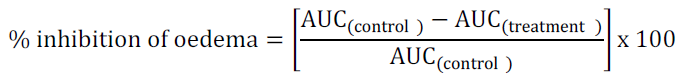ABSTRACT
Inflammation which is a normal phenomenon involved in healing of injurious tissue can be complicated with reactive oxygen resulting in chronic inflammatory state and subsequent tissue damage. Since there are reports on severe side effects associated with the use of synthetic anti-inflammatory medications, there is the need to search for new medications with milder side effects. The study, therefore, investigated the antioxidant and anti-inflammatory properties of methanol extracts of Hilleria latifolia root (HLRE) and Laportea ovalifolia leaf (LOLE). The DPPH free radical scavenging, total phenolic content and phosphomolybdenum antioxidant assays were used to assess the in vitro antioxidant activity, while the carrageenan-induced foot oedema model in rats was employed to investigate the acute anti-inflammatory activity of the extracts. LOLE and HLRE were able to scavenge 1,1-Diphenyl-2-picryl-hydrazyl (DPPH) free radicals with IC50 values of 130.8±0.9 and 233.5±0.5 μg/mL, total phenol content of 56.75±0.3220 and 91.32 ±4.258 mg TAE/g extract whereas total antioxidant capacity of 337.6±6.961 and 408.0±18.70 mg α-TE/g extract, respectively. The extracts at 100 and 300 mg/kg significantly (p<0.001) reduced the induced oedema when administered prophylactically and therapeutically. In conclusion, the methanol extracts of H. latifolia and L. ovalifolia exhibited in vitro antioxidant and in vivo anti-inflammatory properties.
Key words: Hilleria latifolia, Laportea ovalifolia, inflammation, anti-inflammatory, antioxidant.
Inflammation is a complex natural mechanism used by vascular tissue for regeneration when injured or exposed to dangerous stimuli like pathogens or infections and irritants. It is a defensive action taken by an organism to remove injurious stimuli, initiate healing process and generate new tissues to replace impaired ones (Schmid-Schönbein, 2006; Singh et al., 2008). Typical signs that are cardinal to inflammation are pain, redness, swelling, heat and loss of function (Kumar et al., 2010; Rock and Kono, 2008; Shailasree et al., 2012). An organism’s ability to mount an inflammatory response is very crucial for its survival when injured or when infected with pathogens and other harmful stimuli.
Though the inflammatory response is a protective mechanism on its own, it may lead to serious tissue destruction resulting in chronic inflammation when implicated with over production of reactive oxygen species (ROS). These ROS are constantly produced in the body of humans through the process of aerobic respiration and from other external sources (Narayanaswamy and Balakrishnan, 2011) and are acted upon and detoxified by endogenous enzyme called antioxidants. In the presence of excess generation of ROS and insufficient antioxidant defense, oxidative stress sets in and may complicate a lot of diseases like diabetes mellitus, cancer, atherosclerosis, hypertension, ocular diseases, haematological diseases, pulmonary diseases, neurological diseases as well as inflammatory diseases (Cui et al., 2004).
However, key orthodox anti-inflammatory medications though effective, have been reported to be associated with severe side effects (Graham, 2006). This has necessitated the search for new and effective antioxidant and anti-inflammatory medications with milder side effects. The search is ongoing and can never be accomplished without looking out for medicinal plants with such properties, since plants have been known to be rich sources of therapeutic agents (Cragg and Newman, 2013). Phenolic compounds and flavonoids widely distributed in plants have been reported to exert numerous biological activities such as anti-inflammatory activity and have also been known as the main antioxidant compounds of fruits and vegetables (Wu and Ng, 2008). Hilleria latifolia (Lam.) H. Walt (Phytolaccaceae) is locally known in Ghana as ‘Avegboma’ and ‘Anafranaku’ by the Ewes and Asantes, respectively. It is a perennial herb (30 to 120 cm high) with long petiole of 3 to 7 cm high and pink or white flowers. It has ovate-elliptic leaves with numerous short hair-like structures covering the lower surface (Dokosi, 1998; Mshana, 2000). The leaves are used in Ghana for the management of rheumatism, boils and wounds (Agyare et al., 2009). The leaves, added to those of Piper guineense, are used to treat general oedema. In Congo, the leaves are employed to treat some skin diseases (Dokosi, 1998; Mshana, 2000). Woode et al. (2011) reported on the presence of phytochemical constituents such as saponins, tannins, glycosides, steroids, terpenoids, flavonoids and alkaloids in the aerial parts of the plant. In another report, phytochemical screening of methanolic fraction of H. latifolia revealed the presence of glycosides, coumarins and reducing sugars, as well as small amount of triterpens and sterols. However, saponins, tannins, flavonoids and alkaloids were absent (Assob et al., 2011). The leaf extract of H. latifolia has been reported to have anxiolytic and antidepressant-like effects, antimicrobial, antioxidant, anti-nociceptive and some neurobehavioral properties (Abotsi et al., 2012; Assob et al., 2011; Woode et al., 2011; Woode and Abotsi, 2011). The leaf extract has also been found to be able to modify the activity of some selected antibiotics by either enhancing or reducing their activities (Dapaah et al., 2016).
Laportea ovalifolia (Schumach.) Chew (Urticaceae) is known by the Asantes in Ghana as ‘akyekyenwonsa’, ‘abrewa nom taa’ or ‘Kumasi otuo’. It is a herbaceous weed more often creeping than erect and densely covered with stinging hairs. It has cylindrical stem of greenish or sometimes reddish to brownish in colour (Chew, 1969).There are two varieties of L. ovalifolia that is, male and female. These two varieties are related but are different in structure. L. ovalifolia (male) has big leaves and (female) possess small leaves (Essiett et al., 2011). The leaves of L. ovalifolia are used to heal wounds (Agyare et al., 2009), fruits are used as a poison antidote and the roots boiled in water is taken to prevent excessive menstrual bleeding (Sofowora 1996; Bouch, 2004). Phytochemical screening of the leaf extract of L. ovalifolia showed the presence of saponins, tannins, flavonoids, phlobatanins and cardiac glycosides. Anthraquinone was however absent (Essiett et al., 2011). L. ovalifolia has been reported to possess antimicrobial, anti-hyperglycemic activity and it is also effective in reducing oxidative stress in diabetes (Iffen and Usoro, 2010; Okwulehie and Akanwa, 2013). It also has anti-diabetic and hypolipidemic effects in alloxan-induced diabetic rats (Momo et al., 2006). Methanol leaf extract of L. ovalifolia has been found to exhibit antibiotic resistance modifying activity (Dapaah et al., 2016). The aim of study is to investigate the antioxidant and in vivo anti-inflammatory properties of methanol extracts of H. latifolia root (HLRE) and L. ovalifolia leaf (LOLE).
Plant collection
Fresh leaves of L. ovalifolia and leaves and roots of H. latifolia were collected from Aburi (longitude 0.1729°W and latitude 5.8512°N) in the Eastern region of Ghana in February, 2014. The plants were authenticated by Dr. Alex Asase of the Department of Botany, University of Ghana, and voucher specimen AA 71 and AA 63, respectively deposited in the Ghana Herbarium, Department of Botany, University of Ghana, Legon, Accra, Ghana
Plant extraction
The plant parts collected were washed thoroughly under running tap-water and dried under shade at a temperature of 25 to 28°C for two weeks, after which they were pulverized into coarse powder using the laboratory milling machine (Christy and Norris, Chelmsford, England). Eight hundred grams (800 g) each of the powdered plant materials were soaked in 2.5 L of 70% v/v methanol and extracted with the aid of ultra-turrax (T 25 Janke and Kunkel, Labortenik, Germany) under ice-cooling at a speed of 24000 rpm for 3 to 5 min, and then filtered using a laboratory sieve (Retsch, Haan, Germany) of mesh number 200 with aperture of 75 μm and Whatmann ï¬lter paper Number 1. The filtrates were concentrated with the rotary evaporator (Rotavapor BÜCHI R-200 with heating bath B-490, Büchi, Konstanz, Germany) at 40°C under reduced pressure and allowed to dry in the hot air oven (Gallenkamp, London, UK) at 40°C and then stored in air tight containers at 4 to 8°C in a refrigerator. The yields of the extracts relative to the dry powder used were recorded as 11.29 and 7.50% w/w related to the dried material, respectively.
Determination of antioxidant activity
Three different in vitro assays were employed to assess the antioxidant activity of the extracts. These included DPPH free radical scavenging, total phenolic content and phosphomolybdenum antioxidant assays.
DPPH free radical scavenging assay
Different concentrations (15.6 to 1000 μg/mL) of leaf extract of L. ovalifolia (LOLE) and root extract of H. latifolia (HLRE) and reference antioxidant (α-tocopherol) (Sigma-Aldrich, Taufkirchen, Germany) were prepared in methanol. 1,1-Diphenyl-2-picryl-hydrazyl (DPPH) (Sigma-Aldrich, Taufkirchen, Germany) solution (20 μg/mL) was prepared with methanol in the dark. Three milliliters of DPPH solution was added to 1 mL each of the different concentrations of LOLE, HLRE and α-tocopherol, followed by incubation in a dark place for 30 min. Control was prepared by adding 3 mL of DPPH solution to 1 mL methanol and treated in the same way as the test samples. Absorbance of excess DPPH was measured at a wavelength of 517 nm (Braca et al., 2001; Susanti et al., 2007). The experiment was performed in triplicates. The percentage scavenging activity was calculated using the equation below:
% scavenging = [(Absorbance control - Absorbance test)/ Absorbance control] × 100
Total phenolic content
The method described by Škerget et al. (2005) involving the use of Folin-Ciocalteu reagent (Sigma-Aldrich, St. Louis, Missouri, USA) was employed. LOLE and HLRE (0.5 to 10 mg/mL) and standard drug (tannic acid) (Merck BDH, Poole, UK) with concentration range of 0.0156 to 1 mg/mL, were prepared. A volume of 0.1 mL Folin-Ciocalteu reagent was added to 0.5 mL each of the different concentrations of LOLE, HLRE and tannic acid solution, followed by the addition of 2.5 mL 2% sodium carbonate (Sigma-Aldrich, St. Louis, Missouri, USA). The mixtures were incubated for 20 min at room temperature and absorbance read at 760 nm. The experiment was performed in triplicates (independent). A blank was prepared by adding all the reagents with the exception of extracts/standard drug and treated likewise. The total phenolic content was expressed as milligram tannic acid equivalent (TAE) per gram of extract.
Total antioxidant capacity (Phosphomolybdenum antioxidant assay)
The method described by Prieto et al. (1999) was used to determine the ability of LOLE and HLRE to reduce Mo-VI to Mo-V with subsequent formation of green phosphate-molybdate complex in an acidic pH condition. LOLE, HLRE (0.5 to 10 mg/mL) and reference antioxidant (α-tocopherol) ranging from 0.008 to 0.03 mg/mL were prepared. To 1 mL each of the different concentrations of LOLE, HLRE and α-tocopherol, 3 mL of reagent solution (0.6 M sulphuric acid, 28 mM disodium phosphate and 4 mM ammonium molybdate) (Sigma Aldrich, St. Louis, Missouri, USA) was added and incubated at 95°C for 90 min. A blank (all reagents without extracts/standard drug) was treated in the same manner. The mixture was allowed to cool and absorbance was read at 695 nm.
Determination of anti-inflammatory activity of extracts
The acute inflammation model was employed to examine the anti-inflammatory activity of the extracts. Prophylactic determination (drugs given 1 h before inducing the oedema) was made; therapeutic determination (drugs administered 1 h post oedema induction) was also carried out to evaluate the extent of the extracts’ anti-inflammatory effect, since not all drugs or agents that exhibit anti-inflammatory activity when administered prophylactically can do same when administered therapeutically. For instance, as observed with cyclosporine (Kaibara et al., 1983).
Experimental animals
Sprague-Dawley male rats (150 to 200 g) were obtained from the animal house of the Department of Pharmacology, Faculty of Pharmacy and Pharmaceutical Sciences, Kwame Nkrumah University of Science and Technology (KNUST), Kumasi, Ghana, and kept in stainless steel cages with soft wood shavings as bedding. They were maintained under standard environmental conditions of temperature (30±2°C) and adequate humidity, with a twelve hour cycle of light and darkness. The animals were fed with standard pellet diet and provided with water ad libitum.
Ethical approval
The experiments were conducted in accordance with accepted principles for laboratory animal use and care (EU directive of 1986:86/609/EEC) and approval from the Animal Ethical Committee (FPPS-AEC/CA01/13), Faculty of Pharmacy and Pharmaceutical Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana.
Acute anti-inflammatory activity
The carrageenan induced foot oedema model in rats as described by Winter et al. (1962) was employed to assess the extracts’ ability to inhibit/reduce the induced paw swelling. The rats were weighed and assigned randomly into groups consisting of four rats each. The initial paw thickness of the rats were measured using an electronic caliper (Z22855, Milomex Ltd, Bedfordshire, UK) after which reference drug, 100 mg/kg aspirin, (Sigma-Aldrich, St. Louis, Missouri, USA) and extracts (30, 100 and 300 mg/kg), prepared in sterile distilled water, administered orally to the respective groups, with the control group given 0.5 mL sterile distilled water. After an hour of drug administration, oedema was induced by injecting 0.1 ml of 1% w/v carrageenan (Sigma-Aldrich, St. Louis, Missouri, USA) solution (in sterile distilled water) into the sub-plantar tissue of the right foot pads of the rats. paw thickness were again measured at an hourly interval for 6 h. In the therapeutic experimental protocol, eodema was induced and measured 1 h after administering the extracts and reference drug. Inhibition of inflammation was calculated using the relation;
Where Ti is paw thickness before carrageenan injection and Tf is paw thickness at time T.
Raw scores for right foot thickness were individually normalized as percentage of change from their values at time 0 and then averaged. Total pedal oedema was calculated in arbitrary units as the area under the curve (AUC) and to determine the percentage inhibition of oedema, the following equation was used:
Data analysis
Data were presented as mean ± standard error mean (SEM) in the studies. Analysis of results was done using one-way ANOVA followed by the Tukey’s post hoc test in analyzing the antioxidant activity and Dunnett’s post hoc test in the anti-inflammation analysis. Graphs were plotted with Graph Pad Prism for windows version 6 (Graph Pad, San Diego, CA, USA).
Antioxidant activity of extracts
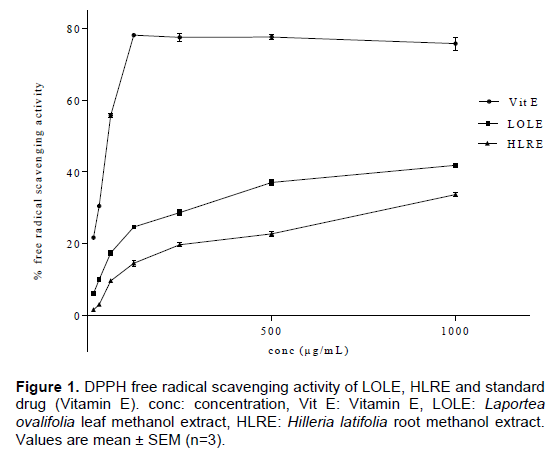
DPPH free radical scavenging activity of LOLE and HLRE
LOLE, HLRE and vitamin E (α-tocopherol) showed antioxidant activity at the test concentrations (0.0156 to 1 mg/mL) (Figure 1). The IC50 values obtained for vitamin E, LOLE and HLRE were 18.9 ± 1.3, 130.8 ± 0.9 and
233.5 ± 0.5 μg/mL, respectively (Table 1).
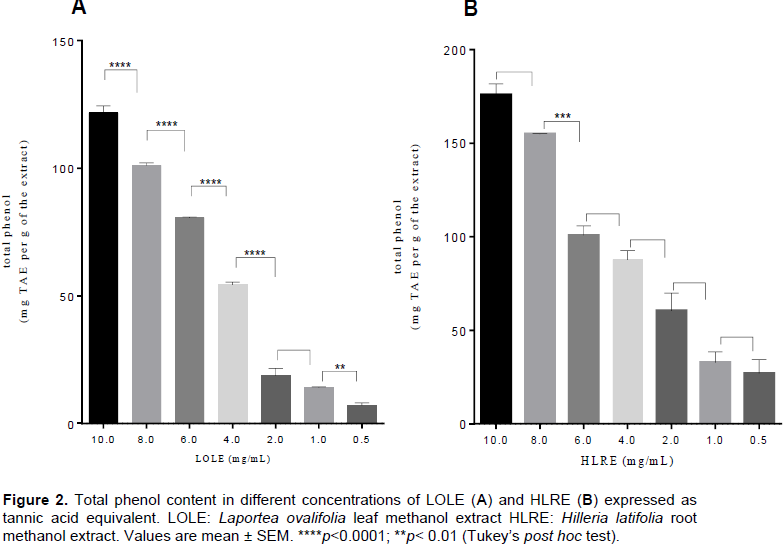
Total phenol content of LOLE and HLRE
LOLE and HLRE at the test concentrations (0.5 to 10 mg/mL) showed an increased phenol content with increasing concentration. LOLE ranged from 7 to 122 mg TAE/g of the extract as the concentration increased and HLRE was from 27 to 175 mg TAE/g of the extract with increasing concentration (Figure 2). The total phenol content in each of the extracts (HLRE and LOLE) was calculated as mean ± SEM as 56.75 ± 0.3220 and 91.32 ± 4.258, respectively.
Total antioxidant capacity of LOLE and HLRE
Total antioxidant capacity of LOLE and HLRE was expressed as α-tocopherol equivalence (α-TE) from the α-tocopherol calibration curve. The total antioxidant capacity of LOLE was 337.6 ± 6.961 mg α-TE/g extract and that of HLMR was 408.0 ± 18.70 mg α-TE/g. At the test concentrations (0.5 to 10 mg/mL) of LOLE and HLRE, it was observed that, antioxidant capacity decreased as the concentrations reduced.
Acute anti-inflammatory activity of LOLE and HLRE
LOLE (30, 100 and 300 mg/kg), when administered
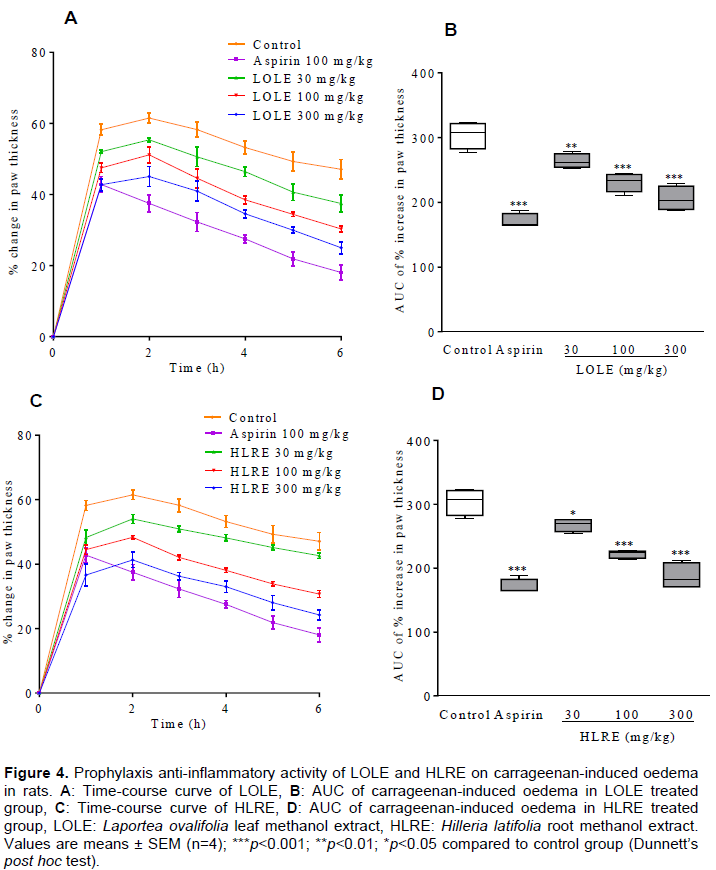
before the induction of the carrageenan paw oedema (prophylaxis), showed a significant (p<0.001) reduction of the oedema with the mean maximal swelling attained at 2 h reduced respectively to 55.38 ± 0.57%, 51.15 ± 2.30% and 45.05 ± 2.81% from the inflamed control response of 61.50 ± 1.44% (Figure 4A). The total paw swellings induced over the 6 h were also significantly (p<0.001) suppressed to 86.96 ± 2.14%, 76.37 ± 3.70% and 67.92 ± 3.77% respectively of the inflamed control response (Figure 4B). Also, HLRE (30, 100 and 300 mg/kg) showed a significant (p<0.001) reduction of the oedema with the mean maximal swelling attained at 2 h reduced to 54.04 ± 1.40%, 48.33 ± 0.54% and 41.30 ± 2.36% respectively, from the inflamed control response of 61.50 ± 1.44% (Figure 4C). The total paw swellings induced over the 6 h were also significantly (p<0.001) suppressed to 88.51 ± 4.30%, 73.38 ± 2.99% and 61.78 ± 3.64% respectively (Figure 4D).
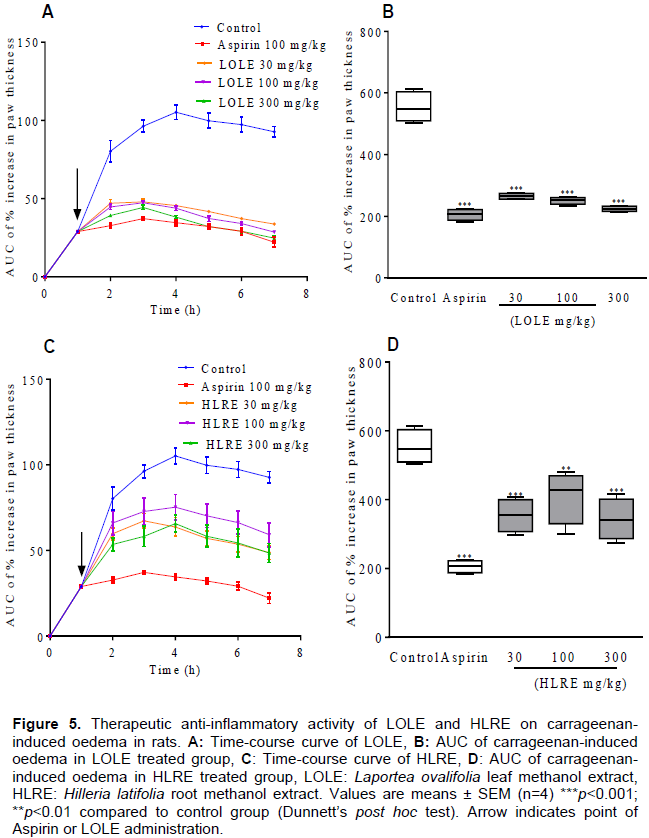
When administered after the induction of the carrageenan paw oedema (therapeutic), LOLE (30, 100, 300 mg/kg) showed a significant (p<0.001) inhibition of the oedema with the mean maximal swelling at 4 h reduced respectively to 45.51 ± 0.6827%, 43.91 ± 1.431% and 38.19 ± 0.9960% of the inflamed control response of 105.2 ± 4.553% (Figure 5A). The total paw swellings induced over the 6 h were also significantly (p<0.001) suppressed to 48.04 ± 1.37%, 45.38 ± 1.57% and 40.79 ± 2.40% respectively of the inflamed control response (Figure 5B). Similarly, HLRE (30, 100, 300 mg/kg) showed a significant (p<0.001) inhibition of the oedema with the mean maximal swelling at 4 h reduced respectively to 63.70 ± 5.490%, 75.22 ± 7.452% and 65.70 ± 4.943% of the inflamed control response of 105.2 ± 4.553% (Figure 5C). The total paw swellings induced over the 6 h were also significantly (p<0.001) suppressed to 64.30 ± 4.66%, 75.05 ± 9.59% and 62.00 ± 4.90% respectively of the inflamed control response (Figure 5D).
Antioxidant activity of the extracts was determined by assessing their DPPH free radical scavenging properties, total phenol content and total antioxidant capacity. LOLE and HLRE were able to scavenge free radicals of DPPH at the various test concentrations (Figure 3), with IC50 values of 130.8 ± 0.9 and 233.5 ± 0.5 μg/mL, respectively. The IC50 gives a notion on the ability of an agent to mop up free radicals indicating its potency as an antioxidant. Lower IC50 values indicate potent antioxidant activity (Apenteng et al., 2014). This implies that, LOLE exhibited a higher antioxidant activity as compared to HLRE. Wu and Ng (2008) report that antioxidant activity exhibited by plant extracts may be due to their phenolic and non-phenolic contents.
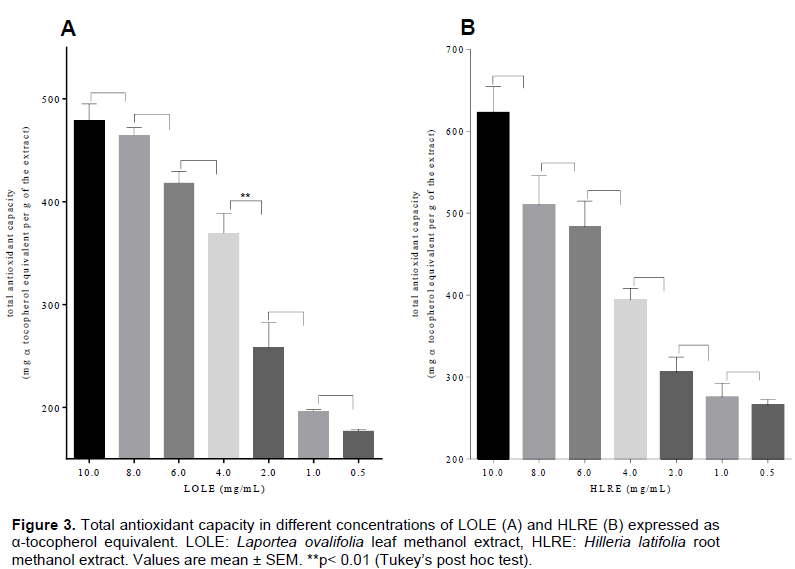
For this reason, the total phenol content of LOLE and HLRE was determined. HLRE had higher total phenol content (91.32 ± 4.258 mg/g) as compared to LOLE of (56.75 ± 0.3220 mg/g). This indicates that the higher free radical scavenging activity of LOLE as compared to HLRE may be due to other non-phenolic compounds present (Conforti et al., 2008).
The total antioxidant capacity of LOLE and HLRE (337.6 ± 6.961 and 408.0 ± 18.70 mg/g, respectively) correlated with their respective total phenol content. This implies that the higher the total phenol content the greater the total antioxidant capacity of the extracts. This confirms the report that phenolic compounds significantly cause the reduction of Mo+6 to Mo+5 (Khan et al., 2012). The acute anti-inflammatory activity of LOLE and HLRE was determined using the carrageenan-induced rat foot oedema model, since the events involved in the vascular response to carrageenan-induced oedema are similar to the early exudative stage of inflammation (Winter et al., 1962; Ozaki, 1990) and hence the use of anti-inflammatory agent to inhibit this acute phase of inflammation. The molecular response to carrageenan-induced oedema is bi-phasic involving the release of diverse inflammatory mediators, characterised by marked oedema formation. Inflammatory mediators such as histamine, serotonin and bradykinin are released during the first phase (1 to 2 h), and sustained by the release of prostaglandins and nitric oxide in the second phase (Thomazzi et al., 2010; Abotsi et al., 2012).
LOLE and HLRE at 300 and 100 mg/kg, significantly (p<0.001) reduced the induced oedema during both prophylactic and therapeutic treatments. This indicates the presence of compounds that can reduce the inflammatory responses. Even though the mechanism(s) of action of LOLE and HLRE are not known, they could be acting by inhibition and/or interference with the role of inflammatory mediators (such as histamine, serotonin, bradykinin, prostaglandins and other cyclooxygenase products) involved in the carrageenan induced oedema (Abotsi et al., 2012; Obiri et al., 2013). Also, the antioxidant potential of LOLE and HLRE may be a contributing factor to the extracts’ anti-inflammatory activity. ROS are released from activated neutrophils and macrophages during inflammatory injury and their overproduction leads to tissue injury or damage. ROS
can also cause the release of pro-inflammatory cytokines such as IL-1β and TNF-α which directly enhance inflammatory response (Dinarello, 2000; Conforti et al., 2008). Hence, the extracts ability to counteract the effects of ROS and free radicals can contribute to their anti-inflammatory activities.
Abotsi et al. (2012) used different in vitro antioxidant models to assess the antioxidant activity of the aerial parts of H. latifolia as well as its anti-inflammatory activity, using the carrageenan induced oedema in 7-day old chick model. The study reported the antioxidant activity and significant reduction of induced oedema (anti-inflammatory activity) by the aerial parts of H. latifolia comparable to what was observed in this study conducted on the root extract. There is need to isolate and characterize the bioactive agent(s) or compound(s) from these two plants responsible for the above pharmacological or biological activities.
The leaf extract of L. ovalifolia and root extract of H. latifolia exhibited antioxidant and acute anti-inflammatory activity at the test concentrations, when administered in both preventive and curative protocols of carrageen-
induced oedema.
The authors have not declared any conflict of interests.
We are most grateful to Dr. A. Asase and Mr. John Amponsah, Ghana Herbarium and Department of Botany, University of Ghana, Legon, Accra, Ghana for the identification and collection of the plant material. Also our gratitude goes to Mr. Thomas Ansah of the Department of Pharmacology and Mr. Jonathan Jato of the Department of Pharmacognosy, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana for their technical support.
REFERENCES
|
Abotsi WKM, Ainooson GK, Woode E (2012). Anti-inflammatory and antioxidant effects of an ethanolic extract of the aerial parts of Hilleria latifolia (Lam.) H. Walt.(Phytolaccaceae). Afr. J. Tradit. Complement. Altern. Med. 9(1):138-152.
|
|
|
|
Agyare C, Asase A, Lechtenberg M, Niehues M, Deters A, Hensel A (2009). An ethnopharmacological survey and in vitro confirmation of ethnopharmacological use of medicinal plants used for wound healing in Bosomtwi-Atwima-Kwanwoma area, Ghana. J. Ethnopharmacol. 125(3):393-403.
Crossref
|
|
|
|
|
Apenteng JA, Agyare C, Adu F, Ayande PG, Boakye YD (2014). Evaluation of wound healing potential of different leaf extracts of Pupalia lappacea. Afr. J. Pharm. Pharmacol. 8(41):1039-1048.
|
|
|
|
|
Assob JCN, Kamga HLF, Nsagha DS, Njunda AL, Nde PF, Asongalem EA (2011). Antimicrobial and toxicological activities of five medicinal plant species from Cameroon Traditional Medicine. BMC Complement. Altern. Med. 11(1):70.
Crossref
|
|
|
|
|
Bouch CH (2004). Laportea ovalifolia (Schumach.) Chew. Vegetable/Legumes Grubben GJH, and Denton AO, (Eds) Porta Press, London.
|
|
|
|
|
Braca A, De Tommasi N, Di Bari L, Pizza C, Politi M, Morelli I (2001). Antioxidant principles from bauhinia tarapotensis. J. Nat. Prod. 64(7):892-895.
Crossref
|
|
|
|
|
Chew WL (1969). Monograph of Laportea (Urticaceae). Gard Bull.
|
|
|
|
|
Conforti F, Sosa S, Marrelli M, Menichini F, Statti GA, Uzunov D (2008). In vivo anti-inflammatory and in vitro antioxidant activities of Mediterranean dietary plants. J. Ethnopharmacol. 116(1):144-151.
Crossref
|
|
|
|
|
Cragg GM, Newman DJ (2013). Natural products: a continuing source of novel drug leads. Biochimica et Biophysica Acta (BBA)-General Subjects 1830(6):3670-3695.
Crossref
|
|
|
|
|
Cui K, Luo X, Xu K, Murthy MRV (2004). Role of oxidative stress in neurodegeneration: recent developments in assay methods for oxidative stress and nutraceutical antioxidants. Progress Neuro-Psychopharmacol. Biol. Psych. 28(5):771-799.
|
|
|
|
|
Dapaah SO, Agyare C, Boakye YD, Appiah T (2016). Modulatory effect of Hilleria latifolia and Laportea ovalifolia on in vitro activity of selected antibiotics. J. Med. Plant Res. (accepted).
|
|
|
|
|
Dinarello CA (2000). Proinflammatory cytokines. Chest 118(2):503-508.
Crossref
|
|
|
|
|
Dokosi OB (1998). Herbs of Ghana. Ghana Universities Press.
|
|
|
|
|
Essiett UA, Edet NI, Bala DN (2011). Phytochemical and physicochemical analysis of the leaves of Laportea aestuans (Linn.) Chew and Laportea ovalifolia (Schumach.) Chew (male and female). Asian J. Plant Sci. Res. 1(2):35-42.
|
|
|
|
|
Graham DJ (2006). COX-2 inhibitors, other NSAIDs, and cardiovascular risk: the seduction of common sense. JAMA, 296(13):1653-1656.
Crossref
|
|
|
|
|
Iffen TS, Usoro CAO (2010). The Effect of Ethanolic Extract of Larpotea ovalifolia Plants Growing in Calabar on Antioxidants Status of Streptozocin Induced Diabetic Rats. Global J. Pharmacol. 4(1):1-5.
|
|
|
|
|
Kaibara N, Hotokebuchi T, Takagishi K, Katsuki I (1983). Paradoxical effects of cyclosporin A on collagen arthritis in rats. J. Exp. Med. 158(6):2007-2015.
Crossref
|
|
|
|
|
Khan RA, Khan MR, Sahreen S, Ahmed M (2012). Assessment of flavonoids contents and in vitro antioxidant activity of Launaea procumbens. Chem. Central J. 6(1):43.
Crossref
|
|
|
|
|
Kumar V, Abbas AK, Fausto N, Aster JC (2010). Robbins and Cotran Pathological Basis of Disease. 8th Edn. Saunders Elsevier. Elsevier, Sauders.
|
|
|
|
|
Momo CEN, Oben JE, Tazoo D, Dongo E (2006). Antidiabetic and hypolipidemic effects of Laportea ovalifolia (Urticaceae) in Alloxan induced diabetic rats. Afr. J. Tradit, Complement. Altern. Med. 3(1):36-43.
|
|
|
|
|
Mshana NR (2000). Traditional medicine and pharmacopoeia: contribution to the revision of ethnobotanical and floristic studies in Ghana. Organization of African Unity/Scientific, Technical & Research Commission.
|
|
|
|
|
Narayanaswamy N, Balakrishnan KP (2011). Evaluation of some medicinal plants for their antioxidant properties. Int. J. Pharm. Technol. Res. 3(1):381-385.
|
|
|
|
|
Obiri D, Osafo N, Dei-Anane S (2013). Anti-Allergic, Anti-inflammatory and analgesic actions of stem bark extract of Lannea welwitschii (Anarcadiaceae) in mice. Int. J. Pharm. Pharm. Sci. 5(4):217–22.
|
|
|
|
|
Okwulehie IC, Akanwa FE (2013). Antimicrobial Activity of ethanol extract of four indigenous plants from south eastern Nigeria. ARPN J. Sci. Tech. 3(4):350-355.
|
|
|
|
|
Ozaki Y (1990). Antiinflammatory effect of Curcuma xanthorrhiza Roxb, and its active principles. Chem. Pharm. Bull. 38(4):1045-1048.
Crossref
|
|
|
|
|
Prieto P, Pineda M, Aguilar M (1999). Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem. 269(2):337-341.
Crossref
|
|
|
|
|
Rock KL, Kono H (2008). The inflammatory response to cell death. Ann. Rev. Pathol. 3:99-126.
Crossref
|
|
|
|
|
Schmid-Schönbein GW (2006). Analysis of inflammation. Ann. Rev. Biomed. Eng. 8:93-131.
Crossref
|
|
|
|
|
Shailasree S, Ruma K, Kini KR, Niranjana SR, Prakash HS (2012). Reveiw Article: Potential anti-inflammatory bioactives from medicinal plants of Western Ghats, India. Pharmacogn. Comm. 2(2):2-12.
Crossref
|
|
|
|
|
Singh A, Malhotra S, Subban R (2008). Anti-inflammatory and Analgesic Agents from Indian Medicinal Plants Anti-inflammatory and analgesic Medicine. Bangladesh J. Pharmacol. 3(1):57-72.
|
|
|
|
|
Škerget M, Kotnik P, Hadolin M, Hraš AR, SimoniÄ M, Knez Ž (2005). Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 89(2):191-198.
Crossref
|
|
|
|
|
Sofowora A (1996). Research on medicinal plants and traditional medicine in Africa. J. Altern. Complement. Med. 2(3):365-72.
Crossref
|
|
|
|
|
Susanti D, Sirat HM, Ahmad F, Ali RM, Aimi N, Kitajima M (2007). Antioxidant and cytotoxic flavonoids from the flowers of Melastoma malabathricum L. Food Chem. 103(3):710-716.
Crossref
|
|
|
|
|
Thomazzi SM, Silva CB, Silveira DCR, Vasconcellos CLC, Lira AF, Cambui EVF (2010). Antinociceptive and anti-inflammatory activities of Bowdichia virgilioides (sucupira). J. Ethnopharmacol. 127(2):451-456.
Crossref
|
|
|
|
|
Winter CA, Risley EA, Nuss GW (1962). Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Exp. Biol. Med. 111(3):544-547.
Crossref
|
|
|
|
|
Woode E, Abotsi WKM (2011). Antinociceptive effect of an ethanolic extract of the aerial parts of Hilleria latifolia (Lam.) H. Walt. (Phytolaccaceae). J. Pharm. Bioallied. Sci. 3(3):384.
Crossref
|
|
|
|
|
Woode E, Abotsi WKM, Mensah AY (2011). Anxiolytic-and antidepressant-like effects of an ethanolic extract of the aerial parts of Hilleria latifolia (Lam.) H. Walt. in mice. J. Nat. Pharm. 2(2).
Crossref
|
|
|
|
|
Wu S-J, Ng L-T (2008). Antioxidant and free radical scavenging activities of wild bitter melon (Momordica charantia Linn. var. abbreviata Ser.) in Taiwan. LWT-Food Sci. Technol. 41(2):323-330.
|
|