases, Antimic. Agen. Chemo. 5:969-976
Crossref
|
|
|
|
Bush K (2008). Extended-spectrum beta-lactamases in North America, 1987–2006. Clin. Microbiol. Infect. (1):134-143.
Crossref
|
|
|
|
Casella T, Rodriguez MM, Takahashi JT, Ghiglione B, Dropa M, Assunção E, Nogueira ML, Lincopan N, Gutkind G, Nogueira MC (2015). Detection of blaCTX-M-type genes in complex class 1 integrons carried by Enterobacteriaceae isolated from retail chicken meat in Brazil. Int. J. Food Microbiol. 197:88-91.
Crossref
|
|
|
|
Chong Y, Shimoda S, Yakushiji H, Ito Y, Miyamoto T, Kamimura T, Shimono N, Akashi K (2013). Community spread of extended-spectrum beta-lactamase-producing Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis: a long-term study in Japan. J. Med. Microbiol. 62:1038-1043.
Crossref
|
|
|
|
Cook EA, de Glanville WA, Thomas LF, Kariuki S, de Clare Bronsvoort BM, Fèvre EM (2017). Working conditions and public health risks in slaughterhouses in western Kenya, BMC Public Health 17(14):1-12.
Crossref
|
|
|
|
Doi Y, Park YS, Rivera JI, Adams-Haduch JM, Hingwe A, Sordillo EM, Lewis 2nd JS, Howard WJ, Johnson LE, Polsky B, Jorgensen JH (2013). Community-associated extended-spectrum β-lactamase-producing Escherichia coli infection in the United States. Clin. Infect. Dis. 56:641-648.
Crossref
|
|
|
|
Greenberger MJ, Strieter RM, Kunkel SL, Danforth JM, Goodman RE, Standiford TJ (1995). Neutralization of IL-10 increases survivalin a murine model of Klebsiella pneumonia. J. Immuno. 155:722-729.
|
|
|
|
Gundogan N, Citak S, Yalcin E (2011). Virulence properties of extended spectrum beta-lactamase-producing Klebsiella species in meat samples. J, Food Prot. 74:559-564.
Crossref
|
|
|
|
Ikegbunam MN, Anagu LO, Nwakile DC, Afunwa RA, Esimone CO (2014). Antimicrobial Activity of Selected Medicinal Plants of South-Eastern Nigeria on Pseudomonas species Expressing Extended Spectrum Beta Lactamase (ESBL). Eur J. Med. Plants 4(11):1367-1377.
Crossref
|
|
|
|
Iroha IR, Amadi ES, Oji AE, Nwuzo AC, Ejike-Ugwu PC (2010). Detection of Plasmid-Borne Extended Spectrum Beta Lactamase Enzymes from Blood and Urine Isolates of Gram- Negative Bacteria from a University Teaching Hospital in Nigeria. Curr. Res. Bact. 3(2):77-83.
Crossref
|
|
|
|
Listrat A, Lebret B, Louveau I, Astruc T, Bonnet M, Lefaucheur L, Picard B, Bugeon J (2016). How Muscle Structure and Composition Influence Meat and Flesh Quality. Sci. World J. 1-15.
Crossref
|
|
|
|
Matthay MA, Ware LB, Zimmerman GA (2012). The acute respira-tory distress syndrome. J. Clin. Invest. 122: 2731 2740.
Crossref
|
|
|
|
Mehmet FG, Mehmet A, Bakir T, Ahmet K, Hakan C (2013). Serum Myeloperoxidase Activity, Total Antioxidant Capacity and Nitric Oxide Levels in Patients with Chronic Otitis Media. J. mem. Bio. 246:519-524.
|
|
|
|
Mun SH, Joung DK, Kim YS, Kang OH, Kim SB, Seo YS, Kim YC, Lee DS, Shin DW, Kweon KT, Kwon DY. (2013). Synergistic antibacterial effect of curcumin against methicillin-resistant Staphylococcus aureus. Phytomed. 20(8-9):714-718.
Crossref
|
|
|
|
Ohkawa H, Ohishi N, Yagi K (1979). Assay of Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Ana. Bio. 95:351-358.
|
|
|
|
Overdevest IT, Heck M, Van Der Zwaluw K, Huijsdens X, Van Santen M, Rijnsburger M, Eustace A, Xu L, Hawkey P, Savelkoul P, Vandenbroucke-Grauls C (2014). Extended-spectrum beta-lactamase producing Klebsiella spp. in chicken meat and humans: A comparison of typing methods. Clin. Microbiol. Infect. 20(3):251-255.
Crossref
|
|
|
|
Tansarli GS, Poulikakos P, Kapaskelis A, Falagas ME (2014). Proportion of extended-spectrum beta-lactamase (ESBL)-producing isolates among Enterobacteriaceae in Africa: evaluation of the evidence-systematic review J. Antimicrob. Chemother. 69:1177-1184.
Crossref
|
|
|
|
Tsai WC, Strieter RM, Zisman DA, Wilkowski JM, Bucknell KA, Chen GH, Standiford TJ (1997). Nitric Oxide is required for Effective Innate Immunity against Klebsiella pneumonia. Infect. Imm. 65:1870-1875.
|
|
|
|
Ward PA (2010). Oxidative stress: Acute and progressive lung injury. Ann. N.Y. Acad. Sci. 1203(1):53-59.
Crossref
|
|
|
|
Yadav V, Sharma S, Harjai k, Mohan H, Chhibber S (2003). Induction and Resolution of Lobar Pneumonia following Intranasal Instillation with Klebsiella pneumonia in Mice. Ind. J. Med. Res. 118:47-52.
|
|
|
|
Yamamoto M, Pop-Vicas AE (2014). Treatment for infections with carbapenem-resistant Enterobacteriaceae: what options do we still have? Crit Care 18(3):229.
Crossref
|
|
|
|
Zhao M, Li C, Shen F, Wang M, Jia N, Wang C (2017). Naringenin ameliorates LPS induced acute lung injury through its anti oxidative and anti inflammatory activity and by inhibition of the PI3K/AKT pathway. Exper. Therapeu. Med. 14:2228-2234.
Crossref
|
|
|
|
Zhu GF, Guo HJ, Huang Y, Wu CT, Zhang XF (2015). Eriodictyol, a plant flavonoid, attenuates LPS induced acute lung injury through its antioxidative and anti inflammatory activity. Exp. Ther. Med. 10:2259 2266.
Crossref
|