ABSTRACT
Lamb meats are nutritious, easily metabolisable, and offer suitable substrates for the growth and metabolism of microorganisms. The main purpose of the present study was to investigate the antagonist effect of Laurus nobilis leaf extract against growth of bacteria present in fresh lamb meat. The 16S rRNA gene sequence analysis was used to identify bacteria present in lamb meat samples. Eighteen bacterial species were detected in 20 samples of fresh lamb meat. The antibacterial activity of L. nobilis leaf extract against growth of the isolated bacteria was examined using agar well diffusion method. Gram-positive and Gram-negative showed differences in their response to L. nobilis leaf extract. Staphylococcus saprophyticus and Proteus vulgaris were the most bacteria affected by L. nobilis leaf extract. Spraying fresh lamb meat with 10% (v/v) L. nobilis leaf extract was found to increase the shelf-life of lamb meat kept at room and refrigeration temperatures from 1 to 3 and 6 to 13 days, respectively. L. nobilis essential oil seems to be a promising tool that can be used as a natural preservative for fresh lamb meat.
Key words: Fresh lamb meat, Laurus nobilis, 16S rRNA gene, antibacterial activity.
Lamb meats, by their nature, are nutritious and easily metabolisable and therefore offer suitable substrates for the growth and metabolism of microorganisms (Thanigaivel and Anandhan, 2015). The microorganisms that eventually cause the spoilage of fresh meat are either present at the time of slaughter or introduced by workmen and their cutting tools, or by water and air in the dressing, cooling and cutting rooms (Newman, 2005). Lamb meat has a short shelf-life of about one day or less at ambient temperature (15-30°C), and few days at refrigerating temperature (0-10°C) (Lucera et al., 2012). This is mainly due to the microbial growth of both pathogenic and non-pathogenic microorganisms, and/or lipid oxidation (Lucera et al., 2012). Staphylococci, Corynebacterium, Streptococci, Micrococcus, Salmonella, Escherichia coli, and yeast have been isolated from fresh lamb meat (Mostafa et al., 2018). In another study, it was reported that the most common bacteria found in fresh meat were bacteria of the genera Acinetobacter species, Pseudomonas species, Brochothrix, Flavobacterium, Psychrobacter, Moraxella, Staphylococcus species, and Micrococcus, lactic acid bacteria and various genera of the Enterobacteriaceae family (Pennacchia et al., 2011).
An assortment of synthetic antimicrobials has been used to decrease bacterial contamination in meat and fresh products (Rafiq et al., 2016). However, synthetic antimicrobials like butylated hydroxyanisole, and carmine have been connected with health problems such as hypersensitivity, allergies, asthma, hyperactivity, and cancer (Anand and Sati, 2013). Furthermore, synthetic antimicrobials can destroy both harmful and beneficial bacteria in the human intestine, and increase bacterial resistance to antimicrobials (Verraes et al., 2013). Natural preservation is considered as a safety parameter in foods with reduced contents of ingredients and additives that usually render to slow down microbial growth (Gharsallaoui et al., 2016).
Laurus nobilis, commonly known as Bay, is a plant belonging to the Lauraceae family (Ghadiri et al., 2014), which comprises about 2500 species (Basak and Candan, 2013). L. nobilis is a native of the southern parts of Europe and the Mediterranean area (Caputo et al., 2017). L. nobilis is an aromatic herb used broadly to add a distinctive aroma and flavor to food (Fernández et al., 2018). Laurus leaf has been used traditionally as herbal medicine to treat rheumatism, ear aches, indigestion, sprains, promote perspiration, and treat a variety of complaints like neuralgia and intestinal cramps (El Malti and Amarouch, 2009). Eucalyptol (1,8-cineole), sabinene, and linalool are the main components of the essential oil isolated from the leaves of L. nobilis (Caputo et al., 2017). All of these components are classified as monoterpenes or modified monoterpenes, which were shown to have antibacterial, antifungal, and antioxidant activities (Basak and Candan, 2013). The antimicrobial activity of the L. nobilis essential oils has taken great importance as an alternative for synthetic antimicrobials because they are a part of the human diet and their biodegradability suggests low poisonous residue problems. Some studies have proved the potential capacity of laurel essential oil as an antimicrobial agent and also the antioxidant property of leaves extracts (Ouibrahim et al., 2013; Ghadiri et al., 2014; Nehir El et al., 2014; Rafiq et al., 2016). L. nobilis was shown to have a strong antagonist activity against 20 strains of bacteria like Salmonella, Staphylococcus aureus, Esherichia coli, Listeria monocytogenes (Ouibrahim et al., 2013). Growth of S. aureus was also found to be inhibited by a hydroalcoholic extract of L. nobilis (Ghadiri et al., 2014). Furthermore, L. nobilis leaf extract was able to inhibit the growth of Salmonella typhimurium and E. coli O157:H7 (Rafiq et al., 2016).
The preservative effect of modified atmosphere and vacuum packaging can be increased by L. nobilis essential oils in chicken meat (Esmer et al., 2011). An aqueous extract of L. nobilis leaf and chitosan was used as a natural edible coating to increase the shelf life of cashew (Azimzadeh and Jahadi, 2018). To our knowledge, no studies have been carried out about the antagonist effect of L. nobilis against bacteria present in fresh lamb meat and the possibility of using L. nobilis leaf extract as a natural preservative to extend the shelf life of fresh lamb meat.
The main objective of the present study was to isolate and identify bacteria from fresh lamb meat and to investigate the antibacterial activity of L. nobilis leaf extract against those bacteria. Furthermore, the ability of L. nobilis leaf extract to extend the shelf life of fresh lamb meat was evaluated.
Fresh lamb meat samples
Twenty samples of fresh lamb meat of 200 g each were collected within 4 h of slaughtering from four different butcher shops located in Tulkarem city, Palestine. Meat samples were placed in clean, dry, and sterile glass bottles, and were transferred in a refrigerator bag to the laboratory. Microbiological analysis of meat samples was carried out within 1 h.
Isolation of bacteria
Meat samples were aseptically cut into thin smaller pieces of 10 g each and were submerged in sterile tubes that contain 90 ml of diluent saline peptone (SPO) [0.1% bactopeptone (Difco, Detroit, MI, USA), 0.85% (w/v) NaCl (Merck, Darmstadt, Germany), 0.03% Na2H2PO4, 2H2O (Merck) and were vortexed for 3 min. Ten-fold dilutions were prepared from each tube and spread onto three types of culture media, which included blood agar (HiMedia M1133-500G Columbia Blood Agar Base with Hemin), Macconkey agar (HiMedia M081B-100G MacConkey Agar Medium H), and chocolate agar (HiMedia M1133-500G Columbia Blood Agar Base with Hemin). Plates were incubated at 37°C for 24 h under aerobic and anaerobic conditions using candle jars (Karoki et al., 2018). The number of colony forming units (CFU) was recorded.
From a suitable dilution of each sample, a representative colony with the same characters of size, morphology, and colour was picked and recultivated on the same culture medium from which it was isolated and incubated at 37°C for 24 h under aerobic and anaerobic conditions using candle jars. Characterization of the isolates was achieved by initial morphological examination of the colonies on the plates (macroscopically) for colony appearance, size, elevation, form, edge, consistency, color, odor, opacity, hemolysis and pigmentation (Nagarajan et al., 2018), and the results were recorded. A colony from each group of colonies that has the same properties was recultivated.
Extraction of deoxyribonucleic acid (DNA)
Heat treatment method was used to extract DNA from bacteria; 2 colonies of each bacterial isolate which were grown overnight at 37°C were placed in an Eppendorf tube filled with 1 ml of ultrapure DNase/RNase-free distilled water and boiled for 10 min in a water bath. The tubes were then centrifuged for 5 min at 1000 ± g (Dashti et al., 2014). The supernatants were used as templates for the PCR reactions.
Polymerase chain reaction (PCR)
Polymerase Chain Reaction-Ready™ (PCR-Ready™) High Specificity kit (Syntezza Company, PCR-S-192, Israel) was used. Forward primer U968-GC (5’-CGC CCG GGG CGC GCC CCG GGC GGG GCG GGG GCA CGG GGG GAA CGA GAA GAA CCT TAC-3) and reverse primer L1401 (5’-GCG TGT GTA CAA GAC CC-3’) were used in the PCR reaction mixture. According to the manufacturer, for each reaction mixture, 11 μl of 0.5 μM of each primer and 3 μl of DNA were added to a PCR-Ready™ master mix tube. The reactions were performed in an automatic thermal cycler (Applied Biosystems™ Veriti™ 96-Well machine) with initial denaturation at 94°C for 3 min; 30 cycles at 94°C for 30 s, 56°C for 30 s and 68°C for 60 s; final extension at 68°C for 10 min. The PCR products were analyzed by electrophoresis in
2% (w/v) Nusieve agarose (FMC BioProducts, Rockland, ME, USA) gel in 1 × TBE [89 mM Tris-base (Sigma), 89 mM boric acid (Sigma), 2 mM EDTA (Sigma)] at 80 V for 2 h. DNA molecular marker (1Kb DNA Ladder RTU, Cat. DM010-R500, Gene DireX) was used as a marker. The amplified fragments were visualized by ethidium bromide staining and UV transillumination.
Sequence analysis of the 16S rRNA gene
The PCR products were purified using QIA quick PCR Purification Kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s instructions. The PCR purified products were sent to the Molecular Genetics Laboratory in Al-Istishari Arab Hospital in Ramallah, Palestine for sequencing. The obtained sequences were aligned to the 16S rRNA gene sequences in the National Center for Biotechnology Information (NBCI) Genbank database using the BLAST algorithm (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Accession numbers were obtained for all sequences.
Leaves of L. nobilis
Dry leaves of L. nobilis leaves were bought from the local market of Tulkarem city in Palestine. A powder was obtained by grinding leaves using a blender, where after the powder was stored in a sterile sealed bottle at room temperature (Caputo et al., 2017).
L. nobilis leaf extracts
Extracts were prepared according to Mostafa et al. (2018) with some modifications, where 100 g of fine powder of L. nobilis were mixed with 500 ml of 99.9% (v/v) methanol. The mixture was kept for 5 days in closed sealed vessels at room temperature, protected from light, and was shaken daily 8 to 10 times. Whereafter, the mixture was filtered by a double layer of filter papers to remove the solid extraction residues. The residues were squeezed to increase the volume of the liquid extract, centrifuged at 5000 × g for 15 min, and finally filtered again through filter paper. The liquid extract was transferred to a vacuum rotary evaporator machine (Peaken Motor Company, China), where the solvent extract was evaporated at 45°C and the extract was concentrated under reduced pressure. The essential oil was sterilized by filtration using 0.45 μm Millipore filter papers. The obtained essential oil was weighted and preserved at 4°C in a tightly sealed bottle for further use.
The antibacterial activity of L. nobilis leaf extract
The antibacterial activity of L. nobilis leaf extract was investigated against bacteria isolated and identified from fresh lamb meat samples as described earlier. Each bacterium was cultured on Mueller-Hilton agar media and incubated at 37°C overnight. The bacterial colonies were harvested using 5 ml of sterile saline 0.85% (w/v) NaCl. The optical densities of all cultures were adjusted to match a 0.5 McFarland standard of 1×108 colony-forming units (CFU/ml). Bacterial concentrations were monitored by measuring turbidity at 610 nm using GP 100 Photometer (Greiner, Germany). Each bacterium was spread using a sterile cotton swab on the Nutrient agar Petri dish. Subsequently, wells of 6 mm diameter were punched into the agar medium. Seven serial dilutions of L. nobilis leaf extract of 20, 15, 10, 7.5, 5, 2.5, and 1% (v/v) were prepared by diluting the extract with 40% (v/v) ethanol. Whereafter, 100 µL of each concentration of L. nobilis leaf extract were added to the wells. Ciprofloxacin disc (Cip) was used as a positive control, and 40% (v/v) ethanol was used as a negative control (Ghadiri et al., 2014). Agar plates were incubated at 37°C for 24 h. The antimicrobial activity of the different concentrations of L. nobilis leaf extract was examined by measuring the zone of growth inhibition using Vernier Calliper. The antimicrobial test was done in triplicates for each bacterium.
The antibacterial effect of L. nobilis leaf extract on fresh lamb meat
To investigate the antibacterial activity of L. nobilis leaf extract, a fresh lamb meat was obtained from a meat shop. Five hundred grams of fresh lamb meat were divided into four parts equally and placed in four sterile boxes. All four parts were divided into small pieces of about 10 g. The first two parts of meat samples were incubated at room temperature (25-30°C), where only one of them was sprayed with 10% (v/v) of L. nobilis extract in 40% (v/v) ethanol. The second two parts were incubated in a refrigerator (4-8°C), where only one of them was sprayed with 10% (v/v) of L. nobilis extract in 40% (v/v) ethanol. Bacterial counts, color, smell, pH, and any physical changes of meat samples were observed and recorded daily. pH measured by making a suspension of small pieces of meat in distilled deionized water using an Electrometrical meter (Milwaukee Electronics Kft, Hungary).
Isolation and identification of bacteria in fresh lamb meat by 16S rRNA gene sequence analysis
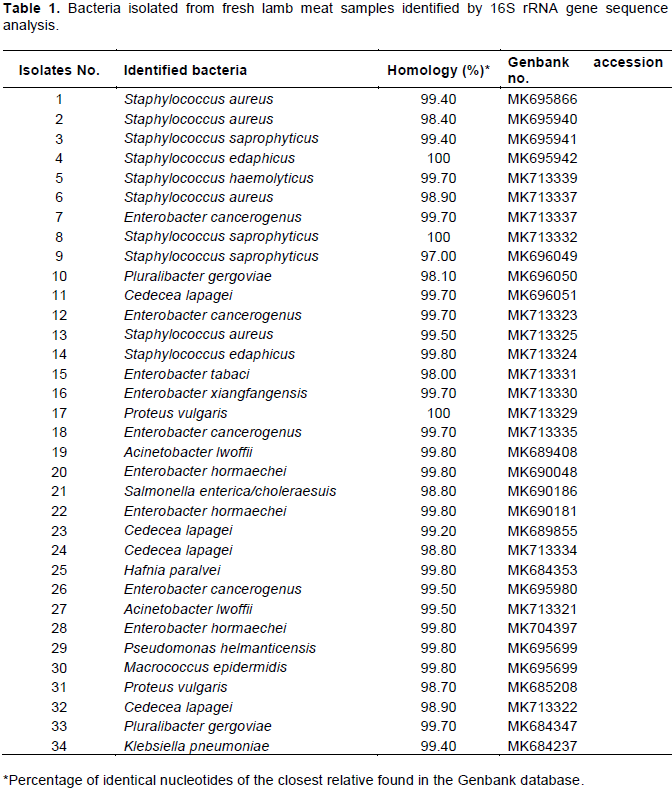
From the 20 collected fresh lamb meat samples, a total of 34 bacterial isolates were obtained and identified. Eighteen bacterial species were identified by sequence analysis of the 16S rRNA gene. The identified bacteria are shown in Table 1. It was observed that Staphylococcus spp., Enterobacter spp. and Cedecea lapagei were the predominant bacteria present in the collected fresh lamb meat samples. Other species of bacteria were detected in one or two fresh lamb meat samples; they include Salmonella choleraesuis, Hafnia alvei, Acinetobacter lwoffii, Pseudomonas helmanticensis, Proteus vulgaris, Klebsiella pneumoniae, and Macrococcus epidermidis. Bacterial counts in fresh lamb meat samples were in a range of 3 × 103 - 1.5 × 105 CFU/g, with S. aureus being the highest in numbers among other bacteria (Table 2).
The antibacterial activity of L. nobilis leaf extract
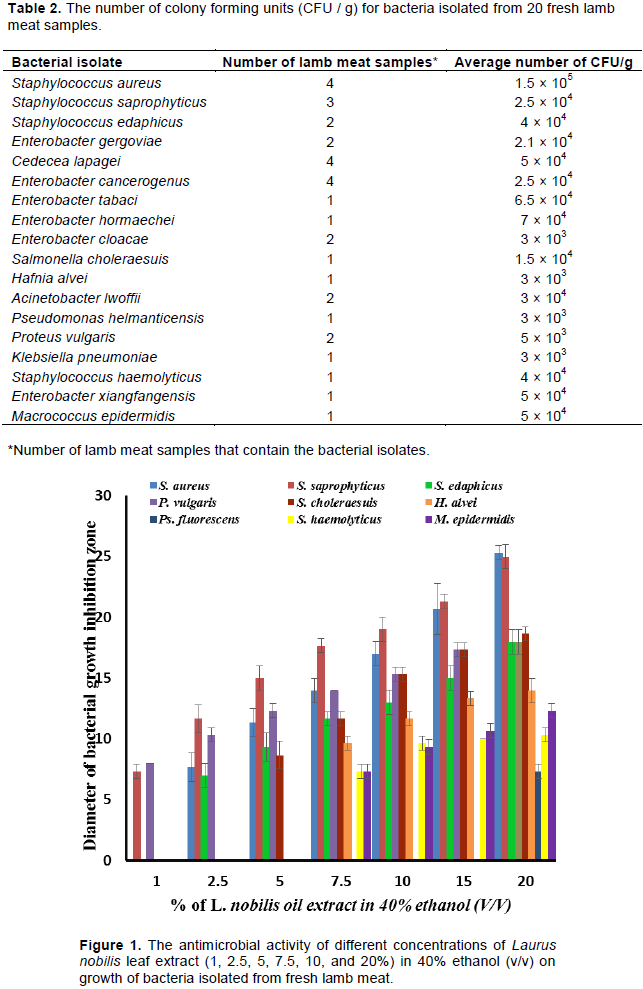
The antibacterial activity of L. nobilis leaves extract were investigated against the 18 bacterial species isolated from fresh lamb meat. The inhibition zones of bacterial growth, which were measured in mm are as shown in Figure 1. It was found that L. nobilis leaf extract has an antibacterial activity against 9 bacterial species (Figure 1). It was observed that Staphylococcus saprophyticus and P. vulgaris were the most bacteria affected by L. nobilis leaf extract where their growth was decreased at all investigated concentrations, that is, 1-20% (v/v). Growth of S. aureus and Staphylococcus edaphicus was reduced at concentrations of 2.5 to 20 (v/v) of L. nobilis leaf extract. S. choleraesuis was affected by concentrations of 5 to 20 (v/v) of L. nobilis leaf extract. Furthermore, concentrations of 7.5 to 20 (v/v) of L. nobilis leaf extract were found to have antagonist effects against growth H. alvei, M. epidermidis, and Staphylococcus haemolyticus. On the other hand, the growth of P. helmanticensis was only affected by a concentration of 20% (v/v) of L. nobilis leaf extract with an inhibition zone of only 8 mm. The remaining bacteria, which include, K. pneumoniae, Enterobacter gergoviae, Cedecea lapagei, Enterobacter cancerogenus, Enterobacter tabaci, Enterobacter hormaechei, Enterobacter xiangfangensis, A. lwoffii, and Enterobacter cloacae were not affected by any tested concentration of L. nobilis leaf extract.
The effect of L. nobilis leaf extract on fresh lamb meat
The effects of spraying of 10% (v/v) L. nobilis essential oil on fresh lamb meat showed changes in microbiological and physicochemical characteristics of lamb meat. Enterobacter hormaechei, P. helmanticensis, S. aureus, P. vulgaris, Cedecea lapagei, Enterobacter cancerogenus and Klebsiella pneumoniae were isolated and identified from the untreated fresh lamb meat (Table 3). After spraying of lamb samples with 10% (v/v) of L. nobilis extract, it was found that S. aureus and P. vulgaris disappeared from the treated meat samples kept at both room temperature and refrigerating temperature (Table 3). The remaining bacteria, which include E. hormaechei, P. helmanticensis, C. lapagei and E. cancerogenus, and K. pneumoniae were still detected in treated meat samples kept at both temperatures but showed a decrease in their numbers compared to untreated fresh meat samples.
The observed physical changes in meat samples are shown in Table 4. It was found that the untreated lamb meat kept at room temperature (25-30°C) showed changes after 24 h. Meat colour was red-yellowish with green dots, pH was about 5.1, strong bad smell, mucoid layer over meat and a large amount of mucus and gas bubbles were noticed on meat. On the other hand, lamb meat treated with 10% (v/v) of L. nobilis essential oil and kept at room temperature did not show any changes in meat appearance until 3 days of incubation. After 3 days, white dots and the mild bad smell started to be noticed in meat (Table 4). Changes in untreated lamb meat, which was kept in refrigerator, were only observed after 6 days (Table 4). Small bubbles of gas, thin mucoid layer on the meat surface and small white dots were observed. Treated meat, which was kept at the refrigerator, showed no changes during the first 13 days, whereafter, very small white dots started to appear on the meat surface.
In the present study, 34 bacteria were detected in fresh lamb meat samples (Table 1), which indicates that meat is a rich medium for the growth of spoilage and pathogenic bacteria. Staphylococcus spp., C. lapagei and Enterobacter spp. were the predominant bacteria found in lamb meat. Enterobacter and Pseudomonas spp. were found to be among the predominant bacteria in lamb meat (Wang et al., 2019). According to Ahmed and Sabiel (2016), the members of the family Enterobacteriaceae are usually associated with the contamination of meat products, their incidence in meat was considered as a public health problem. It was found that S. aureus was the most frequent bacterium present in fresh lamb meat. S. aureus has the ability to colonize on raw meat and spread into meat products during the different processing stages of the meat supply chain (Velasco et al., 2019). The pathogenicity of S. aureus is given by bacterial structures and secondary metabolites, among which are toxins that could cause staphylococcal diseases transmitted by contaminated meat (Velasco et al., 2019).
In the present work, bacterial counts in fresh lamb meat samples were in a range of 3 × 103 - 1.5 × 105 CFU/g (Table 2). In another study, Martineli et al. (2009) found that bacterial counts in lamb meat were in a range of 1.0 × 101 to 8.0 × 104 CFU/cm for mesophiles; 1.0 × 100 to 4.4 × 104 CFU/cm for psychrophiles (Martineli et al., 2009). Contamination of raw meat with bacteria can occur during slaughtering, cutting and inadequate storage conditions.
It was reported that the extraction of L. nobilis showed greater antimicrobial activity against Gram-positive bacteria than Gram-negative, which were shown to have a growth sensitivity to extract of 5 tested herbs (Mostafa et al., 2018). However, in this study, the growth of some of both Gram-positive and Gram-negative bacteria was affected by L. nobilis leaf extract but growth of others was not. El Malti and Amarouch (2009) reported that laurel extract has a significant antimicrobial activity against growth of both Gram-positive and Gram-negative bacteria, which included S. aureus, P. vulgaricus, and Salmonella enteridis. In the present study, growth of K. pneumoniae was not affected by all concentrations of L. nobilis leaf extract. In another study, K. pneumoniae was found to have only a 7 mm zone of inhibition after treatment with L. nobilis leaf extract (El Malti and Amarouch, 2009). This can explain that the effect of L. nobilis extract was investigated against a different strain of K. pneumoniae.
Prevention of food spoilage and food poisoning pathogens is usually achieved by the use of chemical preservatives which have negative effects on human health. No studies have been conducted to examine the antimicrobial activity of L. nobilis leaf extract against bacteria present in fresh lamb meat or to evaluate the antimicrobial effects of L. nobilis leaf extract as a fresh lamb meat preservative. There was a very good agreement between the antimicrobial activity of L. nobilis leaf extract against isolated bacteria and its effect on their presence in treated lamb meat samples. P. vulgaris and S. aureus were not detected in lamb meat samples treated with L. nobilis leaf extract (Table 3), which agrees with the investigations of the antimicrobial activity of L. nobilis leaf extract against those two bacteria (Figure 1). E. hormaechei, C. lapagei, E. cancerogenus, and K. pneumonia were detected in the treated and the untreated lamb meat kept at room and refrigeration temperatures with a slight decrease in their numbers in the treated lamb meat (Table 3). The antibacterial activity of L. nobilis leaf extract showed that there were no effects of 1 to 20% v/v concentrations of L. nobilis leaf extract on the growth of those three bacteria (Figure 1). Growth of the isolated bacterium P. fluorescens was only affected by the highest concentration of L. nobilis leaf extract (20% (v/v)) with a zone of growth inhibition of 8 mm (Figure 1). The effect of spraying lamb meat with L. nobilis leaf extract was found to affect growth of P. fluorescens by decreasing its numbers from 5 × 103 to 4 × 103 CFU/g at room temperature and from 4 × 103 to 2 × 103 CFU/g at refrigerated temperature (Table 3).
It seems that treatment of lamb meat samples with L. nobilis leaf extract plays an important role in delaying spoilage and lipid poisoning of fresh meat. There were differences in the physical characteristics of treated and untreated lamb meat samples. Treatment of lamb meat with L. nobilis leaf extract kept at room and refrigeration temperatures was found to delay physical changes in meat for 3 and 13 days, respectively (Table 4). Azimzadeh and Jahadi (2018) reported that a mixture of L. nobilis leaf extract and chitosan has a significant effect on the growth of mold/yeast and mesophilic bacteria in cashew. Vacuum packaging combined with L. nobilis oil was found to reduce the growth of E. coli in ground chicken meat (Irkin and Esmer, 2010). In addition, the essential oil of L. nobilis was reported to have a significant effect in maintaining the pH and colour of minced beef meat and in reducing microbial counts (Vilela et al., 2016).
This study demonstrated that L. nobilis essential oil appears to be a promising tool that can be used as a natural preservative for fresh lamb meat. However, the present work is a preliminary study. Sequencing of the 16S rRNA gene was shown to be a good and accurate method for the identification of bacterial isolates at the species level.
The authors have not declared any conflict of interests.
This work was financially supported by the Palestine Technical University-Kadoorie and Palestinian Agricultural Academic Cooperation (PAAC) by NUFFIC-Netherlands.
REFERENCES
|
Ahmed A, Sabiel Y (2016). Detection of Microbial Contamination of Processed Beef Meat by Using API Strips and Automated Vitek 2 Compact System. British Microbiology Research Journal 13(2):1-8.
Crossref
|
|
|
|
Anand S, Sati N (2013). Artificial Preservatives and Their Harmful Effects: Looking Toward Nature for Safer Alternatives. International Journal of Pharmaceutical Sciences and Research 4(7):2496.
|
|
|
|
|
Azimzadeh B, Jahadi M (2018). Effect of chitosan edible coating with Laurus nobilis extract on shelf life of cashew. Food Science and Nutrition 1:7.
Crossref
|
|
|
|
|
Basak SS, Candan F (2013). Effect of Laurus nobilis L. essential oil and its main components on α-glucosidase and reactive oxygen species scavenging activity. Iranian Journal of Pharmaceutical Research.
Crossref
|
|
|
|
|
Caputo L, Nazzaro F, Souza L, Aliberti L, De Martino L, Fratianni F, Coppola R, De Feo V (2017). Laurus nobilis: Composition of Essential Oil and Its Biological Activities. Molecules 22(6):930.
Crossref
|
|
|
|
|
Dashti A, Dashti H, Jadaon M (2014). Heat Treatment of Bacteria : A Simple Method of DNA Extraction for Molecular Techniques. Journal of the Kuwait Medical Association 41(2):117-122.
|
|
|
|
|
El Malti J, Amarouch H (2009). Antibacterial effect, histological impact and oxidative stress studies from laurus nobilis extract. Journal of Food Quality.
Crossref
|
|
|
|
|
Esmer OK, Irkin R, Degirmencioglu N, Degirmencioglu A (2011). The effects of modified atmosphere gas composition on microbiological criteria, color and oxidation values of minced beef meat. Meat Science.
Crossref
|
|
|
|
|
Fernández NJ, Damiani N, Podaza EA, Martucci JF, Fasce D, Quiroz F, Meretta PE, Quintana S, Eguaras MJ, Gende LB (2018). Laurus nobilis L. Extracts against Paenibacillus larvae: Antimicrobial activity, antioxidant capacity, hygienic behavior and colony strength. Saudi Journal of Biological Sciences pp. 4-10.
Crossref
|
|
|
|
|
Ghadiri E, Ahmadi R, Moridikyia A, Mahdavi E, Tavakoli P (2014). Laurus nobilis Has Antibacterial Activity Against Staphylococcus aureus, International Conference on Food, Biological and Medical Sciences (FBMS-2014) Jan. 28-29, 2014 Bangkok, 75-76.
|
|
|
|
|
Gharsallaoui A, Oulahal N, Joly C, Degraeve P (2016). Nisin as a Food Preservative: Part 1: Physicochemical Properties, Antimicrobial Activity, and Main Uses. Critical Reviews in Food Science and Nutrition 56(8):1262-1274.
Crossref
|
|
|
|
|
Irkin R, Esmer OK (2010). Control of Listeria monocytogenes in ground chicken breast meat under aerobic, vacuum and modified atmosphere packaging conditions with or without the presence of bay essential oil at 4°C. Food Science and Technology Research.
Crossref
|
|
|
|
|
Karoki WH, Karanja DN, Bebora LC, Njagi LW (2018). Isolation, Characterization, and Quantification of Bacteria from African Sausages Sold in Nairobi County. International Journal of Food Science, Kenya 2018:3861265 9 p.
Crossref
|
|
|
|
|
Lucera A, Costa C, Conte A, Nobile MADel (2012). Food applications of natural antimicrobial compounds. Frontiers in Microbiology 3:1-13.
Crossref
|
|
|
|
|
Martineli TM, Rossi Junior OD, Cereser ND, Cardozo MV, Fontoura CL, Perri SHV (2009). Microbiological counting in lamb carcasses from an abattoir in São Paulo, Brazil. Ciência Rural 39(6):1836-1841.
Crossref
|
|
|
|
|
Mostafa AA, Al-Askar AA, Almaary KS, Dawoud TM, Sholkamy EN, Bakri MM (2018). Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi Journal of Biological Sciences 25(2):361-366.
Crossref
|
|
|
|
|
Nagarajan V, Wahab A, Alex L (2018). Study of bacterial contamination of raw meat in Hyderabad. Medcrave - MOJ Proteomics & Bioinformatics 7(1):46-51.
Crossref
|
|
|
|
|
NehirEl S, Karagozlu N, Karakaya S, Sahın S (2014). Antioxidant and Antimicrobial Activities of Essential Oils Extracted from Laurus nobilis L. Leaves by Using Solvent-Free Microwave and Hydrodistillation. Food and Nutrition Sciences 5:97-106.
Crossref
|
|
|
|
|
Newman MJ (2005). Food Safety: Take life easy; eat, drink and be merry. Luke 12: 19b. Ghana Medical Journal 39(2):44-45.
|
|
|
|
|
Ouibrahim Y, Tlili-Ait-kaki S, Bennadja S, Amrouni AGD, Djebar MR (2013). Evaluation of antibacterial activity of Laurus nobilis L., Rosmarinus officinalis L. and Ocimum basilicum L. from Northeast of Algeria. African Journal of Microbiology Research 7(42):4968-4973.
|
|
|
|
|
Pennacchia C, Ercolini D, Villani F (2011). Spoilage-related microbiota associated with chilled beef stored in air or vacuum pack. Food Microbiology 28(1):84-93.
Crossref
|
|
|
|
|
Rafiq R, Hayek S, Anyanwu U, Hardy B, Giddings V, Ibrahim S, Tahergorabi R, Kang H (2016). Antibacterial and Antioxidant Activities of Essential Oils from Artemisia herba-alba Asso., Pelargonium capitatum × radens and Laurus nobilis L. Foods 5(2):28.
Crossref
|
|
|
|
|
Thanigaivel G, Anandhan AS (2015). Isolation and Characterization of Microorganisms from Raw Meat Obtained from Different Market Places in and Around Chennai. Journal of Pharmaceutical, Chemical and Biological Sciences 3:295-301.
|
|
|
|
|
Velasco V, Quezada-Aguiluz M, Bello-Toledo H (2019). Staphylococcus aureus in the Meat Supply Chain: Detection Methods, Antimicrobial Resistance, and Virulence Factors . In Staphylococcus and Streptococcus [Working Title].
Crossref
|
|
|
|
|
Verraes C, Van Boxstael S, Van Meervenne E, Van Coillie E, Butaye P, Catry B, Herman L (2013). Antimicrobial resistance in the food chain: A review. International Journal of Environmental Research and Public Health.
Crossref
|
|
|
|
|
Vilela J, Martins D, Monteiro-Silva F, González-Aguilar G, de Almeida JMMM, Saraiva C (2016). Antimicrobial effect of essential oils of Laurus nobilis L. and Rosmarinus officinallis L. on shelf-life of minced "Maronesa" beef stored under different packaging conditions. Food Packaging and Shelf Life.
Crossref
|
|
|
|
|
Wang T, Guo H, Zhang H, Ren F, Zhang M, Ge S, Luo H, Zhao L (2019). Dynamics of Bacterial Communities of Lamb Meat Packaged in Air and Vacuum Pouch during Chilled Storage. Food Science of Animal Resources 39(2):209-221.
Crossref
|
|