ABSTRACT
This study aimed to evaluate the in vitro antimicrobial effect of exposing raw water intended for human consumption to light (λ= 450 nm) and to investigate the correlation between the results obtained and physical and chemical parameters. Fifteen (15) samples of raw water were collected from households in a rural area of ​​Santo Antônio de Jesus – Bahia (Brazil), from November to December 2016. A 100 mL aliquot of each sample was exposed to a lighting system consisting of two high intensity light emitting diodes, with a wavelength of 450 nm and luminous flux of 200 lumens per 10 h. Quantifications of heterotrophic bacteria, total coliforms and temperature started at time zero and were done every two hours until the end of exposure to light. Bacteriological analysis was repeated after 72 h of being exposed to light. pH, dissolved oxygen and salinity analyses were performed before each experiment. After a 10h illumination at 450 nm light emitting diodes (LEDs), the dosage of light received by the water samples was 581.8 J/cm2. There was a significant reduction in the two bacteriological parameters analyzed after treatment (p = 0.000). There was an average decrease in heterotrophic bacteria counts from 3.44 to 1.86 log CFU/mL and total coliforms from 2.45 to 1.02 log CFU/mL. Mean reductions of heterotrophic bacteria were 97.01% and total coliforms were 95.61%. After 72 h, both counts increased; there was significant growth between heterotrophic bacteria (p = 0.000), but there was no significant growth for total coliforms (p = 0.058). pH (p = -0.981, p = 0.000), dissolved oxygen (ρ = -0.529, p = 0.043) and temperature (ρ = 0.521, p = 0.047) were related to the percentage reduction of heterotrophic bacteria. The method is shown to be effective in disinfecting raw water in vitro under different physical and chemical conditions.
Key words: Blue light emitting diode (LED), indicator microorganisms, potability standards, contaminated water.
Light has been used for decades as a bacterial inactivation tool in several areas. Sunlight is used for
water disinfection because it has combined antimicrobial action of ultraviolet A (UVA), is responsible for the alteration of microorganisms’ DNA and infrared radiation that causes water temperature to increase. However, with low light intensity, the process is not effective (Boyle et al., 2008; Mcguigan et al., 2012).
Artificially produced ultraviolet (UV) radiation is also used for water treatment. Ultraviolet band C (UVC - 254 nm) is used as germicide. However, in sublethal doses, microorganisms can recover their metabolic activity, in addition to requiring a physical barrier to protect the operator due to its carcinogenic potential (Wisbeck et al., 2011; Di-Bernardo et al., 2017; Mbonimpa et al., 2018).
However, recently, with the introduction of new technologies, researches evaluating the antimicrobial effect of light emitting diode (LED) in visible light spectrum and ultraviolet light spectrum have emerged (Maclean et al., 2014). A LED is a compact electronic device that emits light within a monochromatic wave-length spectrum, when electric current passes through it. The main advantage LED has over traditional devices is its high durablility, with a life expectancy of approximately 30,000 h, and consumes low energy when producing a high luminous flux. In the visible light spectrum, due to its safety, it does not require additional protection of its operator (Ghate et al., 2013; Yeh et al., 2015).
Recent studies using this apparatus demonstrate that after the exposure of bacteria to violet or blue light at a wavelength between 405-520 nm, they are inactivated. The device can be potentially applied in food and clinical microbiology, since it has the ability to inactivate pathogenic bacteria, such as Staphylococcus aureus, Streptococcus pyogenes, Bacillus cereus, Listeria monocytogenes, Escherichia coli O157: H7, Salmonella spp., Staphylococcus epidermidis, Methicillin-resistant Staphylococcus aureus (MRSA), Acinetobacter baumannii, Proteus vulgaris, Klebsiella pneumoniae (Enwemeka et al., 2008; Maclean et al., 2009; Ghate et al., 2013; Kumar et al., 2015). Its cytotoxic action is widely documented and is characterized from the formation of reactive oxygen species (Guffey and Wilborn, 2006; Luksiene and Zukauskas, 2009). Bacteria, such as Salmonella spp., S. aureus, Escherichia coli, and Bacillus cereus are amongst the predominant species identified as etiological agents responsible for outbreaks of foodborne diseases in Brazil, from 2007 to 2016. These microorganisms account for 77.8% of all outbreaks in that time interval in which the etiological agent was identified (Brazil, 2016).
Considering the possibility of bacterial inactivation after exposure to visible light at monochromatic wavelengths, the efficiency of this system against pathogenic bacteria causing foodborne diseases and the lack of researches on the use of this device for treatment of water meant for human consumption, this study aims to evaluate the in vitro antimicrobial effect of exposing raw water intended for human consumption to light at 450nm and also to investigate the relationship of the results with physical and chemical parameters.
Samples
Raw water samples were collected from 15 households located in a rural area of Santo Antônio de Jesus – Bahia (Brazil), from November to December 2016. 500 mL of water was collected and stored in polyethylene bottles used for the first time. Samples were labeled and stored in thermal boxes, and kept at refrigeration temperature (+2 to + 8°C). They were transported to the Laboratory of Microbiology and Parasitology of the Center of Food and Nutrition Security (SANUTRI) of Health Science Center (CCS), Federal University of Recôncavo of Bahia (UFRB), where they were kept in the refrigerator for 12 h.
Characterization of the light emitting diodes system
The lighting system consists of two high-intensity LEDs, each of 10 W, with a wavelength of 450 nm (blue) and luminous flux of 200 lumens. The LED was put on a heat dissipating plate in order to minimize heat transfer from the LED to the samples. It involved using an acrylic plate to isolate the samples from the environment. The entire system was overlaid with an autoclaved glass vat. This way, the LED was positioned directly above each sample (Ghate et al., 2013; Kumar et al., 2015) (Figure 1).
Experimental arrangement
A 100 mL aliquot of the refrigerated sample was transferred to the glass vat. The LED system was put on the vat, switched on and transferred to the refrigerator. It was kept under this condition for 10 h. Bacteriological analysis started at time zero and was performed every two h for 10 h. After 10 h, the samples were stored in polyethylene bottles used for the first time under the same temperature conditions, but they were protected from light. After 72 h, a new analysis was performed. Consequently, there were seven analyses done for each sample. A control sample was maintained under the same refrigeration conditions, but it was not exposed to the LED light. Bacteriological analysis in the control sample was performed at the beginning of the experiment and after 72 h (Ghate et al., 2013; Kumar et al., 2015). The dosage of light received by each water sample was calculated using the equation:
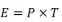
Where, E = Dose in J/cm2; P = Irradiance in W/cm2; and t = time in seconds (Ghate et al., 2013).
Two indicator microorganisms present in Ordinance MS 2914/2011 (Brazil, 2011) were used for bacteriological analysis. Quantification of heterotrophic bacteria was used to identify flaws in water disinfection and quantification of total coliforms, to evaluate the efficiency of the treatment (Brazil, 2011). Heterotrophic bacteria and total coliforms populations were estimated by the methods of Petrifilm Aqua Heterotrophic Count Plate (AQHC, 3M Company™) and Petrifilm Aqua Coliform Count Plate (AQCC, 3M Company™), respectively. 1 mL of the sample was diluted in 9 mL of 0.9% NaCl solution. 1 mL of the dilution was inoculated into each Petrifilm plate. After complete gel solidification, plates were incubated in a bacteriological oven at 36 ± 1°C for 44 ± 4 h and 36 ± 1°C for 24 ± 2 h, respectively. Results were expressed as log CFU / mL (APHA, 2012). pH, dissolved oxygen and salinity analyses were performed before each experiment with a AK88 multiparameter meter (AKSO®), using an approximate aliquot of 100 mL of water. Temperature was checked with an infrared digital thermometer MT-350 (Minipa®) every two hours. Color and turbidity analysis was performed with a spectrophotometer SP22 (Biospectro), using wavelengths of 455 nm and 860 nm, respectively (APHA, 2012).
Statistical analysis
Data were processed and analyzed using Microsoft Office Excel version 2007 (Microsoft Corporation™) and Statistical Package for the Social Sciences (SPSS) version 23 (International Business Machines™). The data normality test (Shapiro-Wilk) was performed with all quantitative variables. Descriptive and analytical statistics, such as mean, median, maximum and minimum, logarithmic and percentage reduction, Spearman’s correlation coefficient, paired t test and analysis of variance (ANOVA) were performed. The level of significance was 5% (p <0.05).
Figure 2 shows the means of the bacterial populations of the fifteen samples analyzed within 10 h of exposure to light as well as bacterial growth after 72 h. There was a decrease in heterotrophic bacteria and total coliforms counts during their exposure to light (3.44 to 1.86 log CFU/mL) and total coliforms (2.45 to 1.02 log CFU/mL). After 72 h, both counts increased, with significant growth of heterotrophic bacteria (p = 0.000), but there was no significant growth for total coliforms (p = 0.058). Even after 72 h, samples exposed to light, when compared to control samples, obtained values 26 times lower for heterotrophic bacteria and 30 times lower for total coliforms. After a 10 h illumination by the 450 nm LEDs, the dosage received by the water samples was 581.8 J/cm2.
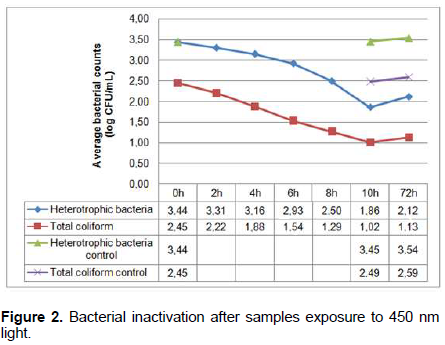
Quantifications of heterotrophic bacteria before their exposure to light reached a maximum value of 3.75 log CFU/mL, median of 3.63 log CFU/mL and a minimum value of 3.09 log CFU/mL. All the values were above the ones recommended by the Brazilian Legislation (Brazil, 2011), 11.4 times higher than the recommended ones (limit of 2.7 log CFU/mL). After 10 h of the bacteria exposure to light, the maximum value found was of 2.51 log CFU/mL and the minimum was 1.30 log CFU/mL, meaning all samples were within the legislation standards. Within 8 h of their exposure, most samples were already within this legislation standard (73.3%). Regarding total coliform counts, a maximum value of 3.08 log CFU/mL, median of 2.48 log CFU/mL and a minimum value of 1.90 log CFU/mL were found before the bacteria exposure to light. After 10 h of their exposure, maximum value was reduced to 1.48 log CFU/mL, median and minimum values to <1 log CFU/mL. In eight samples there were no counts of total coliform after 10 h of the bacteria exposure to light, nor growth after 72 h (Figure 3).
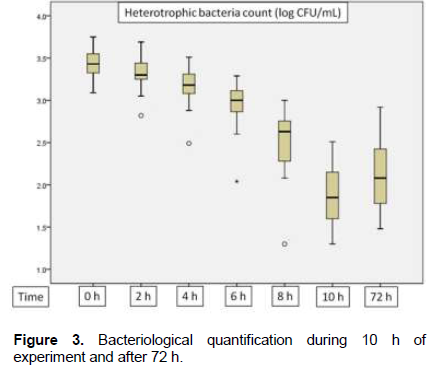
There was a significant reduction in the two bacteriological parameters analyzed after treatment (p = 0.000). Reductions in bacterial counts reached 98.98% for total coliforms and 98.96% for heterotrophic bacteria. The lowest reductions found were 88.75% in total coliform analysis in sample 12 and 93.57% in the heterotrophic bacteria analysis in sample 8. Mean reduction of the heterotrophic bacteria was 97.01% and of total coliforms, 95.61%. During pre and post treatment periods, there is strong positive and significant correlation in the heterotrophic bacteria counts (ρ = 0.912; p = 0.000) and a moderate positive and significant correlation in total
coliform counts (ρ = 0.581; p = 0.023) (Table 1).
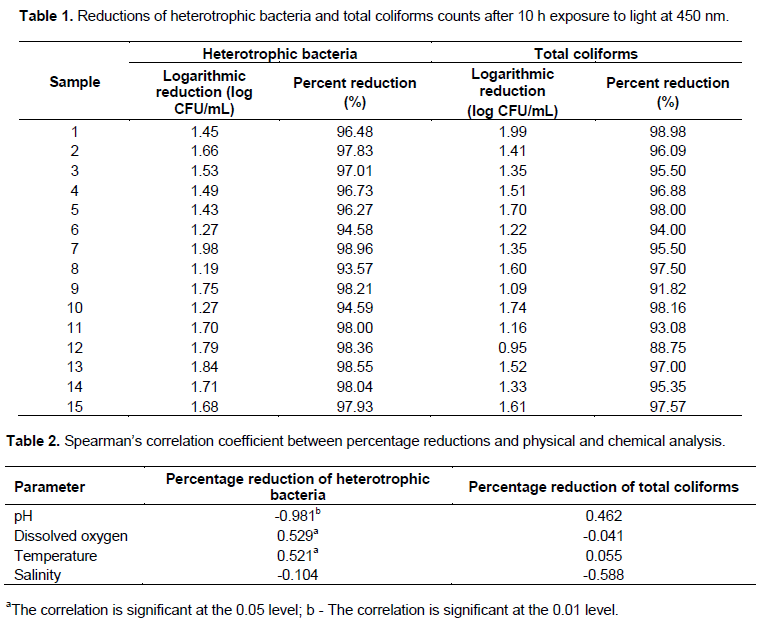
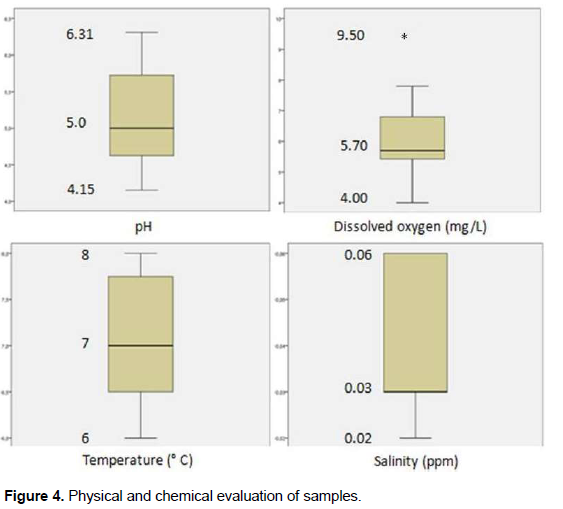
Results of Spearman’s correlation coefficient between percentage reductions of bacteriological parameters and physical and chemical analysis showed a strong negative and significant correlation between heterotrophic bacteria percentage reduction and pH (p = 0.000), moderate positive and significant correlation between heterotrophic bacteria percentage reduction and dissolved oxygen (p = 0.043) and moderate negative and significant correlation between the percentage reduction of heterotrophic bacteria and temperature (p = 0.047) (Table 2). pH values reached a maximum of 6.31; median, 5.0 and a minimum, 4.15. Dissolved oxygen had a maximum value of 9.50 mg/L; median, 5.70 mg/dL and minimum, 4.0 mg/dL. Temperature reached a maximum value of 8°C; median, 7°C and minimum, 6°C. There was an increase of 0.5-1.0°C in each experiment, but temperature did not exceed 8°C. Salinity had the lowest variation, with a maximum value of 0.06 ppm, median of 0.03 and 0.02 ppm. Color and turbidity obtained zero values in all samples (Figure 4).
Results demonstrate the antimicrobial effect of high intensity light with a 450 nm wavelength under refrigeration temperature on tested indicator micro-organisms. Reductions were significant during the experiment, indicating the efficiency of the process in small scale. The bacterial inactivation process has been shown to be dose dependent, because reductions result from longer exposure time. Inactivation curves and percentage reductions were similar and sometimes better compared to previous studies done with pathogenic bacteria like S. aureus, S. pyogenes, Bacillus cereus, Listeria monocytogenes, Escherichia coli O157:H7 (Guffey and Wilborn, 2006; Enwemeka et al., 2008; Maclean et al., 2009; Ghate et al., 2013; Kumar et al., 2015).
Microorganisms are sensitive to environmental changes. There is an inversely proportional relationship between pH and percentage reduction of heterotrophic bacteria, and, the more the pH moves away from neutrality, the greater the percentage of bacterial reduction. Optimum pH ranges from 6.5 to 7.5 for most bacteria, thus lower values can inhibit or delay bacterial multiplication. A few species of bacteria, such as E. coli have survival mechanisms at more acidic pH in short periods. However, bacterial growth may fall by up to five times at low pH (Cotter and Hill, 2003; Rousk et al., 2009).
The process of bacterial inactivation using visible light is oxygen dependent, according to Feuerstein et al. (2005). Photodynamic inactivation involves excitation of photosensitizing molecules, such as endogenous porphyrins. Excitation of porphyrin leads to energy transfer, which, on the other hand, generates reactive oxygen species, especially singlet oxygen. These reactive oxygen species oxidize constituents of the cell membrane, such as unsaturated fatty acids, proteins, in addition to DNA, promoting a cytotoxic and bactericidal effect (Hamblin and Hasan, 2004; Guffey and Wilborn, 2006; Luksiene and Zukauskas, 2009).
It is possible that species less tolerant to oxygen are more susceptible to these cytotoxic effects, due to the lower presence of oxidative regulatory mechanisms compared to aerobic species (Luksiene and Zukauskas, 2009; Murdoch et al., 2010; 2012). Even at low temperatures, some studies have shown that bacterial damage can rise with higher temperatures; as bacteria increase their metabolic rates in these situations, their metabolic load and cytotoxic reactions that aid their inactivation also increase (Song et al., 2011; López-Velasco et al., 2012). However, the increased susceptibility of bacteria observed in this and other studies may be related to the increase of unsaturated fatty acids in the cell membrane. These unsaturated fatty acids have a greater tendency to be oxidized compared to saturated fatty acids, therefore a greater damage will occur to this important cellular constituent. The optimal temperature for bacterial inactivation in this light spectrum is close to 10°C (Ghate et al., 2013; Kumar et al., 2015).
Bacterial growth after 72 h can be explained by the presence of particulate matter and colonies in the medium with bacterial aggregation; this negatively affects the water disinfection process with light exposure, as they lead to the formation of areas with no direct exposure to light and consequently oxidative stress will be affected. Thus, raw water needs to be filtered (Bohrerova and Linden, 2006; Cantwell and Hofmann, 2008). Another possibility would be the susceptibility of species to inactivation at this wavelength. For example, Pseudomonas aeruginosa requires higher doses compared to S. aureus (Guffey and Wilborn, 2006). Salmonella entericae Enterococcus faecalis are two studied bacteria with greater resistance to inactivation by exposure to visible light, requiring long periods of exposure. The reasons for these different susceptibilities are still indeterminate, but analysis indicates that gram-positive bacteria tend to be more susceptible to inactivation than gram-negative (Maclean et al., 2009; 2014; Murdoch et al., 2012). Therefore, there will be cells that cannot be injured and can multiply in the time interval. Even after 72 h, there was no growth of total coliforms in 53.3% samples and 86.7% were within the recommended standard for heterotrophic bacteria (Brazil, 2011).
Compared to UV light, the germicidal efficacy of blue light is lower, since its lethal dose is much lower, thus it has a slower inactivation. However, the fact that this wavelength lies within the visible light range and does not require the necessary protections for UV light, it can be continuously for treatment of water, food as well as surfaces. It can be safely used in the presence of people in the same enclosure, it is easy to operate, and it has high penetration power in water, plastics and glass (Maclean et al., 2014).
In summary, exposure to light at 450 nm wavelength led to mean reductions in bacterial counts above 95%. All samples were within the standards recommended by the Brazilian Legislation for heterotrophic bacteria and 53.3% of the samples were within the recommended standards for total coliforms. pH, temperature and dissolved oxygen were directly related to the percentage reduction of heterotrophic bacteria.
This is an unpublished study involving raw water meant for human consumption, addressing the correlation between physical and chemical parameters and indicator microorganisms. No other study so far has worked with these parameters or indicator microorganisms; they have used only pathogenic bacteria and experimental inoculations in Petri dish with growth medium or saline solution. Because of its uniqueness, this study presents itself as an initial effort to find new methods for water disinfection. It proves to be a safe, effective method against indicator microorganisms, allowing continuous use without the need for special protection and it is easy to use. In the same way, it demonstrates the necessity of expanding subsequent studies, seeking to perfect the system to leave experimental work in vitro for in loco evaluations.
The authors have not declared any conflict of interests.
This work was supported by the Coordination for the Improvement of Higher Education Personnel (CAPES) and Federal University of Recôncavo of Bahia (UFRB).
REFERENCES
|
American Public Health Association (APHA) (2012). Standard methods for the examination of water and wastewater. 22nd edition. Washington, USA.
|
|
|
|
Bohrerova Z, Linden KG (2006). Ultraviolet and chlorine disinfection of Mycobacterium in wastewater: effect of aggregation. Water Environment Research 78:565-571.
Crossref
|
|
|
|
|
Boyle M, Sichel C, Fernández-Ibá-ez P, Arias-Quiroz GB, Iriarte-Pu-a M, Mercado A, Ubomba-Jaswa E, McGuigan KG (2008). Bactericidal effect of solar water disinfection under real sunlight conditions. Applied and Environmental Microbiology 74:2997-3001.
Crossref
|
|
|
|
|
Brazil, General Coordination of Communicable Diseases (2016). Epidemiological Surveillance of Foodborne Diseases (VE-DTA). Available at:
View
|
|
|
|
|
Brazil, Ministry of Health (2011). Ordinance Nº 2.914, 12 of December of 2011. Brasília, Brazil.
|
|
|
|
|
Cantwell RE, Hofmann R (2008). Inactivation of indigenous coliform bacteria in unfiltered surface water by ultraviolet light. Water Research 42:2729-2735.
Crossref
|
|
|
|
|
Cotter PD, Hill C (2003). Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiology and Molecular Biology Reviews 67:429-453.
Crossref
|
|
|
|
|
Di-Bernardo L, Dantas AB, Voltan PEN (2017). Methods and techniques of water treatment. 3rd edition. São Paulo, Brazil.
View
|
|
|
|
|
Enwemeka CS, Williams D, Hollosi S, Yens D, Enwemeka SK (2008). Visible 405 nm SLD light photo-destroys methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers in Surgery and Medicine 40:734-737.
Crossref
|
|
|
|
|
Feuerstein O, Ginsburg I, Dayan E, Veler D, Weiss EI (2005). Mechanism of visible light phototoxicity on Porphyromonas gingivalis and Fusobacterium nucleatum. Photochemistry and Photobiology 81:1186-1189.
Crossref
|
|
|
|
|
Ghate VS, Ng KS, Zhou W, Yang H, Khoo GH, Yoon WB, Yuk HG (2013). Antibacterial effect of light emitting diodes of visible wavelengths on selected foodborne pathogens at different illumination temperatures. International Journal of Food Microbiology 166:399-406.
Crossref
|
|
|
|
|
Guffey JS, Wilborn J (2006). In vitro bactericidal effects of 405-nm and 470-nm blue light. Photomedicine and Laser Surgery 24:684-688.
Crossref
|
|
|
|
|
Hamblin MR, Hasan T (2004). Photodynamic therapy: a new antimicrobial approach to infectious disease?. Photochemical and Photobiological Sciences 3:436-450.
Crossref
|
|
|
|
|
Kumar A, Ghate, V, Kim MJ, Zhou W, Khoo GH, Yuk HG (2015). Kinetics of bacterial inactivation by 405 nm and 520 nm light emitting diodes and the role of endogenous coproporphyrin on bacterial susceptibility. Journal of Photochemistry and Photobiology B: Biology 149:37-44.
Crossref
|
|
|
|
|
López-Velasco G, Tomás-Callejas A, Sbodio A, Artés-Hernández F, Suslow TV (2012). Chlorine dioxide dose, water quality and temperature affect the oxidative status of tomato processing water and its ability to inactivate Salmonella. Food Control 26:28-35.
Crossref
|
|
|
|
|
Luksiene Z, Zukauskas A (2009). Prospects of photosensitization in control of pathogenic and harmful micro-organisms. Journal of Applied Microbiology 107:1415-1424.
Crossref
|
|
|
|
|
Maclean M, MacGregor SJ, Anderson JG, Woolsey G (2009). Inactivation of bacterial pathogens following exposure to light from a 405-nanometer light-emitting diode array. Applied and Environmental Microbiology 75:1932-1937.
Crossref
|
|
|
|
|
Maclean M, McKenzie K, Anderson JG, Gettinby G, MacGregor SJ (2014). 405 nm light technology for the inactivation of pathogens and its potential role for environmental disinfection and infection control. Journal of Hospital Infection 88:1-11.
Crossref
|
|
|
|
|
Mbonimpa EG, Blatchley ER, Applegate B, Harper WF (2018). Ultraviolet A and B wavelength-dependent inactivation of viruses and bacteria in the water. Journal of Water and Health 71:1-11.
|
|
|
|
|
McGuigan KG, Conroy RM, Mosler HJ, Preez M, Ubomba-Jaswa E, Fernandez-Iba-ez P (2012). Solar water disinfection (SODIS): a review from bench-top to roof-top. Journal of Hazardous Materials 235:29-46.
Crossref
|
|
|
|
|
Murdoch LE, Maclean M, Endarko E, MacGregor SJ, Anderson JG (2012). Bactericidal effects of 405 nm light exposure demonstrated by inactivation of Escherichia, Salmonella, Shigella, Listeria, and Mycobacterium species in liquid suspensions and on exposed surfaces. Scientific World Journal 2012:1-8.
Crossref
|
|
|
|
|
Murdoch LE, Maclean M, MacGregor SJ, Anderson JG (2010). Inactivation of Campylobacter jejuni by exposure to high-intensity 405-nm visible light. Foodborne Pathogens and Disease 7:1211-1216.
Crossref
|
|
|
|
|
Rousk J, Brookes PC, Baath E (2009). Contrasting Soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Applied and Environmental Microbiology 75:1589-1596.
Crossref
|
|
|
|
|
Song L, Farrah SR, Baney RH (2011). Bacterial inactivation kinetics of dialdehyde starch aqueous suspension. Polymers 3:1902-1910.
Crossref
|
|
|
|
|
Wisbeck E, Sandri EK, Soares ALM, Medeiros SHW (2011). Disinfection of rainwater by ultraviolet radiation. Revista Engenharia Sanitária e Ambiental 16:337-342.
Crossref
|
|
|
|
|
Yeh N, Ding TJ, Yeh P (2015). Light-emitting diodes light qualities and their corresponding scientific applications. Renewable and Sustainable Energy Reviews 51:55-61.
Crossref
|
|