ABSTRACT
This study was conducted to determine the bacteriological quality of retail raw meat. Twenty one raw meat samples were randomly collected from four administrative towns: Bure, Debre-Markos, Dejen and Fenoteselam meat-stalls of Gojjam area. Samples were collected and transported kept in cold sterile screw cap bottles with ice contained icebox. It was cultured on plate count agar and mannitol salt agar plates for enumeration of aerobic and pathogenic staphylococci bacteria respectively. Bacterial isolation was identified by culturing on selective medium and biochemical test. The mean total aerobic plate count (APC) ranged 6.325 to 6.477 log cfu/g was not significantly different (P<0.05); but enumeration of pathogenic Staphylococci ranged 3.588 to 4.251 log cfu/g was significantly different (P<0.05) between places. According to international standards microbial quality acceptability of ready-to-eat food and raw meat aerobic plate and pathogenic staphylococci count of almost all samples were categorized in borderline and unsatisfactory quality. A total of 65 gram positive cocci isolates were identified. The dominant bacterial pathogens isolates were Staphylococcus epidermidis, Staphylococcus aureus and Streptococcus pyogenes in a ratio of 0.86, 0.71 and 0.71 respectively. The high bacteria count and isolates of aerobic plate count and pathogenic Staphylococci is an indication of higher risk for retail raw meat consumption. Hence needs improved hygienic practice at all levels in the raw meat production industry.
Key words: Raw meat, aerobic plate count, pathogenic Staphylococci, Gojjama.
Foodborne diseases are an important cause of morbidity and mortality in worldwide but the full extent and cost of unsafe food, and especially the burden arising from contaminants in food, is still unknown. Foodborne bacterial pathogens are any bacteria in food with the potential to cause an adverse health effect (WHO, 2015). Normally bacterial pathogens associated with meats can pose risks with food poisoning; and contamination may be associated with the animals themselves, or be introduced to a clean carcass through cross contamination (FSA, 2015).
To supply healthy food for the consumer’s microbiological quality guidelines of food have been developed. Microbiological quality of ready-to-eat food in general has limits for consist of aerobic colony count; hygiene indicator organisms; and specific foodborne pathogens (CFS, 2014). According to Commission Regulation EC (2014) and FSANZ (2016), the aerobic colony count of beef of more than 105 cfu/cm2, and ready-to-eat sliced meat of more than 107 cfu/g, respectively has unsatisfactory quality standard and also hazards for consumption. In general the aerobic colony count of raw meat microbiological quality is classified into three classes: Satisfactory, if the test results indicating good microbiological quality; borderline or marginal, if the test results are not unsatisfactory and also not satisfactory; and unsatisfactory, if the test results indicate high bacterial count (FAO, 2007; CFS, 2014; Commission Regulation EC, 2014; FSANZ, 2016).
In addition testing pathogenic microorganisms associated with food is significantly important from public health point of view. From these Gram Positive Cocci (GPC) like Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pneumonia, Streptococcus pyogenes and Enterococcus faecalis are pathogenic microorganisms and responsible for human infections (Todar, 2008; CFS, 2014; FSANZ, 2016). According to CFS (2014) and FSANZ (2016) count of S. aureus in ready-to-eat food is more than 104 and 103 cfu/g respectively has unsatisfactory microbiological quality. In Ethiopia the passion for eating raw meat is at highest and the way it is eaten is sometimes shocking.
Meat-stalls are often packed with people who come to eat raw meat; but there is no microbiological standard and also monitoring system for retail raw meat products. On the other hand food consumers in Ethiopia suffer from food-borne bacterial illnesses with the likes of Staphylococcus aureus (Ayana et al., 2015). The trend of raw meat consumption in administrative towns of Gojjam area is known. However there has no microbial quality standard finding of retail raw meat in the study area. Therefore this research was undertaken to check microbial quality of raw meat in meat-stalls; and isolation of S. aureus and other pathogenic gram-positive cocci.
Sampling
The study was conducted in selected four administrative towns of Mirab-Gojjam and Misraq-Gojjam Zones. In each of these zones two administrative towns; Debremarkos and Dejen towns from Misraq-Gojjam zone and Bure and Finoteselam towns from Mirab-Gojjam zone were selected. Twenty-one meat-stalls: Four from Bure, eight from Debremarkos, four from Dejen and five from Fenoteselam were randomly selected. From each meat-stall subsamples were taken from different parts of the available carcass; butchers comminute the subsamples with them knife and thoroughly mixed to form a composite sample. Samples were collected cross-sectionally between April and August 2014; and transported kept in cold sterile screw cap bottles with ice contained icebox to DebreMarkos University College of Agriculture and Natural Resource Management Animal Science Laboratory for analysis.
Preparation of test sample and serial dilution
Blended 25 g of comminuted composite meat sample with 225 ml of peptone water (HIMEDIA) solution. Further decimal dilutions were carried out at 1:10 ratios with peptone water diluents according to EAS (2008).
Aerobic plate counts
Inoculated 0.1 mL of 4
th to 2
nd (the highest to the lowest dilution) consecutive serial dilutions in duplicate at the center of accordingly labeled pre dried Petri dishes of aerobic Plate Count Agar (PCA) (HIMEDIA) plates. The inoculum spread using sterilized hockey glass spreader and incubated at 35 to 37°C for

. Then plates containing 30 to 300 colonies were selected and recorded average of the colonies counted as number of colony forming units per gram (cfu/g) for each sample.
Enumeration of pathogenic Staphylococci
Inoculated 0.1 mL of 3
rd to 1
st serial dilutions at the center of accordingly labeled pre dried Petri dishes of Mannitol Salt Agar (MSA) (HIMEDIA) plates into duplicate and incubated at 35 to 37°C for
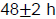
. Then plates containing 20 to 200 colonies were selected and recorded average of the colonies counted as number of colony forming units per gram (cfu/g) for each sample.
Identification and conformation of gram positive cocci species
From the last dilution inoculum of PCA plates 5 to 10 different colonies were sub-cultured separately on labeled nutrient agar (NA) plates (HIMEDIA) and incubated at 35 to 37°C for 28 to 48 h. Grown colonies on NA plates were subjected for potassium hydroxide test according to PHE (2010) to identify gram positive organisms, and catalase test according to PHE (2014) to identify catalase positive and negative reaction. Only gram positive and catalase negative colonies were sub-cultured on 5% sheep blood enriched Azide Blood Agar Base (HIMEDIA) plates and Bile Esculin Agar (HIMEDIA) slanted test tube medium. Species identifications were undertaken regarding hemolytic and esculin fermentation activity of the inoculum. In addition other gram positive cocci species were isolated on the mannitol fermention character from MSA plates.
Method of data analysis
Microbial count of log10 transformed value was analyzed with mixed procedure using Statistical Analysis Software (SAS) 9.2 to determine the difference between fixed effect of places and random effect of meat-stalls. For the fixed effect (administrative town), least square difference was used to separate means when the parameter tests were significantly different at P< 0.05. The PROC MIXED estimates the variance components for the meat-stalls and the residual by default used Restricted Maximum Likelihood Estimation (REML). The convergence criteria of colony forming unit bacterial count of PCA plates and MSA plates were met at the first and second iteration respectively. In addition standardization of colony counts and isolates were analyzed with a descriptive statistics.
Bacterial counts and standardization
The mean aerobic plate count was not significantly (P≥0.05) different; but the mean count of pathogenic staphylococci was significantly (P≥0.05) different between places. The least square mean differences indicate that the highest pathogenic staphylococci count (4.251log cfu/g) was found from Debremarkos but it was not significantly (P≥0.05) different from the mean count (4.063 log cfu/g) of Fenoteselam. On the contrary the least count (3.588 log cfu/g) of pathogenic staphylococci was found from Bure however statistically there was no significant (P≥0.05) difference with Dejen (3.788log cfu/g) samples. On the other hand there was no significant (P≥0.05) difference between Fenoteselam and Dejen (Table 1).

The variance estimate of meat-stalls and residual are presented in Table 2. It indicates that both variance components are significantly (P≥0.05) equals to 0. These estimates suggested that no difference in their mean aerobic plate counts and pathogenic Staphylococci among meat-stalls between and within places. Generally the aerobic plate counts and pathogenic staphylococci were standardized in to three category: Satisfactory, borderline and unsatisfactory illustrated in Figure 1. According to FAO (2007) recommonded microbial criteria for fresh meat, and Commission Regulation EC (2014) process hygine criteria of aerobic colony count for cattle carcass, of all meat samples were categorized in unsatisfactory quality.


On the other hand according to aerobic colony count levels in ready-to-eat foods, most (95.24%) of the meat samples were categorized in borderline microbial quality of aerobic colony count; while only few (4.76%) samples had satisfactory quality. In addition according to CFS (2014), S. aureus and other coagulase-positive staphylococci criteria in ready-to-eat foods, 47.62 and 52.38% of the samples were categorized in unsatisfactory and borderline quality respectively; but regarding FSANZ (2016), S. aureus in ready-to-eat food, all meat samples were categorized in unsatisfactory quality. Even if, similarly with this finding the mean viable count 4.52×106 cfu/cm2 was found from raw meat displayed for sale at Sokoto, Sokoto State, Nigeria (Danlami et al., 2013). Jahan, Mahbub-E-Elahi and Siddique (2015) also found 1.6 × 107 to 4.23 × 107cfu/g mean viable count from fresh raw beef samples collected from seven major markets of Sylhet Sadar.
In addition 1.73 × 107 cfu/g of Staphylococcal count was found from spice used for traditionally dried and grilled meat product at Dandalin Fagge, Kano State, Nigeria (Shamsuddeen, 2009). In all these findings in lined and catagorized in unsatisfactory microbial quality regarding of international standardes. The most probable reasons of unsatisfactory quality of aerobic plate count and pathogenic staphylococci count in retail raw meat might be transferred from butcher’s hands, tools, working surfaces, equipment, water, pests, cleaning equipment, packaging or other meat and/or offal. In addition, the inadequate temperature contro can enhance the microbial load during transportation and storage(Melngaile et al., 2014; FSA, 2015).
Prevalence of Gram positive cocci isolates
From the total meat samples S. epidermidis (0.86), S. aureus (0.71) and S. pyogenes (0.71) were found in higher ratio and followed by Streptococcus species (0.48) other than S. pyogenes and S. pneumonia; E. faecalis (0.24) and S. pneumonia (0.10). The occurrence of gram positive cocci bacterial isolate per sample size of the study area, Finoteselam was the leading with 4.20 ratios; and tailed by Bure, DebreMarkos and Dajen with ratio of 3.25, 2.63 and 2.5 respectively. From the total 65 isolates equivalently a ratio of 0.32 was found from DebreMarkos and Fenoteselam; when 0.20 and 0.15 ratios isolated from Bure and Dajen respectively (Table 3).

In line with this finding S. epidermidis (Gundogan and Ataol, 2013; Igbinosa et al., 2016; Jackson et al., 2013), S. aureus (Danlami et al., 2013; Gundogan and Ataol, 2013; Jackson et al., 2013; Adzitey, 2016; Igbinosa et al., 2016; Raji et al., 2016; Martinez, 2017) and Streptococcus species (Danlami et al., 2013) were found from retail raw meat and/or meat products. Isolate of S. epidermidis in higher ratio of DebreMarkos might be contaminated with skin, conjunctiva, nose, pharynx, mouth, lower intestine, and anterior urethra; because it is highly adapted to the diverse environments of human body (Todar, 2008).
Isolated bacterium might be transmitted from the animals during dressing and/or butcher’s hands (FSA, 2015). Concerning the bacteriological culture of the nose and skin of normal humans invariably yields staphylococci. S. pyogenes is estimated that between 5 and 15% of normal individuals harbor, usually in the respiratory tract; S. pneumoniae also a normal inhabitant of the human upper respiratory tract. E. faecalis is a regular component of the intestinal flora, that many European countries use it as the standard indicator of fecal pollution (Todar, 2008).
Staphylococcal food-borne disease is one of the most common food-borne disease and is of major concern in public health programs worldwide (Kadariya et al., 2014). S. aureus causes a variety of supportive (pus-forming) infections and toxinoses in humans. In food it causes food poisoning by releasing enterotoxins into food, and toxic shock syndrome by release of superantigens into the blood stream (Todar, 2008; USDA, 2012). S. pyogenes is introduced or transmitted to vulnerable tissues, a variety of types of supportive infections can occur. S. pyogenes is the leading cause of uncomplicated bacterial pharyngitis and tonsillitis commonly referred to a strep throat. Other respiratory infections include sinusitis, otitis, and pneumonia. S. pneumonia can cause pneumonia, usually of the lobar type, paranasal sinusitis and otitis media, or meningitis which is usually secondary to one of the former infections. It is currently the leading cause of invasive bacterial disease in children and the elderly. In recent years, E. faecalis has emerged as a significant, antibiotic-resistant, nosocomial pathogen (Todar, 2008).
CONCLUSION AND RECOMMENDATION
High aerobic plate and pathogenic Staphylococci count beyond international microbial standards indicates poor production and handling practices of retail raw meat. Moreover the isolates of S. aureus in a higher concentration could result in public health risk for raw meat consumers. This suggests the need for improved hygiene practices at all levels in the raw meat production industry. In addition the responsible body of governmental and non-governmental organizations should strengthen awareness campaigns on improved hygiene practices and the rate of microbial infections with food poisons.
The authors have not declared any conflict of interests.
Authors would like to thank Debre-Markos University research directorate for financing this study.
REFERENCES
|
Adzitey F (2016). The prevention and control of bacterial foodborne hazards in meats and meat products-An overview. J. Meat Sci. Technol. 4(1):1-10. |
|
|
|
Ayana Z, Yohannis M, Abera Z (2015). Food-Borne Bacterial Diseases in Ethiopia. Acad. J. Nutr. 4(1):62-76. |
|
|
|
CFS (2014). Microbiological Guidelines for Food For ready-to-eat food in general and specific food items. Revised. Centre for Food Safety. Available at: http://www.cfs.gov.hk/english/food_leg/files/food_leg_Microbiological_Guidelines_for_Food_e.pdf. |
|
|
|
Commission Regulation EC (2014). Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. |
|
|
|
Danlami S, Magajia AA, Garbaa B, Saidub B, Aliyuc M, Suliemanb N, Wurno BS (2013). Bacteriological quality of raw meat displayed for sale at Sokoto, Sokoto state, Nigeria. Sci. J. Microbiol. 2(7):134-139. |
|
|
|
EAS (2008). Microbiology of food and animal feeding stuffs — Preparation of test samples, initial suspension and decimal dilutions for microbiological examination — Part 1-2: General rules for the preparation of the initial suspension and decimal dilutions'. Available at: https://law.resource.org/pub/eac/ibr/eas.217.1.2.2008.pdf. |
|
|
|
FAO (2007). Meat processing technology for small- to medium- scale producers. Bangkok: Food and agriculture organization of the United Nations regional office for Asia and the pacific. Available at: http://www.fao.org/docrep/010/ai407e/AI407E00.htm. |
|
|
|
FSA (2015). Meat Industry Guide. Food Standards Agency. Available at: https://www.food.gov.uk/business-industry/meat/guidehygienemeat. |
|
|
|
FSANZ (2016). Compendium of Microbiological Criteria for Food. Food Standards Australia New Zealand. Available at: www.foodstandards.gov.au. |
|
|
|
Gundogan N, Ataol O (2013). Biofilm, protease and lipase properties and antibiotic resistance profiles of staphylococci isolated from various foods. Afr. J. Microbiol. Res. 7(28):3582-3588. |
|
|
Jackson CR, Davis JA, Barrett JB (2013). Prevalence and Characterization of Methicillin-Resistant Staphylococcus aureus Isolates from Retail Meat and Humans in Georgia. J. Clin. Microbiol. 51(4):1199-1207.
Crossref |
|
|
Jahan F, Mahbub-E-Elahi ATM, Siddique AB (2015). Bacteriological Quality Assessment of Raw Beef Sold in Sylhet Sadar. The Agriculturists 13(2):9-16.
Crossref |
|
|
|
Kadariya J, Smith TC, Thapaliya D (2014). Staphylococcus aureus and Staphylococcal Food-Borne Disease : An Ongoing Challenge in Public Health', BioMed Res. Intern. 2014, P 9. |
|
|
|
Melngaile A, Ciekure E, Valcina O (2014). Microbiological quality of meat preparations and meat products, in foodbalt, pp. 61-65. |
|
|
|
PHE (2010). UK Standards for Microbiology Investigations Potassium Hydroxide Test', Public Health England. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/394289/TP_30i3.pdf. |
|
|
|
PHE (2014). UK Standards for Microbiology Investigations Catalase Test', Public Health England. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/376073/TP_8i3.pdf. |
|
|
|
Shamsuddeen U (2009). Microbiological quality of spice used in the production of Kilishi a traditionally dried and grilled meat product', Bayero J. Pure Appl. Sci. 2(2):66-69. |
|
|
|
Todar K (2008). Todar's Online Textbook of Bacteriology. Available at: http://textbookofbacteriology.net/. |
|
|
|
USDA (2012). Introduction to the Microbiology of Food Processing. United States Department of Agriculture (USDA) Food Safety and Inspection Service (FSIS). Available at: |
|
|
|
WHO (2015). WHO estimates of the global burden of foodborne diseases: food borne disease burden epidemiology reference group 2007-2015. World Health Organization. Available at: http://apps.who.int/iris/bitstream/10665/199350/1/9789241565165_eng.pdf |
 . Then plates containing 30 to 300 colonies were selected and recorded average of the colonies counted as number of colony forming units per gram (cfu/g) for each sample.
. Then plates containing 30 to 300 colonies were selected and recorded average of the colonies counted as number of colony forming units per gram (cfu/g) for each sample.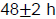 . Then plates containing 20 to 200 colonies were selected and recorded average of the colonies counted as number of colony forming units per gram (cfu/g) for each sample.
. Then plates containing 20 to 200 colonies were selected and recorded average of the colonies counted as number of colony forming units per gram (cfu/g) for each sample.