ABSTRACT
Cassia sieberiana DC. (Caesalpiniaceae), Guiera senegalensis JF. Gmel (Combretaceae) and Excoecaria grahamii (Hochst) Pax. (Euphorbiaceae) are used in traditional medicine in Burkina Faso to treat gastro-intestinal parasites. Standard methods were used for phytochemical screening and to evaluate the in vitro anthelmintic activity of the aqueous extracts of the three (3) plants using the egg hatch test against Haemonchus contortus eggs. The phytochemical analysis revealed the presence of anthocyanosides, tannins, saponosides, reducing compounds, carbohydrates, flavonoids and triterpenic steroids in the extracts of the root bark of C. sieberiana and the leaves of C. sieberiana, G. senegalensis and E. grahamii. All extracts inhibited significantly (p< 0.05) the eggs hatch of H. contortus. At the concentration of 14.25 mg/mL, the percentages of inhibition varied from 60.33 ± 8.43 to 76.6 ± 4.91%. These results indicate that the studied plants possess anthelminthic activities and thus justify their use for the treatment of gastrointestinal parasites infections.
Key words: Anthelmintic activity, medicinal plants, phytochemical, in vitro, Haemonchus contortus, Burkina Faso.
Gastrointestinal parasites infections are public health problem in humans and livestock. In particular, the high prevalence of intestinal nematodes, with an estimated 3.5 billion people being infected every year has been reported (Horton, 2003; Bethony et al., 2006; Grzybek et al., 2016). Thus, more than a quarter (1/4) of the world's population is affected by parasites infections including helminthosis. They are often responsible for nutritional deficiency with heavy consequences for the children, girls of childbearing age and pregnant women (WHO, 2008). Indeed, these diseases undermine the intellectual growth, the cognitive development and the school performance in children (Hotez, 2009; WHO, 2015). These gastrointestinal parasites are also responsible for high morbidity, weight loss, poor reproductive status and likely mortality in livestock (Ndao et al., 1995; Wolstenholme et al., 2004; Grzybek et al., 2016).
Their control is based on the use of modern anthelmintic drugs (Hounzangbe-Adote et al., 2005; Hoste et al., 2011). However, the excessive use of these anthelmintics leads increasingly to the development of resistance of helminths. Due to this factor and other reasons including the problem of accessibility to the modern drugs at the appropriate time and place and the lack of financial resources (Asase et al., 2008), many people in the developing countries use the plants for their primary health care (Stangeland et al., 2008; Tanner et al., 2011; Asase et al., 2008). Thus, the importance and the use of medicinal plants in countries such as Burkina Faso are no longer to be demonstrated. But, the therapeutic efficacy and the chemical contents of many plants remain to be scientifically documented. Several medicinal plants are used in Burkina Faso to treat human and animal gastrointestinal parasites (Kaboré et al., 2007).
Surveys in four areas of the country have shown that Cassia sieberiana DC. (Caesalpiniaceae), Guiera senegalensis JF. Gmel. (Combretaceae) and Excoecaria grahamii (Hochst) Pax. (Euphorbiaceae) are among the most frequently cited plants for the treatment of gastro intestinal parasitosis (Traoré et al., 2013). Several studies have demonstrated the antimicrobial, analgesic, anti-inflammatory, antihypertensive, anti-venomous, spasmolytic, diuretic and laxative effects of these medicinal plants (Anton and Duquenois, 1968; Kerharo and Adam, 1974). Fall et al. (2004) have demonstrated the anticoccidial effects of the butanol extract of C. sieberiana. Trypanocidal properties of the leaves of this plant have also been reported (Hoet et al., 2004). The use of G. senegalensis in the treatment of diarrhoea, as febrifuge, antineuralgic, and diuretic have been reported by Kerharo et al. (1948). This paper reported the results of the chemical screening of the extracts of C. sieberiana, G. senegalensis and E. grahamii used in Burkina Faso against gastro intestinal parasites in human and livestock. The extracts of the plants were also evaluated for their anthelminthic activities using the eggs hatch test on the nematode parasite Haemonchus contortus.
Study species
C. sieberiana DC. (Caesalpiniaceae). Synonyms: Cassia abbreviata Oliv. ; Cassia kotschyana Oliv
Cassia sieberiana is a small tree branched near the base and can reach 5 m high. The stem bark is fissured and lamellar, blackish in old subjects. The leaves are alternate and composed, 10-30 cm long with 6-12 pairs of opposite leaflets. The inflorescences are long hanging clusters of yellow flowers up to 35-50 cm long. The fruits are long and straight cylindrical pods up to 60 to 80 cm long by 10 to 15 mm in diameter, indehiscent, dark brown or blackish at maturity, persisting for a very long time on the tree. The fruit is transversely partitioned, each lobe containing a seed surrounded by a more or less farinaceous yellow pulp. C. sieberiana is widespread in West Africa and is very common in all savannah woodlands or shrubs of the Sudanian zones up to the edge of the Guinean forest in Casamance. The plant is widely used in traditional medicine as an analgesic in dysmenorrhea, body pain in humans and in veterinary medicine (Sam et al., 2011). The in vitro anthelmintic activity against Caenorhabditis elegans of the leaves extract have been reported (Waterman et al., 2010).
Guiera senegalensis J.F. Gmel. (Combretaceae) ; Synonym: Guiera glandulosa Sm. G. senegalensis is an erect shrub, branched almost from the base with several silvery pubescence branches. The leaves are opposite, oval, softly pubescent on both sides, with black glands below and white hairs giving a general green-gray silvery shade to shrubs. The fruit is elongated, densely and long hairy, silver and pink, radiating like hairy legs of a spider. The plant is semi-evergreen and can reach 3 m high. The species is widespread in Africa throughout the Sudano-Sahelian zone; from Senegal to Cameroon up to Sudan. It is widely distributed in Nigeria, Senegal, The Gambia, Mali, Niger and Burkina Faso where it is locally gregarious and very abundant. G. senegalensis is regarded by traditional medicine practitioners in West Africa as a panacea, as its medicinal uses are important and varied (Akuodor et al., 2013). It is used to treat cough, pneumonia, and malaria, gastrointestinal pain, dysentery, diarrhea and used against intestinal worms (Maleš et al., 1998).
Excoecaria grahamii (Hochst) Pax. (Euphorbiaceae); Synonym Sapium grahamii (Stapf) Prain
E. grahamii is an African small shrub glabrous, unbranched, up to 60 to 90 cm tall with milky and sticky latex. The stems are herbaceous or sub herbaceous. The stem with lignified base is derived from a tuberous creeping root. The fruit is a three-lobed capsule of 2-2.5 cm diameter, hard, brown, with explosive dehiscence, containing three seeds. The seeds are almost globular, brown to yellow and about 5 mm long. The genus Excoecaria occurs in the tropics of the Old World and the Pacific islands and comprises 35 species. There are about 6 species in the continental Africa and about 5 species in Madagascar. E. grahamii is common in savannahs and at the edge of forests, usually on moist soils, from Guinea to Nigeria. The whole plant, the leaves and the latex are used in traditional medicine in Benin, Burkina Faso, Ivory Coast, Niger and Nigeria against skin affections, edema, leprosy, dysentery, guinea worms, and hallucinations. In Burkina Faso, the leaves decoction is known to have purgative effects and is also used to induce abortion (Nacoulma, 1996). The roots latex is used for ritual scarifications and tattooing.
Plants material
The leaves and the roots bark of the three (3) plants were collected in the suburbs of Ouagadougou (savanna zone). The plants C. sieberiana and G. senegalensis were collected at the beginning of the rainy season (June) in Gampéla, located at 15 km in the East of Ouagadougou. E. grahamii was collected during the rainy season (August) at Loumbila at 15 km at the North-East of Ouagadougou. The plant's species were confirmed at the herbarium of the National Centre for Scientific and Technical Research (CNRST) in Ouagadougou, where specimens are deposited under the numbers of HNBU00179 (C. sieberiana), HNBU00252 (G. senegalensis) and HNBU01032 (E. grahamii).
Eggs of H. contortus
The H. contortus eggs were collected from adult worms harvested from the stomach of naturally infected goats or sheep. The stomachs were bought from the refrigerated slaughterhouse of Ouagadougou.
Plants extracts
The leaves and the roots bark were washed with water and dried from dust. They were finely powdered. The powders were used to make decoctions for the leaves of C. sieberiana, E. grahamii and G. senegalensis and maceration for the roots barks of C. sieberiana. For decoctions, 100 g of plant powder were extracted in 1000 mL of distilled water. The mixture was boiled for 1 h. After cooling, the extract is filtered through fine nylon mesh. The filtrates obtained from C. sieberiana (DFCS), from G. senegalensis (DFGS) and from E. grahamii (DFEG) were centrifuged at 2000 rpm for 10 min. The supernatant obtained from each sample was frozen and then lyophilized. For extraction, 100 g of powder was mixed with 1000 mL of distilled water and allowed to macerate for 48 h before being filtered as for the decoctions. The filtrate obtained from C. sieberiana (MERCS) was frozen and then lyophilized. For the assay, 150 mg of the dried extracts were diluted in 10 mL of distilled water to get a stock solution which was serially diluted to obtain five different concentrations.
Collection of eggs of H. contortus
The method used was that described by Jabbar et al. (2006). For this purpose, the adult worms collected in phosphate buffer solution (PBS; pH = 7.2) were washed with distilled water and crushed lightly in a mortar using a porcelain pestle to release the eggs. The solution obtained was filtered through mesh sieves of various sizes (1 mm and 100 μm). Then, a smaller mesh sieve (38 μm) was used to collect the eggs released by the crushed worms. After several rinses with distilled water, the eggs were recovered in solution and the concentration was adjusted to approximately 1000 eggs/mL of distilled water.
Phytochemical screening of the extracts
The decoctions and the macerate obtained were used for phytochemical tests to identify the chemical groups present in the plants. The characterization methods described by Ciulei (1982) were used.
Egg hatch assay
The egg hatch assay was carried out according to the method described by Coles et al. (2006). 100 μL of the egg suspension adjusted to approximately 1000 eggs/mL was deposited in each well of a multiwell (24 wells) plate. In each well, 1900 μL of the lyophilized extracts were added at concentrations of 0.1, 1, 3, 10 and 15 mg/mL giving final concentrations of 0.095, 0.95, 2.85, 9.50 and 14.25 mg/mL. PBS and albendazole were used as negative and positive controls, respectively. Then, the plate was incubated at the laboratory temperature (32°C) for 48 h. At the end of the incubation, a drop of formalin was deposited in each well to stop the evolution of the eggs. Then, the eggs and died or alive larvae were counted under a microscope (Olympus BH-2, Optical Co. LTD, Japan) at 40 X, to establish the egg hatching rate. The test was carried out in triplicate. The results were expressed as % inhibition of eggs hatch. For each extract concentration, the % inhibition of eggs hatch was calculated using the modified formula of Coles et al. (1992):
Percentage (%) inhibition of egg hatch = (1-X2 / X1) × 100
Where, X1 = initial number of eggs in the well and X2 = final number of eggs in the well.
Reference drug
The reference drug, albendazole (tablets, 400 mg) used in this study was bought from a local pharmacy. This drug was chosen due to its availability and also because some authors have used it for in vitro trials using different concentrations. The tablets were powdered and the powder was diluted in distilled water to obtain a stock solution with a concentration of 15 mg/mL, which was serially diluted to obtain the same concentrations as with the extracts.
Statistical analysis
For the egg hatch assay, the tests were carried out in triplicate and repeated twice (2) or three (3) times. The statistical analysis was performed with the GraphPad Prism software, version 6.01 (Graph Pad Software Inc., San Diego, CA). Results were expressed as mean ± SEM. Values of 50% inhibitory concentrations (IC50: the concentration capable of inducing 50% inhibition of egg hatching) were determined using a nonlinear regression analysis. The ANOVA was used for the analysis of the data. The differences were considered statistically significant when p <0.05.
Phytochemical analysis
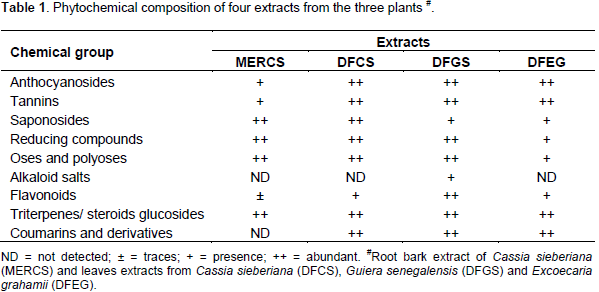
The results of chemical screening of the four (4) extracts from the 3 plants are presented in Table 1. According to these results, anthocyanosides, tannins, saponosides, reducing compounds, oses and polyoses, flavonoids and triterpene steroids were found in all extracts. Thus, the 4 extracts showed approximately the same phytochemical profile except for coumarins which were not detected in the roots bark extract of C. sieberiana and the alkaloids salts which were found only in the leaves extract of G. senegalensis.
Ovicidal activity
The results of the egg hatch assay, testing of the four (4) extracts of the three (3) plants are presented in Figure 1. All the extracts showed a dose-dependent inhibition of the H. contortus egg hatching. The values of the % inhibition produced by the extracts and the positive control (albendazole) were statistically different (p <0.05) compared to that of the negative control (PBS) (2.27 ± 0.39 %). At the concentration of 14.25 mg/mL, the macerate of root bark of C. sieberiana (MERCS), the decoction of leaves of C. sieberiana (DFCS), the decoction of leaves of G. senegalensis (DFGS) and the decoction of leaves of E. grahamii (DFEG) showed egg hatch inhibition between 60 and 73%.
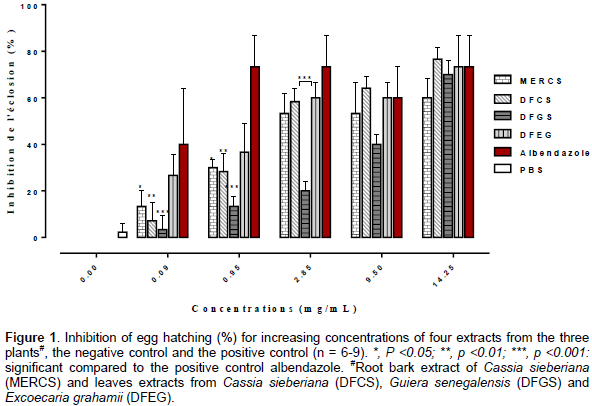
These effects were similar to the inhibition induced by albendazole (73.34 ± 13.33%) and were significantly different (p <0.05) compared to the negative control (PBS). Comparison between the four (4) extracts showed that there was no significant difference (p > 0.05) between the effect of the leaves extract (DFCS) and the root bark extract (MERCS) of C. sieberiana. But the effect induced by DFCS was significantly different (p <0.05) from the effects of DFGS and DFEG. Similarly, MERCS induced a significantly different (p <0.05) effect from that of DFGS. The IC50 values for the extracts were determined and are presented in Table 2.
The main chemical groups highlighted in the extracts of C. sieberiana, G. senegalensis and E. grahamii were anthocyanosides, tannins, saponosides, triterpene and steroids. These findings corroborate those of Abubakar et al. (2000), Kouamé et al. (2009), Shettima et al. (2012) and Nartey et al. (2012) who reported the presence of these chemical compounds in the three plants. The chemicals found in G. senegalensis leaf extracts were alkaloids, saponosides, coumarins, flavonoids, and tannins. These data corroborate the results previously reported. Indeed, the presence of tannins, flavonoids (Abubakar et al., 2000) and alkaloids (Fiot et al., 2005) was revealed in leaves extracts of this plant. Nacoulma (1996) also reported the presence of these constituents in the leafy stems of G. senegalensis. In addition to tannins, our results show the presence of saponosides and triterpene/steroids in the leaves and the roots bark of C. sieberiana.
These results are in agreement with those of Tamboura et al. (2005) which revealed the presence of tannins and saponosides in this plant. These active substances probably explain the current uses of the three plants by traditional healers in the treatment of many human and animal diseases including parasitic infections (Nacoulma, 1996). Indeed, the therapeutic properties of these secondary metabolites have been reported (Cowan, 1999). According to Waterman et al. (2010), tannins and other phenolic compounds possess proteins coagulant properties that would give rise to a broad spectrum of vermicidal activities. It is generally considered that the anthelminthic effects of forage containing tannins are related to their content of condensed tannins (Hoste et al., 2011). Some in vitro results suggest also that secondary metabolites such as flavonoid and alkaloïds (Molan et al., 2003;; Barrau et al., 2005; Brunet and Hoste, 2006; Eguale et al., 2008; Chagas et al., 2008; Muthee et al., 2011), saponins (Ademola and Eloff, 2010; Waterman et al., 2010) and terpenoids (Marley et al., 2006; Githiori et al., 2003) may possess some anthelminthic properties.
The dose-dependent relationship between the concentration of tannins and/or flavonoid compounds and the anthelminthic activity has been demonstrated under both in vitro (Molan et al., 2002; Brunet and Hoste, 2006) and in vivo conditions (Hoste et al., 2011; Brunet et al., 2007; Terrill et al., 2009). The high egg hatching inhibition (> 60%) induced by the extracts at 14.25 mg/mL and the few inhibition rate (≈ 2.27%) with PBS (negative control), confirm that changes in egg hatch observed with the extracts, result from the ovicidal action of these substances. They induced an inhibition of the egg hatching in the nematode parasite H. contortus. Thus, they possess in vitro anthelmintic properties. These results showed clearly that the leaves extract of E. grahamii (IC50 = 0.39 mg/mL) was more active than the other extracts and 10 times less active than the positive control, albendazole (IC50 = 0.03 mg/mL).
Based on the IC50 values (Table 2), the extracts and the positive control could be classified, in order of power, as follows: albendazole > DFEG > DFCS > MERCS > DFGS. This ovicidal activity could support the use of these plants by traditional healers against gastrointestinal parasites and the chemical groups found in the plant's extracts should be the basis of the biological activities attributed to these plants, including their anthelminthic properties (Paolini et al., 2003). It has been reported that the active molecules with ovicidal activity penetrate the eggshell and prevent its development or paralyze the L1 larval stage inside the egg (Wabo et al., 2011). The four extracts could have exerted their ovicidal effect through this mechanism. Thus, the hatching process could have been inhibited by active compounds such as nitrogen-containing compounds which are known to have ovicidal properties (Maciel et al., 2006). However, more investigation is needed to identify the active components responsible for this action.
The results from this study revealed that the extracts from C. sieberiana, G. senegalensis and E. grahamii inhibit the egg hatching in H. contortus and could be a potential source of anthelmintics. The activity of these extracts can be attributable to the secondary metabolites such as tannins, flavonoids, alkaloids, steroids, terpenoids and/or saponins or to the combined interaction of these components. However, further studies like larvicidal assays and in vivo trials should be carried out to confirm the efficacy of these plant species.
The authors have not declared any conflict of interests.
REFERENCES
|
Abubakar MS, Sule MI, Pateh UU, Abdurahman EM, Haruna AK, Jahun BM (2000). In vitro snake venom detoxifying action of the leaf extract of Guiera senegalensis. J. Ethnopharmacol. 69(3):253-257.
Crossref
|
|
|
|
Ademola IO, Eloff JN (2010). In vitro anthelmintic activity of Combretum molle (R. Br. ex G. Don) (Combretaceae) against Haemonchus contortus ova and larvae. Vet. Parasitol. 169(1):198-203.
Crossref
|
|
|
|
|
Akuodor GC, Essien AD, David-Oku E, Chilaka KC, Akpan JL, Ezeokpo B, Ezeonwumelu JOC (2013). Gastroprotective effect of the aqueous leaf extract of Guiera senegalensis in Albino rats. Asian Pac. J. Trop. Med. (2013):771-775.
Crossref
|
|
|
|
|
Anton R, Duquenois P (1968). L'emploi des Cassias dans les pays tropicaux et subtropicaux examiné sous quelques-uns des constituants chimiques de ces plantes médicinales. Pltes Méd. Phytother. 2:225-268.
|
|
|
|
|
Asase A, Kokubun T, Grayer RJ, Kite G, Simmonds MSJ, Oteng-Yeboah AA, Odamtten GT (2008). Chemical constituents and antimicrobial activity of medicinal plants from Ghana: Cassia sieberiana, Haematostaphis barteri, Mitragyna inermis and Pseudocedrela kotschyi. Phytother. Res. 22(8):1013-1016.
Crossref
|
|
|
|
|
Barrau E, Fabre N, Fouraste I, Hoste H (2005). Effects of bioactive compounds from Sainfoin (onobrychis viciifolia Scop.) on the in vitro larval migration of Haemonchus contortus: role of tannins and flavonol glycosides. Parasitol. 131(4):531-538.
Crossref
|
|
|
|
|
Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, Hotez PJ (2006). Soil-transmitted helminth infections: Ascariasis, trichuriasis, and hookworm. Lancet 367:1521-1532.
Crossref
|
|
|
|
|
Brunet S, Aufrere J, El Babili F, Fouraste I, Hoste H (2007). The kinetics of exsheathment of infective nematode larvae is disturbed in the presence of a tannin- rich plant extract (sainfoin) both in vitro and in vivo. Parasitol. 134(9):1253-1262.
Crossref
|
|
|
|
|
Brunet S, Hoste H (2006). Monomers of condensed tannins affect the larval exsheathment of parasitic nematodes of ruminants. J. Agric. Food Chem 54(20):7481-7487.
Crossref
|
|
|
|
|
Chagas ACS, Vieira LS, Freitas AR, AraÏŠjo MRA, AraÏŠjo-Filho JA, Araguγo WR, Navarro AMC (2008). Anthelmintic efficacy of neem (Azadirachta indica A. Juss) and the homeopathic product Fator Vermes® in Morada Nova sheep. Vet. Parasitol. 151(1):68-73.
Crossref
|
|
|
|
|
Ciulei I (1982). Practical manuals on the industrial utilization of chemical and aromatic plants. Methodology for analysis of vegetable drugs. Ed. Ministry of Chemical Industry, Bucharest, p. 67.
|
|
|
|
|
Coles GC, Bauer C, Borsgsteede FHM, Geerts S, Klei TR, Taylor MA, Waller PJ (1992). World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 44:35-44.
Crossref
|
|
|
|
|
Coles GC, Jackson F, Pomroy WE, Prichard RK, von Samson-Himmelstjerna G, Silvestre A, Taylor MA, Vercruysse J (2006). The detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 136(3):167-185.
Crossref
|
|
|
|
|
Cowan MM (1999). Plant products as antimicrobial agents. Clin. Microbiol. Rev. 12(4):564-82.
|
|
|
|
|
Eguale T, Tilahun G, Debella A, Feleke A, Makonnen E (2008). Haemonchus contortus: In vitro and in vivo anthelmintic activity of aqueous and hydro-alcoholic extracts of Hedera helix. Exp Parasitol. 116(4):340-345.
Crossref
|
|
|
|
|
Fall AD, Gbati AOB, Diatta W, Sow B, Gueye F, Bassene E, Pangui L (2004). Etude de la fraction anticoccidienne de l'extrait de racines de Cassia sieberiana D.C. (Caesalpiniaceae) chez le lapin. J. Pharm. Méd. Trad. Afr. 13:49-55. Available at :
View
|
|
|
|
|
Fiot J, Sanon S, Azas N, Mahiou V, Jansen O, Angenotc L, Balansard G, Ollivier E (2005). Phytochemical and pharmacological study of roots and leaves of Guiera senegalensis J.F. Gmel (Combretaceae). J. Ethnopharmacol. 106 (2):173-178.
Crossref
|
|
|
|
|
Githiori J B, Höglund J, Waller PJ, Baker RL (2003). The anthelmintic efficacy of the plant Albizia anthelmintica, against the nematode parasite Haemonchus contortus of sheep and Heligmosomoides polygyrus in mice. Vet. Parasitol. 116(1):23-34.
Crossref
|
|
|
|
|
Grzybek M, Kukula-Koch W, Strachecka A, Jaworska A, Phiri AM, Paleolog J, Tomczuk K (2016). Evaluation of anthelmintic activity and composition of pumpkin (Cucurbita pepo L.) seed extracts—in vitro and in vivo studies. Int. J. Mol. Sci. 17 (9):1456-1467.
Crossref
|
|
|
|
|
Hoet S, Opperdoes F, Brun R, Adjakidj V, Quetin-Leclercq J (2004). In vitro antitrypanosomal activity of ethnopharmacologically selected Beninese plants. J. Ethnopharmacol. 91(1):37-42.
Crossref
|
|
|
|
|
Horton J (2003). Human gastrointestinal helminth infections: Are they now neglected diseases? Trends Parasitol. 19(11):527-531.
Crossref
|
|
|
|
|
Hoste H, Manolaraki F, Brunet S, Arroyo LC, Martínez-Ortiz de Montellano C, Sotiraki S, Torres AF (2011). The anthelmintic properties of tannin-rich legume forages: from knowledge to exploitation in farm conditions. In: Ranilla M.J. (ed.), Carro M.D. (ed.), Ben Salem H. (ed.), Morand-Fehr P. (ed.). Challenging strategies to
|
|
|
|
|
Hotez PJ (2009). One world health: Neglected Tropical Diseases in a Flat World. PLoS Negl. Trop. Dis. 3:e405.
Crossref
|
|
|
|
|
Hounzangbe-Adote M, Paolini V, Fouraste I, Moutairou K, Hoste H. (2005). In vitro effects of four tropical plants on three life-cycle stages of the parasitic nematode, Haemonchus contortus. Res. Vet. Sci. 78(2):155-160.
Crossref
|
|
|
|
|
Kaboré A, Tamboura HH, Belem AMG, Traoré A (2007). Traitements ethno-vétérinaires des parasitoses digestives des petits ruminants dans le plateau central du Burkina Faso. Int. J. Biol. Chem. Sci. 1(3):297-304.
|
|
|
|
|
Kerharo J, Adam JG (1974): Pharmacopée sénégalaise traditionnelle: Plantes médicinales et toxiques. Edition Vigot et frères, Paris.
|
|
|
|
|
Kerharo J, Bouquet A, Heintz R (1948). Le Wilinwig-a des Mossi (Guiera senegalensis, Lam.), ses usages thérapeutiques indigènes et son application au traitement des diarrhées cholériformes. Acta Trop. 5:345.
|
|
|
|
|
Kouamé J, Gnoula C, Palé E, Bassolé H, Guissou IP, Simporé J, Nikiéma J -B (2009). Etude des propriétés cytotoxiques et anti-radicalaires d'extraits de feuilles et de galles de Guiera senegalensis J. F. Gmel (Combretacae). Sci. et technique-Sciences de la santé, pp. 9-23.
|
|
|
|
|
Maciel MV, Morais SM, Bevilaqua CML, Camurça-Vasconcelos ALF, Costa CTC, Castro CMS (2006). Ovicidal and larvicidal activity of Melia azedarach extracts on Haemonchus contortus. Vet. Parasitol. 140(1):98-104.
Crossref
|
|
|
|
|
Maleš Ž, Medic-Šarić M and Bucar F (1998). Flavonoids of Guiera senegalensis J. F. Gmel.Thin-layer Chromatography and Numerical Methods. Croatica Chemica Acta 71(1):69-79.
|
|
|
|
|
Marley CL, Cook R, Barrett J, Keatinge R, Lampkin NH (2006). The effects of birdsfoot trefoil (Lotus corniculatus) and chicory (Cichorium intybus) when compared with perennial ryegrass (Lolium perenne) on ovine gastrointestinal parasite development, survival and migration. Vet. Parasitol. 138(3):280-290.
Crossref
|
|
|
|
|
Molan AL, Meagher LP, Spencer, PA, Sivakumaran S (2003). Effect of flavan-3-ols on in vitro egg hatching, larval development and viability of infective larvae of Trichostrongylus colubriformis . Int. J. Parasitol. 33(14):1691-1698.
Crossref
|
|
|
|
|
Molan AL, Waghorn GC, McNabb WC (2002). Effect of condensed tannins on egg hatching and larval development of Trichostrongylus colubriformis in vitro. Vet. Rec. 150:65-69.
Crossref
|
|
|
|
|
Muthee JK, Gakuya DW, Mbaria JM, Kareru PG, Mulei CM, Njonge FK (2011). Ethnobotanical study of anthelmintic and other medicinal plants traditionally used in Loitoktok district of Kenya. J. Ethnopharmacol. 135(1):15-21.
Crossref
|
|
|
|
|
Nacoulma OG (1996). Plantes médicinales et pratiques médicales traditionnelles au Burkina Faso : cas du plateau central. Thèse de Doctorat ès Sciences Naturelles. Faculté des Sciences et Techniques, Université de Ouagadougou, 320p.
|
|
|
|
|
Nartey ET, Ofosuhene M, Kudzi W, Agbale CM (2012). Antioxidant and gastric cytoprotective prostaglandins properties of Cassia sieberiana roots bark extract as an anti-ulcerogenic agent. BMC Complem. Altern. Med. 12(1):1-10.
Crossref
|
|
|
|
|
dao M, Belot J, Zinsstag J, Pfister K (1995). Épidémiologie des helminthoses gastro-intestinales des petits Ruminants dans la zone sylvo-pastorale au Sénégal. Vet. Res. 26(2):132-139.
|
|
|
|
|
Paolini V, Frayssines A, De La Farge F, Dorchies P, Hoste H (2003). Effects of condensed tannins on established populations and on incoming larvae of Trichostrongylus colubriformis and Teladorsagia circumcincta in goats. Vet. Res. 34(3):331-339.
Crossref
|
|
|
|
|
Sam GH, Mensah MLK and Nyakoa-Ofori N (2011). Pharmacognostic studies and standardization of Cassia sieberiana roots. Pharmacogn. J. 3(21):12-17.
Crossref
|
|
|
|
|
Shettima YA, Tijjani MA, Karumi Y, Sodipo OA (2012). Phytochemical and anti-diarrhoeal properties of methanol root extract of Guiera senegalensis J.F.Gmel. Inter. Tes. J. Pharm., 3 (11):61-65. Available at:
View
|
|
|
|
|
Stangeland T, Dhillion S S, Reksten H (2008). Recognition and development of traditional medicine in Tanzania. J. Ethnopharmacol. 117(2):290-299.
Crossref
|
|
|
|
|
Tamboura HH, Bayala B, Lompo M, Guissou IP, Sawadogo L, (2005). Ecological distribution, morphological characteristics and acute toxicity of aqueous extracts of Holarrhena floribunda (G. Don) Durand and Schinz., Leptadenia hastata (Pers.) Decne and Cassia sieberiana (DC) used by veterinary healers in Burkina Faso. Afr. J. Tradit. Complem. Altern. Med., 2(1):13-24.
|
|
|
|
|
Tanner S, Chuquimia-Choque ME, Huanca T, McDade TW, Leonard WR, Reyes-García V (2011). The effects of local medicinal knowledge and hygiene on helminth infections in an Amazonian society. Soc. Sci. Med. 72(5):701-709.
Crossref
|
|
|
|
|
Terrill TH, Dykes GS, Shaik SA, Miller JE, Kouakou B, Kannan G, Burke JM, Mosjidis JA, (2009). Efficacy of sericea lespedeza hay as a natural dewormer in goats: Dose titration study. Vet. Parasitol. 163(1):52-56.
Crossref
|
|
|
|
|
Traoré A, Ouedraogo S, Lompo M, Traoré S, Somé N, Guissou IP (2013). Ethnobotanical survey of medicinal plants used to treat gastrointestinal parasites in human and livestock in four geographic areas of Burkina Faso (West Africa). Arch. Appl. Sci. Res. 5(6):172-177.
|
|
|
|
|
Wabo PJ, Kenne TF, Mpoame MP, Tedonkeng E, Bilong Bilong CF (2011). In vitro activities of acetonic extracts from leaves of three forage legumes (Calliandra calotyrsus, Gliricidia sepium and Leucaena diversifolia) on Haemonchus contortus. Asian Pac. J. Trop. Med. 4(2):125-128.
Crossref
|
|
|
|
|
Waterman C, Smith RA, Pontiggia L, Dermarderosian A (2010). Anthelmintic screening of Sub-Saharan African plants used in traditional medicine. J. Ethnopharmacol. 127(3):755-759.
Crossref
|
|
|
|
|
Wolstenholme AJ, Fairweather I, Prichard R, von Samson-Himmelstjerna G, Sangster NC (2004). Drug resistance in veterinary helminths. Trends Parasitol. 20(10):469-476.
Crossref
|
|
|
|
|
World Health Organization (WHO) (2008). Chimio prévention des helminthiases chez l'homme: utilisation coordonnée des médicaments anthelminthiques pour les interventions de lutte : manuel à l'intention des professionnels de la santé et des administrateurs de programmes. / D.W.T. Crompton; Genève, 64p.
|
|
|
|
|
World Health Organization (WHO) (2015). Relevé épidémiologique hebdomadaire, 18 Décembre 2015, N° 51/52: 701-712. Available at:
View
|
|