ABSTRACT
Folklore medicine in Sudan used medicinal materials to treat inestinal infections caused by bacterial infections or contamination of food. Methanolic and aqueous extracts of different parts of Acacia nilotica (L.) Delile gum, Haplophyllum tuberculatum Juss.aerial parts, Hydnora abyssinica A. Br.fruits, Nigella sativa L seeds, Rhynchosia minima (L.) DC. roots,and Usnea molliuscula lichen were tested for antibacterial properties at a concentration of 100 mg/mL against 20 intestinal isolates including Escherichia coli, Pseudomonas aeruginosa, Proteus mirabilis, Salmonella typhi, Salmonella para typhi B,Staphylococcus aureus, and standard bacterial strains (Bacillus subtilis (NCTC 8236), Escherichia coli (ATCC 25922), Klebsiella pneumoniae (ATCC 35657), Salmonella typhi (ATCC 1319106) and Staphylococcus aureus (ATCC 25923), using the Agar Diffusion Method. Standard antibiotics were used as standards drug for antibacterial effect. The highest enhancing properties were observed in methanol extracts and the lowest in aqueuos extract. U. molliuscula methanolic extract was the most active among all tested plant extracts, while, the aqueous extract of H. tuberculatum had a promising level of efficacy among the aqueous extracts tested. Most responsive Gram-negative clinical isolates bacteria were S. para typhi B and P. aeruginosa. Most susceptible standard bacteria were B. subtilis (NCTC 8236). Obtained results from investigated plants confirm their antibacterial potential and usefulness in the treatment of intestinal infections.
Key words: Phytomedicine, traditional uses, antibactrial activity.
The increasing incidence of antibiotic resistance has been increasing throughout the world in the last few decades (Abdallah, 2011).This has led to higher mortality in humans. Consequently, one of the most intensive researched fields is the search for material with high antibacterial potency, with folklore medicine being a prime area.In this regard Sudan has a long history of traditional use of plants to treat primry health issues.However, very little research is directed towards understanding their potential for curing gastrointestinal tract infections,caused by various bacteria. Spread and prevelence of microbial resistance is geting more frequent worldwide (WHO, 2001). Search for new antimicrobial substances is the major weapon to combat the microbial resistance through developing new antibacterial materials to substitute with inefficient ones.
Combination of native cultures and different traditions are factors formed by Sudanese traditonal medicine.The extremely large diversity of plants in the area, different cultures due to the variation of climatic zones, and the distinctive geography created Sudanese herbal medicine. 11% of the population has access to prescribed health care.Therefore, research on the best pharmacological influence and possible undesirable side effects or toxicity is essential to enhance potency and protection of Sudanese folklore medicine (Khalid et al., 2012).
Ethnobotanical survey in the Blue Nile State, South-eastern Sudan of medicinal materials used by folklore healers was carried out. Fifty three plant species within 31 families and 47 genera were detected as being used to treat one or more diseases. The most commonly mentioned manifestations were gastro intestinal tract disorders, diseases/infestations, stiffness, and respiratory tract disorders (Musa et al., 2011).
Traditional medicine in Sudan still has the most rational source of therapy of several ailments and bacterial infections.Traditional medicine is distinguished by a special fusion of Islamic, Arabic, and African tradition. Sudanese folk plants have been reported to be characterized with a wide range of folk medicinal uses including different bacterial infections and digestive system disorders (Karar and Kuhnert, 2017). Different extracts of Usnea revealed a variable effect of antibacterial properties against S. aureus, E. coli, V. cholerae, S. dysenteriae and S. flexneris.The methanol extracts were the most active against tested bacteria; all tested organisms showed no antibacterial susceptibility against aqueous extracts of tested lichens (Sinha and Biswas, 2011). U.molliuscula extract showed high antimicrobial potential against all tested Gram (+ve) bacteria inclusive penicillin-resistant S aureus and methicillin-resistant S.aureus (Weckesser et al., 2007). Studies revealed the effects of N.sativa seed extracts have dose dependent antibacterial activities on the tested organisms (Hosseinzadeh et al., 2007; Chaudhry and Tariq, 2008; Yoruk et al., 2010; Mishra, 2011; Pichette et al., 2011; Haloci et al., 2012; Monika et al., 2013).
H. tuberculatum showed antimicrobial evaluation against a variety of strains, revealed moderate effect against B. subtilis, S. choleraesuis and E. coli, gentamycin sulphate; it has 75% potency as antibacterial agent on S.aureus and E.coli. It is the most potent inhibitor against plant pathogenic bacteria and fungi (Al-Burtamani et al., 2005; Sabry et al., 2016; Abdelgaleil et al., 2020). Available literature shows that no earlier study has been performed on the antibacterial characters of A.nilotica gum until 2013.Study of methanolic extract of A. nilotica revealed moderate antibacterial effect (14-18 mm) against E. coli (ATCC 25922);aqueous extracts of A. nilotica had no effects of antibacterial characters against all tested bacteria (Mahjoub, 2013). Studies of antimicrobial evaluation of different extracts of A.nilotica revealed high (˃18 mm) to modrate (14-18 mm) activity for different extracts which consider a good source of natural antibiotic for the therapy of different transmittable diseases (Sravani et al.,2014; Banjar et al., 2017; Al Alawi et al., 2018; Shehu et al., 2018; Ali et al., 2020). Ethanolic extracts of 8 species of R.minima were investigated for their antibacterial activity and phytochemical screening against clinical isolates (B.subtilis,E.coli, P.aeruginosa and S.aureus); they showed equal or nearly equal antibacterial characters against all tested bacteria (El-Kamali and El-Amir, 2010).The essential oils of R.minima exhibited antioxidant and antimicrobial activities against E. coli, S aureus and C. albicans, but not active against C. perfringens and K. pneomoniae (Gundidza et al., 2009). Methanolic extract of H.abyssinica showed moderate antimicrobial potential. Analysis there are tanins and phenols in the plant root extracts (Saadabi and Ayoub, 2009).
A research was done to determine the antibacterial potential of six medicinal materials U. molliuscula lichen, N .sativa seeds, H. tuberculatum aerial parts, A. nilotica gum, R. minima roots and H.abyssinica fruits. They were obtained from Omdurman market on the origin of antimicrobial potentials with documented details for antibacterial assay against bacterial strains associated with intestinal infections. These investigations determine antibacterial potential efficacy of selected medicinal plants.This selection was guided in the first place by ethnobotanical claim in traditional medicine suggestive of their antibacterial activity and secondly by defficiency or insufficiency of information in published works on antibacterial and antioxidants potency of their extracts. This will provide baseline data for developing new antibacterial compounds in the treatment of intestinal infections, based on their folklore use.
Plants’ taxonomy and authentication were established by Prof.Hatil H.Elkamali via differentiation with herbs specimens of Botany Department, Faculty of Science and Technology, Omdurman Islamic University during the spring season of 2012. The plants wew dried in the shade for three weeks.The air-dried plants’ parts were crushed and turned into coarse powder. It was further reduced to powder using a mechanical grinder.
Two hundred grams of all air-dried plants were soaked for 24 h with 50% methanol (MeOH) in a round bottomed flask. Liquor (crude extract) was filtered with whatman grade1qualitative filter papers and then rotor-evaporated.The filtrate was dried at room temperature.Then the dried extracts were sterilized and kept in air-tight containers at room temperature until used for further tests. At the time of testing for antibacterial activities extracts were prepared at a concentration of 100 mg/mL in methanol. One hundred g of dried plant material was crushed coarsely into powder, which was further reduced to powder using a mechanical grinder.Then it was dissolved with purified water (1L), and left for 24 h at room temperature.With whatman grade 1 qualitative filter papers the mother liquor was filtered.Thus, the aqueous extract (10%) was obtained.
The antibacterial testing was investigated by well-agar diffusion technque (Cheesbrough, 1984). Two hundard and 50 millilitres of decontaminate nutrient agar (Oxoid) were used for antibacterial testing.Suspension of 106 cells was monitored in the inoculum size of each test bacteria.Two millilitres of the inoculum suspention obtained from 24 h cultures of bacteria were supplemented with 250 ml of nutrient agar (Oxoid) and then mixed; they were inoculated on soft agar (20 mL) and flowed on 10 cm diameter sterilized petri dishes and agar plates and allowed to solidify. After solidifying, a sterile cooled-flamed cork borer using four wells (10 mm in diameter) was bored in the agar and the agar discs were removed. One hundred microliters (100 µl) of each extract solution to each plant extract was added to each well with a pipette and the plate was held for 2 h at room temperature for diffusion of extract into agar. The plates were incubated at 37°C for 24 h. Result of tested plants were evaluated to test their antibacterial activities and expressed as the diameters of the inhibition zones which were calculated as the adjacent mm. Methanol serves as negative control. Axiom laboratories, New Delhi 1100055 Multidisc was used for antimicrobial susceptibility testing of tested clinical isolates (4 E. coli, 5 P. aeruginosa, 5 P. mirabilis, 3 S. aureus, 2 S. typhi, and 1 S. para typhi B ).
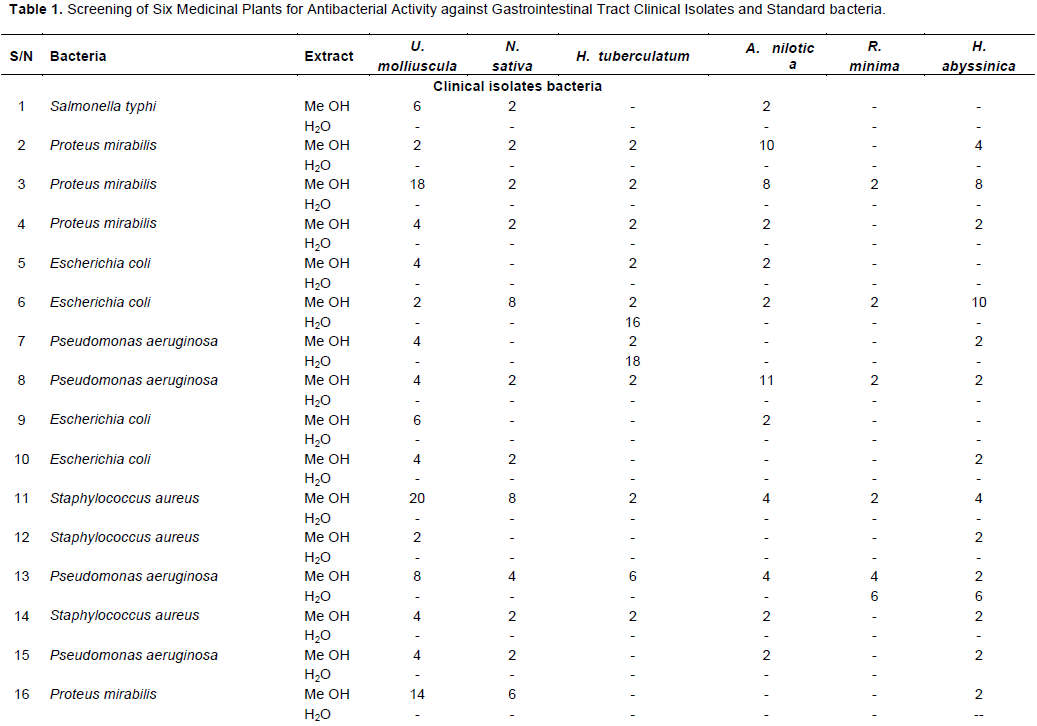
Methanolic extracts of U.molliuscula revealed high (˃18 mm) antibacterial properties (1Z = 20 mm) against P. aeruginosa no. (20), S.aureus, P.mirabilis no. (11) and S. para typhi, B no.(17). B. subtilis (NCTC 8236) was (˃18 mm) (1Z = 24mm) to methanolic extracts of U.molliuscula (Table 1). E.coli (ATCC 25922), S. aureus (ATCC 25923), K. pneumoniae (ATCC 35657), and S.typhi (ATCC1319106) were found to be not sensitive to the tested plants (<14 mm) (Table 1). All clinical isolates were found to be resistant (< 14 mm) to methanolic extracts of N.sativa except S.para typhi B no.(17), that showed moderate (14-18 mm) antibacterial potency (1Z = 16 mm). All standard bacteria were resistant (1Z < 14 mm) (Table 1).
Methanolic extracts of H.tuberculatum, A.nilotica and H.abyssinica were not effective against all tested bacteria. K.pneumoniae (ATCC 35657), B.subtilis (NCTC 8236), S. aureus (ATCC 25923) and S.typhi (ATCC1319106) were resistant to the tested plant extracts (< 14 mm), except E. coli (ATCC 25922) that moderately (1Z = 14-18 mm) resisted (1Z = 16 mm) the methanolic extract of A. nilotica (Table 1).
All tested bacteria were less susceptible to aqueous extracts of all plants except H,tuberculatum. Aqueous extract of H. tuberculatum showed promising result against P.aeruginosa isolate no.(7) (1Z=18 mm), but moderate (1Z=14-18 mm) efficacy was observed against E.coli no.(6) (1Z=16mm)(Table 1).Aqueous extract of U.molliuscula, Nigella sativa, Acacia nilotica.R.minimas, and H. abyssinica were ineffective against all tested bacteria. Their methanolic extracts exhibited strong antioxidant properties as compared to other plants.Further work should be on antioxidant and anti-inflammatory activities of isolated compounds from active extracts (Elkamali and Mahjoub, 2015).
Co-Trimoxazole (BA) at concentration of 25 mcg showed promising effect (1Z=24 mm) against S.para typhi B no. (17), and moderate effect (14-18 mm) (1Z=16-14 mm) against E.coli no.(9), P.aeruginos no.(15) and S. aureus no.(14) (Table 2). P. mirabilis no. (3) exhibits antibiotic-resistant (<14 mm) to BA and showed a high degree (˃18 mm) of sensitivity (1Z=18 mm) to the methanolic extracts of U. molliuscula. P. mirabilis has the ability to cause various human diseases; it is primarily corresponding with urinary system disorders and is a major health concern due to its complications and frequent recurrence. Usnic acid rich in phenols is accountable for the antioxidant activities of U.molliuscula methanolic extract; it possesses strong antioxidant activities against different antioxidant systems in vitro. It is considered as the source of natural antioxidants. It can be used simply as possible food complement, and in pharmaceutical implementations (Elkamali and Mahjoub, 2015). B.subtilis (NCTC 8236) showed promsing (˃18 mm) degree of sensitivity (1Z =24mm) to methanolic extracts of U.molliuscula, which exhibit antibiotic-resistant to Co-Trimoxazole and Ceftizoxim; same goes to Piperacillin/Tazobactam, Chloramphenicol and Ciprofloxacin. Our results support that the traditional therapeutic information for U.molliuscula, in near future, can surely change the traditional antimicrobial agents to which there is increased occurrence of drug interactions. The study recommends that U.molliuscula is promising for increasing phytomedicines with antibacterial potential. More studies on the selection and distinguishing of active concepts and assessing possible synergism among extract components for their antimicrobial potentiels depending on the main results obtained might be considered enough (Ali et al., 2012).
Piperacillin/Tazobactam (TZP) at concentration of 100/10 mcg showed good (14-18 mm) efficacy (1Z=16 mm) against E.coli no. (6) (Table 2); it also showed promising (˃18 mm) result (1Z=20 mm) against B.subtilis (NCTC 8236); whereas S. typhi (ATCC1319106), S. aureus (ATCC 25923) and E. coli (ATCC 25922) were found to be resistant (< 14 mm). E. coli no.(6) exhibited antibiotic-resistant to most synthetic antibiotics and showed good (14-18 mm) degree of sensitivity (1Z=16 mm) to the methanolic extracts of H.tuberculatum in the same way as TZP (1Z = 16 mm). The phytochemical profile of leaf extracts of H.tuberculatum revealed the presence of alkaloid and polyphenolic compounds may be basic contributors to the antioxidant potential of these extracts (Hamdi et al., 2018).
Chloramphenicol (CH) at concentration of 30 mcg showed moderate (14-18 mm) efficacy (1Z=14 mm) against clinical isolates P.aeruginosa no.(15) S.aureus no. (14), P. mirabilis no. (19) and E. coli no. (9) showed promising (˃18 mm) result (1Z=20 mm) against B. subtilis (NCTC 8236); while other standard bacteria S. aureus (ATCC 25923), E.coli (ATCC 25922),S. typhi (ATCC1319106) and K. pneumoniae (ATCC 35657) were found to be chloramphenicol resistant (<14mm). Bacillus subtilis (NCTC8236) showed high sensitivity (1Z=24 mm) to methanolic extract of U.molliuscula and antibiotics resistant, except Piperacillin/ Tazobactam, Ciprofloxacin, Chloramphenicol, and Levofloxacin .U. molliuscula extracts can be used in the therapy of infectious diseases caused by resistant bacteria due to their great potential as chemotherapeutic agents against microorganisms.
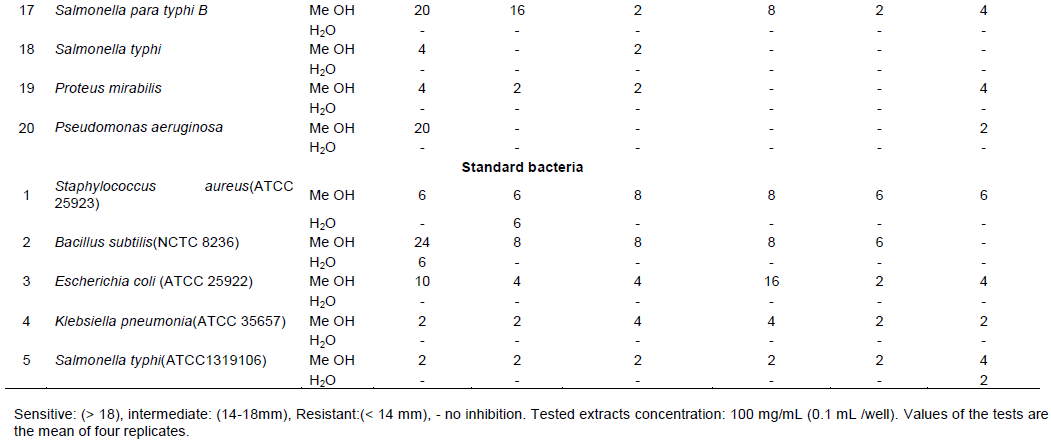
Ceftizoxime (CI) at concentration of 30 mcg was found effective (1Z=20 mm) against P. aeruginosa no. (13), P. mirabilis no.(2), moderate effect (1Z=16 mm) was observed against P. aeruginosa no.(15), it was ineffective to S. aureus (ATCC 25923),B. subtilis (NCTC 8236),K. pneumoniae (ATCC 35657) and S.typhi (ATCC 1319106) except E.coli (ATCC 25922).Standard bacteria E.coli (ATCC 25922) exhibit moderate sensitivity to most synthetic antibiotics,showed good (14-18 mm) degree of sensitivity (1Z=16 mm) to the methanolic extracts of A.nilotica in the same extent as to CI and AK (1Z=16 mm).The pharmacognostical study revealed that A.nilotica species can be characterized on the basis of its macroscopic, microscopic and phytochemical properties. It was found to contain different secondary metabolites such as alkaloids, saponins, tannins and flavanoids (Saini et al., 2008). This information represents an ample chance to establish drug based design depending on their significant role in the folk medicine, efficacy against many pathogenic microoganisms, and their significant phytochemical coumpounds.
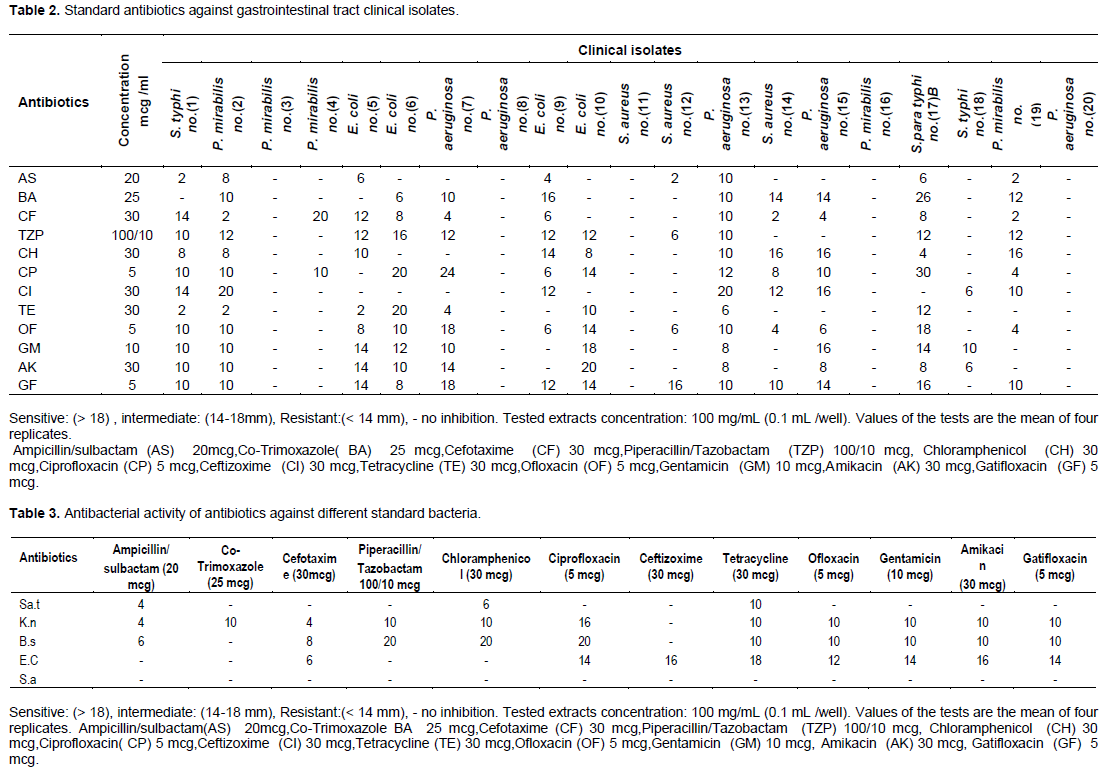
Ofloxacin (OF) at concentration of 5 mcg showed good effective result (1Z=18 mm) against P. aeruginos no.(7),and S.para typhi B no.(17) (Table 2). S. typhi (ATCC1319106), S. aureus (ATCC 25923), B. subtilis (NCTC 8236), E. coli (ATCC 25922), and K. pneumoniae (ATCC 35657) exhibit antibiotic-resistant to OF (<14 mm) (Table 3). S. aureus no.(11), P. mirabilis no. (3) P. aeruginosa no.(20) exhibit antibiotic-resistant to OF and showed high (˃ 18 mm) degree of sensitivity (1Z = 20-18 mm) to the methanolic extracts of U. molliuscula. The appearance and rapid development of antibiotic resistance by infectious bacterial isolates are critical threats to the international public health; it leads to a significant threat to public health worldwide due to the bounded therapy options and unconcerned discovery of new types of antibiotics (Trojan et al., 2016).Incorporating sensitivity data within study may be worth it in fulfilling treatment strategies for diseases.P.mirabilis no.(16) showed antibiotic-resistant to synthetic antibiotics and showed good (14-18 mm) degree of sensitivity (1Z=14 mm) to the methanolic extracts of U. molliuscula. S. para typhi B no.(17) showed promising degree of sensitivity (1Z=20 mm) to the methanolic extracts of U.molliuscula same extent as to OF. Plant chemical constituents as alkaloids, flavonoids, tannins,and phenolic compounds work as protection mechanisms contrary to predation by many microbs,insects and herbivores. Flavonoids have antibacterial potential through their capability to complex with extracellular and disolved proteins and to complex with bacterial outer cell walls (Vijayasanthi et al., 2012). E.coli no.(6) exhibited antibiotic-resistant to OF and showed a good (14-18 mm) degree of sensitivity (1Z=16 mm) to the methanolic extracts of H.tuberculatum same extent as to Piperacillin/Tazobactam. P.aeruginosa no.(7) showed fairly high effect (1Z = 18 mm) against H.tuberculatum methanolic extracts in the same extent as to OF.Phytochemical constituents of H tuberculatum revealed polyphenolic compound as resveratrol,myricetin and quercetin flavonol kaempferol and rutinand rosmarinic acid (Abdelkhalek et al.,2012). Evidence indicated that bioactive substances (antioxidants) possess extranutritional charateristics and advanced role in food-disease association.Radical scavenging activity under physiological conditions needs additional research to show and to determine whether there is any link between their radical scavenging properties and their antimicrobial potential (Ramadan et al., 2003).
U. molliuscula methanolic extracts revealed high (˃18 mm) antibacterial effect (1Z = 20 -18 mm) against P.aeruginosa, S.aureus, and P.mirabilis. There is need for more research on the isolation and recognition of active ingradients and to assess the probable synergism between extract constituents for their antibacterial activities based on the results obtained (Ali et al., 2012). P. aeruginosa, S. aureus, and P. mirabilis showed antibiotic-resistant against U.molliuscula extracts. There is need to discover and develop new antimicrobial medications for the treatment of diarrhoea and other bacterial infections due to P.mirabilis and E. coli through support scientific base and inclusion of traditional practices in present system of medicines.
Only one of all S. aureus clinical isolates was more sensitive and effective against methanolic extract of U.molliuscula than Gram (-ve) antibiotics (Tables 2 and 3). Lichen metabolite usnic acid exhibits antimicrobial activity against plant and man infectious microorganism, including antimicrobial efficacy against antibiotic-resistant bacterial strains (Ingólfsdóttr, 2002). Aqueous extract of U. molliuscula lichen did not exhibit antibacterial properties against all tested bacteria.The obtained results may account for the reason people in Sudan refuse to use aqueous extract countiniously as a treatment of gastrointestinal tract. It is speculated that it may possess anti-inflammatory activities, where it is used in Sudan as a bitter stomachic, for cough and also by women to relive menstrual pain (Kheir, 1966).
Nigella sativa methanolic extract showed moderate (1Z=14-18 mm) potency (1Z=16 mm) against S.para typhi B (Table 1).More and more beneficial effects of N.sativa should be explored in order to maximize its utility in effective treatment and cure for various diseases.A single strain of S. typhi B clinical isolates was resistant to most of the plant extracts analyzed.This bacterial species is of importance in gastrointestinal tract infections and deserves a wider investigation, including a large number of strains.Aqueous extract did not exhibit antibacterial potentials against all tested bacteria.Thymoquinon possesses anti-inflammatory activities,protects the cell membrane integrity through inhibition of lipid peroxidationm and alters levels of leukotrienes and prostaglandins favoring cytoprotection of the gastric mucosal cells (Kanter et al., 2005).Scavenging activity of the free radical ions was increased due to effectiveness of human serum albumin (HSA) isoforms (‘N’ form at pH 7.4 and ‘B’ form at pH 9.0) in the presence of Thymoquinone (TQ), the main constituent in N. sativa (Ishtikhar et al., 2015).
All plant aqueous extracts except H. tuberculatum do not possess significant antibacterial efficacy against tested bacteria.Phytochemical investigation revealed the presence of Terpenes and β-phellandrene,limonene, β-ocimene, β-caryphyllene,myrcene, and α-phellandrene, the most rich oil components (Al-Burtamani et al., 2005).It is speculated that they may possess anti-inflammatory characteristics. The potential therapeutic assessment of the medicinal plants has been the subject of continual research for their anti-inflammatory componants, including the terpenes which have pharmacological actions (Souza et al., 2014). H. tuberculatum aqueous extract showed moderate (1Z=14-18 mm) potency (1Z=18-16 mm) against clinical isolates E. coli and P. aeruginosa. Antibacterial activity of the extract showed promising result against P. aeruginosa and E. coli clinical isolates, the most common one and problematic among opportunistic pathogens.Resistance to antimicrobial drugs may be a problem. The use of an appropriate combination therapy is important. This plant plays vital role in man health, possesses different pharmacological properties and bioactive materials; therefore, it might participate in various drug productions. Its cultivation is very important.
U. molliuscula, H.tuberculatum, and N.sativa extracts show positive microbial potency. These plants may be considered as important sources for new antimicrobial drugs. Therefore they will contribute to the devolopment of new methods to treat infectous diseases and intestinal disorders caused by some pathogenic strains.U molliuscula in folk medicine is used widely for the treatment of different diseases,but scientifically few of them have been invetigated .Thus more scientific research should be harmonized to investigate unutilized activities. Thymoquinone is a phytochemical compound found in the plant N.sativa; it was found to be effective against Gram -positive bacteria (Kokoska et al., 2008). Depending on doses the oil showed antibacterial activity against all tested bacteria (Salman et al., 2008). Thymoquinone and Thymohydroquinone may be used for the treatment of infections alone or in combination with some antibiotics, especially in case of highly susceptible Gram (+ve) bacteria S.aureus (Halawani, 2009).More and more beneficial effects of N. sativa should be explored in order to maximize its utility for effective treatment and curing of various diseases (Naz, 2011).
Methanolic extract of A. nilotica, R. minima, H. abyssinica and aqueous extracts of C. phelypaea did not exhibit antibacterial properties against all tested bacteria except A. nilotica against E. coli (ATCC 25922) (Tables 1).It is speculated that it may possess anti-inflammatory activities. The antioxidant activity of H. abyssinica aqueous extract and A. nilotica extracts reported by Mahjoub (2013) revealed that the solvent extracts exhibited strong to modrate antioxidant activity as compared to other plants .Aqueous and methanolic extracts of R. minima showed the presence of alkaloids, flavonoids, tannins, trepenoids, glycoside and steroid were absent (Mali and Mahale, 2008). In recent years, the trend towards natural products that are considered as antioxidant, antimicrobial, anti-inflammatory and similar agents is rapidly increasing in the prevention and treatment of diseases. Accordingly, various speculations on natural products could arise and lead to information on pollution (Sevindik et al., 2017).
The results demonstrate that the extremely active plant was U. molliuscula. Methanolic extracts of U. molliuscula of all extracts possessed significant antibacterial efficacy. All the plants’ aqueous extracts did not clearly show antibacterial activities against all tested bacteria except H. tuberculatum. U. molliuscula showed high antibacterial activity against P. aeruginosa, S. aureus, P.mirabilis and S.para typhi, B clinical isolates; also B. subtilis (NCTC 8236) showed high sensitivity (1Z = 4mm) to methanolic extracts of U.molliuscula . All plants’ aqueous extracts were less effective against the bacterial growth of all tested Bacteria except H. tuberculatum; they showed high effect against P.aeruginosa and moderate effect against E.coli clinical isolates.Aqueous extract of U.molliuscula, N. sativa, A. nilotica. R. minima, and H. abyssinica were found to be ineffective against all tested bacteria. Studied medicinal materials of U. molliuscula were satisfactory on the basis of their antibacterial properties. An adequate toxicological testing must be carried out to confirm the capability of using these plants to fight against infectious dieases.
The author has not declared any conflict of interests.
The author expresses his gratitude to Prof.Hatil Hashim El-kamali, for his encouragement and support; also to Departments of Biochemistry and Microbiology of Medicinal and Aromatic Plants Research Institute for providing the basic research facilities.
REFERENCES
|
Abdallah EM (2011). Plants: An Alternative Source for Antimicrobials. Journal of Applied Pharmaceutical Science 1(06):16-20.
|
|
|
|
Abdelgaleil SA, Saad MM, Ariefta NR, Shiono Y (2020). Antimicrobial and Phytotoxic Activities of Secondary Metabolites from Haplophyllum Tuberculatum and Chrysanthemum Coronarium. South African Journal of Botany 128:35-41.
Crossref
|
|
|
|
|
Abdelkhalek A, Salem MZ, Hafez E, Behiry SI, Qari SH (2020). The Phytochemical, Antifungal, and First Report of the Antiviral Properties of Egyptian Haplophyllum tuberculatum Extract, Biology 9:248-265.
Crossref
|
|
|
|
|
Al Alawi SM, Hossain MA, Abusham AA (2018). Antimicrobial and Cytotoxic Comparative Study of Different Extracts of Omani and Sudanese Gum acacia. Beni-Suef University Journal of Basic and Applied Sciences 7(1):22-26.
Crossref
|
|
|
|
|
Al-Burtamani SK, Fatope MO, Marwah RG, Onifade AK, Al-Saidi SH (2005). Chemical Composition, Antibacterial and Antifungal Activities of the Essential Oil of Haplophyllum Tuberculatum from Oman. Journal of Ethnopharmacology 96(1):107-112.
Crossref
|
|
|
|
|
Ali A, Akhtar N, Khan BA, Khan MS, Rasul A, UZ-Zaman Sh, Khalid N, Waseem Kh, Mahmood T, Ali L (2012). Acacia nilotica: A Plant of Multipurpose Medicinal Uses. Journal of Medicinal Plants Research 6(9):1492-1496.
Crossref
|
|
|
|
|
Ali KS, Salih TA, Daffalla HM (2020). In vitro Phytochemical, Larvicidal and Antimicrobial Activities of Gum Arabic Extract. Walailak Journal of Science and Technology 17(3).
|
|
|
|
|
Banjar MM, Khafaji AM, Maher YA (2017). Antimicrobial Activity of Hydrogen Peroxide, Sesame and Gum Arabic against Streptococcus. International Journal of Health Sciences and Research 7(1):97-104.
|
|
|
|
|
Chaudhry NM, Tariq P (2008). In Vitro Antibacterial Activities of Kalonji, Cumin and Poppy Seed. Pakistan Journal of Botany 40(1):461-467.
|
|
|
|
|
Cheesbrough M (1984). Culture Media. In: Medical Laboratory Manual for Tropical Countries. Tropical Health Technology and Butterworth-Heineman. Cambridge 3(60-9):407-428.
|
|
|
|
|
El-Kamali HH, El-Amir MY (2010). Antibacterial Activity and Phytochemical Screening of Ethanol Extracts Obtained from Selected Sudanese Medicinal Plants. Current research Journal of Biological Sciences 2(2):143-146.
|
|
|
|
|
Elkamali HH, Mahjoub SE (2015). Antioxidant potential of Some Sudanese Medicinal Plants used in Traditional Medicine.International Journal of Scientific World 3(2):192-198.
Crossref
|
|
|
|
|
Gundidza M, Gweru N, Magwa ML, Ramalivhana NJ, Humphrey G, Samie A, Mmbengwa V (2009). Phytochemical Composition and Bio- logical Activities of Essential Oil of Rhynchosia minima (L) (DC) (Fabaceae). African Journal of Biotechnology 8(5):721-724.
|
|
|
|
|
Halawani F (2009). Antibacterial Activity of Thymoquinone and Thymohydroquinone of Nigella sativa L. and Their Interaction with Some Antibiotics. Advances in Biological Research 3(5-6):148-152.
|
|
|
|
|
Haloci E, Manfredini S, Toska V, Ziosi SV, Topi I, Kolani H (2012).Antibacterial and Antifungal Activity Assesment of Nigella Sativa Essential Oils. International Journal of Medical, Health, Biomedical, Bioengineering and Pharmaceutical Engineering 6(6):270-272.
|
|
|
|
|
Hamdi A, Viane J, Mahjoub MA, Majouli K, Hussein M, Gad H, Kharbach M, Demeyer K, Marzouk Z, Heyden YV (2018). Polyphenolic Contents, Antioxidant Activities and UPLC-ESI-MS analysis of Haplophyllum tuberculatum A. Juss leaves extracts. Macromolecules106:1071-1079.
Crossref
|
|
|
|
|
Hosseinzadeh H, Bazzaz BS, Haghi MM (2007). Antibacterial Activity of Total Extracts and Essential oil of Nigella Sativa L. Seeds in Mice. Pharmacolgyonline 2:429-435.
|
|
|
|
|
Ingólfsdóttr K (2002) .Usnic acid. Phytochemistry 61(7):729-736.
Crossref
|
|
|
|
|
Ishtikhar M, Rabbani G, Khan Sh, Khan RH (2015). Biophysical Investigation of Thymoquinone Binding to 'N' and 'B' Isoforms of Human Serum Albumin: Exploring the Interaction Mechanism and Radical Scavenging Activity .Journal of Royal Society of chemistry 24.
Crossref
|
|
|
|
|
Kanter M, Demir H, Karakaya C, Ozbek H (2005). Gastroprotective Activity of Nigella sativa L Oil and its Constituent, Thymoquinone against Acute Alcohol-Induced Gastric Mucosal Injury in Rats. World Journal of Gastroenterology 11(42):6662-6666.
Crossref
|
|
|
|
|
Karar MG, Kuhnert N (2017). Herbal Drugs from Sudan: Traditional Uses and Phytoconstituents. Journal of Pharmacognosy Review 11(22):83-103.
Crossref
|
|
|
|
|
Khalid H, Abdalla WE , Abdelgadir H, Opatz T, Efferth Th (2012). Gems from Traditional North-African Medicine: Medicinal and Aromatic Plants from Sudan.Natural Products and Bioprospecting 2:92-103.
Crossref
|
|
|
|
|
Kheir YM (1966). Investigation of Certain Plants used in Sudanese Local Medicine. M.Pharm.Thesis. Khartoum University.
|
|
|
|
|
Kokoska L, Havlik J, Valterova I, Sovova H, Sajertova M, Jankovska I (2008). Comparison of Chemical Composition and Antibacterial Activity of Nigella sativa Seed Essential Oils Obtained by Different Extraction Methods. Journal of Food Protection 71(12):2475-2480.
Crossref
|
|
|
|
|
Mahjoub SE (2013). Antibacterial and Antioxidant Activities of Some Medicinal Plants Used in Sudanese Traditional Medicine. PHD Thesis. Omdurman Islamic University. Omdurman, Sudan.
|
|
|
|
|
Mali RG, Mahale NB (2008). Evaluation of Rhynchosia minima (Linn.) DC Leaves for Anthelmintic Activity. International Journal of Pharmaceutical Sciences and Nanotechnology 1(2):191-194.
|
|
|
|
|
Mishra RP (2011). Effect of Metal Ions and Drugs on Antibacterial Activities of Nigella Sativa (L.) Seeds.Webmed Central Ayurvedic Medicine 2(8):WMC002074.
|
|
|
|
|
Monika T, Sasikala P, Bhaskara MV (2013). A Investigational Study of Antibacterial Activities of Nigella sativa on Mastits in Dairy Crossbred Cowst. International Journal of Advanced Scientific and Technical Research 4(3):277-283.
|
|
|
|
|
Musa MS, Abdelrasool FE, Elsheikh El A, Ahmed LA, Mahmoud AL, Yagi SM (2011). Ethnobotanical Study of Medicinal Plants in the Blue Nile State, South-eastern Sudan. MPR 17:4287-4297.
|
|
|
|
|
Naz H (2011). Nigella sativa: the Miraculous Herb.Pak. Journal of Biochemistry and Molecular Biology 44(1):44-48.
|
|
|
|
|
Pichette A, Marzouk B, Legault J (2011). Antioxidant, Antiâ€inflammatoyy, Anticancer and Antibacterial Activities of Extracts form Nigella sativa (Black Cumin) Plant Parts. Journal of Food Biochemistry.
|
|
|
|
|
Ramadan MF, Kroh LW, Mörsel JT (2003). Radical Scavenging Activity of Black Cumin (Nigella sativa L.),Coriander (Coriandrum sativum L.), and Niger (Guizotia abyssinica Cass.) Crude Seed Oils and Oil Fractions. Journal of Food Chemistry 1-9.
Crossref
|
|
|
|
|
Saadabi AM, Ayoub SM (2009). Comparative Bioactivity of Hydnora abyssinica A. Braun against Different Groups of Fungi and Bacteria. Journal of Medicinal Plants Research 3(4):262-265.
|
|
|
|
|
Sabry OM, El Sayed AM, Sleem AA (2016). Potential Anti-Microbial, Anti-Inflammatory and Anti-Oxidant Activities of Haplophyllum tuberculatum Growing in Libya. Journal of Pharmacognosy 2(1):1000116.
Crossref
|
|
|
|
|
Salman MT, Khan RA, Shukla I (2008). Antimicrobial activity of Nigella sativa Linn. Seed oilagainst multi-drug resistant bacteria from clinical isolates. CSIR.
|
|
|
|
|
Saini ML, Saini R, Roy Sh, Kumar A (2008). Comparative Pharmacognostical and Antimicrobial Studies of Acacia Species (Mimosaceae) Mohan Lal. Journal of Medicinal Plants Research 2(12):378-386.
|
|
|
|
|
Sevindik M, Akgul H, Pehlivan M, Selamoglu Z (2017). Determination of Therapeutic Potential of Mentha Longifolia ssp. Longifolia. Fresenius Environmental Bulletin 26(7):4757-4763.
|
|
|
|
|
Shehu Z, Lamayi DW, Sabo MA, Shafiu MM (2018). Synthesis, Characterization and Antibacterial Activity of Kaolin/Gum Arabic Nanocomposite on Escherichia Coli and Pseudomonas Aeruginosa. Research Journal of Nanoscience and Engineering 2(2):23-29.
|
|
|
|
|
Sinha SN, Biswas M (2011).Evaluation of Antibacterial Activity of Some Lichen from Angla, Sikkim, India. International Journal of pharma and Bio Sciences 2(4):23-28.
|
|
|
|
|
Souza MT, Almeida JR, Araujo AA , Duarte MC, Gelain DP , Moreira JC , Santos MR, Quintans LJ (2014). Structure-Activity Relationship of Terpenes with Anti-Inflammatory Profile - A Systematic Review. Basic and Clinical Pharmacology and Toxicology115:244-256.
Crossref
|
|
|
|
|
Sravani P, Kiranmayee Y, Narasimha MS, Reddy VS, Asha S, Kumar RB (2014). In vitro Experimental Sstudies on Selected Natural Gums and Resins for their Antimicrobial Activity. Research Journal of Pharmaceutical, Biological and Chemical Sciences 5(1):154-172.
|
|
|
|
|
Trojan R, Razdan L, Singh N (2016). Antibiotic Susceptibility Patterns of Bacterial Isolates from Pus Samples in a Tertiary Care Hospital of Punjab, India. International Journal of Microbiology.
Crossref
|
|
|
|
|
Vijayasanthi M, Kannan V, Venkataswamy R, Doss A (2012). Evaluation of the Antibacterial Potential of various solvent extracts of Acacia nilotica linn Leaves. HYGEIA - Journal for Drugs and Medicines 4(1):91-96.
|
|
|
|
|
Weckesser S, Engel K, Simon-Haarhaus B, Wittmer A, Pelz K, Schempp CM (2007). Screening of Plant Extracts for Antimicrobial Activity against Bacteria and Yeasts with Dermatological Relevance. Phytomedicine1-9.
Crossref
|
|
|
|
|
WHO Publication (2001).
View. 22-4-2003.
|
|
|
|
|
Yoruk O, Gur FO, Uyanik H, Yasar M, Mutlu V, Altas E, Baysal E, Taysi S (2010). Antioxidant Effects of Nigella Sativa in the Treatment of Experimentally Induced Rhinosinusitis. Macedonian Journal of Medical Sciences 3(2):132-137.
Crossref
|
|