Respiratory syncytial virus (RSV) causes viral infection of a global proportion. Currently, there is no approved vaccine or useful antiviral drug against it. Natural products hold potential for discovery of anti-RSV agents. The present study assessed the anti-RSV activities of Alchornea cordifolia (AC) (Shumach. and Thonn.) MÏ‹ll. Arg. and Alchornea floribunda (AF) MÏ‹ll. Arg., Antiviral activities of AC and AF leaf extracts were evaluated by a modified viral plaque reduction assay. Parallel assays for effect of the antiviral agents on cell viabilities were carried out the cells using the 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide (MTT) reduction assays. Mechanism of anti-RSV action of the extracts was done based on time-of-addition assay. Extracts of AC and AF, showed anti-RSV activities with IC50 = 5.64 ± 1.16, and 75.62 ± 3.38 µg/ml, respectively while the cell cytotoxic effect of extracts were TC50 = 103.14 ± 3.98, and 333.82 ± 6.32 µg/ml, respectively. Time-of-addition assay results suggested that AC and AF interfere with viral replication at a viral post-entry step. The extracts of AC and AF exhibited profound anti-RSV activities warranting their development for further possible clinical utility against RSV.
Respiratory syncytial virus (RSV) belongs to the
Pneumovirus genus of the Paramyxoviridae family. It remains globally the most important cause of viral lower respiratory tract illness (LRI) in infants and children. RSV global prevalence is on the increase, especially among infants and young children (
Hall, 1994; Collins et al., 1996; NIH, 2011; Jorquera and Tripp, 2017). In the developing countries and Africa about 17% of acute respiratory infections in children admitted to hospital are attributed to RSV (Adegbola et al., 1994; Nwankwo et al., 1994; Martin et al., 1998; Peltola and Ruuskanen, 2008). Unfortunately, there have been many obstacles to development of vaccines against RSV (Wright et al., 2000; Tregoning and Schwarze, 2010). Drug therapeutics is equally presently a big challenge (Simoes et al., 2015). Ribavirin, a broad-spectrum antiviral agent, seldom employed as adjunctive therapy for the very sick patients is the only therapeutic option; however, its efficacy has been called into question warranting its disuse by most institutions. It is also costly and quite difficult to administer (
Kneyber et al., 2000; Prince 2001; Lafeuillade et al., 2001; Seetharama and Narayana 2005).
Therefore, the search for novel antiviral inhibitors of RSV has become more intensive with efforts directed towards medicinal plants as lead sources for potent antiviral agents.
Some plants materials have been reported with inhibitory effects against the RSV, although none has made it to clinical stage (Esimone et al., 2007; 2008; Lai et al., 2013) Accordingly
, our focus was to screen
Alchornea cordifolia and
Alchornea floribunda based on currently available folkloric and
scientific reports about their wide range biological effect (Okoye et al., 2010; Okoye et al., 2015).
A. cordifolia MÏ‹ll. Arg. (Shumach. and Thonn.) is an erect, sometimes scrambling, bushy, perennial shrub or small tree, up to 4 m high reproducing from seeds. The plant has found usefulness in African folklore medicine. It has been utilized in various capacities as anti-inflammatory, antifungal and
antibacterial agent (Okoye et al., 2015; Noundou et al., 2016). Previous research findings have reported its anti-inflammatory, immunomodulatory and anti-oxidant activities (Osadebe and Okoye, 2003, Nworu et al., 2010). Despite its greatly desirable potential biologic effects, there is yet to be any reported investigation for anti-respiratory syncytial virus property of
A. cordifolia extracts.
A. floribunda MÏ‹ll Arg. is a laxly-branched shrub or small tree usually growing up to 4.5 m tall, occasionally to 7 m.
A. floribunda is a widely used medicinal plant in Africa, where it enjoys a high reputation as a stimulating intoxicant and aphrodisiac. The plant is sometimes cultivated for its medicinal uses and is also often traded.
A. floribunda (Euphorbiaceae)
leaves are widely used in African ethnomedicine for the treatment of bacterial (Noundou et al., 2014), fungal, parasitic infection. It has equally been used in the management of acute and chronic inflammatory disorders (Okoye et al., 2010).
Although various biological activities have been reported for both
A. cordifolia (AC) and
A. floribunda (AF), no assessment had been carried and recorded against the RSV. In traditional medicine both AC and AF are utilized against a wide range of aliments (Noundou et al., 2014; 2016; Okoye et al., 2015; 2016). Often times these disease conditions may overlap with underlying viral infection with both benefitting from overall treatment with either AC or AF traditional preparations. Moreover, AC and AF are known to be rich in vital phytochemicals including flavonoids which have been documented to contain a huge class of antimicrobial and antiviral agents (
Orhan et al., 2010; Zakaryan et al., 2017). Whether AC and AF could be beneficial in the control of RSV infection remains to be investigated. In this study, antiviral actions of extracts from
two medicinal plants (AC) and (AF) have been examined, as a way of identifying lead compounds with potencies against RSV.
Plant material and extraction
The plant materials were collected in Nsukka, Nigeria, and identified as
A. cordifolia (Shumach. and Thonn.) MÏ‹ll. Arg. (Fam: Euphorbiaceae) and
A. floribunda MÏ‹ll. Arg. (Fam: Euphorbiaceae) by Mr. A.O. Ozioko of the Bioresources Development and Conservation Programme, Nsukka, Enugu State, Nigeria. Voucher specimens
A. cordifolia (0012) and
A. floribunda (06/085), have been deposited at the herbarium of the Department of Pharmacognosy and Environmental Medicine, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka. The leaves of both plants were cleaned, dried and extracted by cold maceration using 90% v/v aqueous methanol (for isolation of most of the polar and non-polar constituents) to obtain the crude methanol extracts, respectively.
Reagents
Dulbecco's Modified Eagle's medium (DMEM) medium: Life Technologies, UK; MTT (thiazolyl blue tetrazolium bromide): Applichem; Dimethyl sulfoxide (DMSO): J.T Baker; SDS (sodium dodecylsulphate): Applichem; purity 99.5%; DMF (dimethyl formamide): Fischer Scientific; purity 99.96%; ribavirin: Sigma-Aldrich; purity: ≥ 98%.
Cell lines and respiratory syncytial virus (RSV)
We utilized the Human larynx epidermoid (HEp-2). HEp-2 was used to culture either HRSV (Long A2 strain: ATCC VR-26) or rgRSV which is a green fluorescent protein-displaying recombinant RSV strain. Moreover, rgRSV construction has been described (Zhang et al., 2002). HEp-2 Cells were propagated in DMEM supplemented with 10% fetal calf serum (FCS) and 1% penicillin/streptomycin antimicrobials while RSV was propagated in DMEM with 0.5% FCS and antimicrobials 1% penicillin/streptomycin. Viral titer was always determined and expressed as plaque forming units per ml (pfu/ml) (Esimone et al., 2008; Lai et al., 2013). Virus was purified and preserved at 80°C until used.
Cytotoxicity assay
The MTT assay for cytotoxicity evaluation has been described (Esimone et al., 2008; Lai et al., 2013). Briefly, HEp-2 cells at a 6000 cells/well population density were seeded onto a 96 well plate in 200 µl of DMEM-5 (5% FCS DMEM medium) per well. Well defined concentration ranges (100, 50, 25, 12.5 and 6.25 µg/ml) of extracts were solubilized in DMEM medium containing not more than 0.6% DMSO. DMEM-5 containing 0.6% DMSO was used as the “no drug” control. Set-up was incubated at 37°C under 5% CO2 for 2 days. Thereafter, MTT solution (5 mg/ml, 50 µl per well) was introduced to each well and additionally incubated at 37°C + 5% CO2 for 1 h to allow for formazan production. Formed blue formazan crystals trapped within the cells (after medium removal) was dissolved by addition of 200 µl of 20% sodium dodecyl sulphate (SDS) solution in water/dimethylformamide (1:1) (pH 4.7) overnight. Using a multi-well microtiter plate reader (Tecan, Austria) optical densities of triplicate values were determined at 550 nm. From the triplicate measurements of drug treated and “no drug” control cultures the mean and standard error of the percent values of optical densities were calculated for each drug concentration. The concentration of 50% cellular toxicity (TC50) of the test compounds were calculated by simple regression analysis.
Activity against rgRSV
Determination of antiviral activity against rgRSV was done as previously described (Ternette et al., 2007 ;
Lai et al., 2013). Briefly, HEp-2 cells at a 6000 cells/well population density were seeded onto a 96 well plate in 200 µl of DMEM-5 (5% FCS DMEM medium) per well and incubated overnight. Next day, extract were prepared as above and added to the well, followed by the virus at multiplicity of infection (
MOI of 0.01) and further incubated for 48 h at 37°C + 5% CO
2. Cell culture wells seeded with virus alone served as control. After 48 h incubation, fluorescence microscopy (green fluorescence) was employed to enumerate the number of viral plaque forming units (pfu) in drug treated and untreated HEp-2 cells. Percentage viral plaques reduction were then calculated, and IC
50 determined by simple regression analysis.
Activity against RSV A2 strain
RSV A2 inhibition was evaluated using the immunocytochemical viral plaque reduction technique (Esimone et al., 2008; Lai et al., 2013). Experimental set-up was described as above except that 0.03 MOI of virus (RSV A2) is employed. The numbers of plaque forming units (pfu) in drug treated and untreated HEp-2 cells following 48 h incubation were determined. Immunocytochemical labeling was achieved with a monoclonal antibody targetting the RSV-P protein (3C4) (Lai et al., 2008). After removal of the supernatant, cells were fixed for 10 min with 80% ethanol, allowed to dry in air before re-hydrating with PBS-T for 5 min followed by incubation with 1:250 dilution of 3C4 antibody in PBS-T for 60 min at 37°C. Next cells were incubated with a peroxidase conjugated rabbit anti-mouse IgG secondary antibody (Dako, Germany) (1:400 dilution in PBS-T) after a three times wash step. Finally, cells were incubated AEC substrate (10 ml phosphate citrate pH 5.0, 200 ml AEC and 10 ml hydrogen peroxide) which stains red in RSV localized regions. Enumeration of reddish-brown plaques is done microscopically. Each single value of the triplicates was expressed as percent of the mean of triplicates of control cultures (infected with same MOI of virus in the absence of the drugs) and the mean and standard error of the percent values was calculated for each triplicate.
Time course assay (Time-of-addition studies)
Time of addition assay is based on the determination of antiviral activity at different time points before and after viral inoculation of susceptible cells (Lai et al., 2013; Esimone et al., 2008). HEp-2 cells were incubated overnight in 96 well plates (6000 cells/well). AC and AF (10 µg/ml), respectively and Ribavirin (3 µg/ml) were added to the triplicate wells together with the virus (MOI of 0.01), respectively and incubated for 48 h at 37°C + 5% CO2. Control wells containing virus alone at the same MOI in DMEM-5 (containing 0.6% DMSO) but without drugs were equally set up. Additionally, triplicate wells were also inoculated with same MOI of virus and incubated for 2 h or 4 h at 37°C with 5% CO2. Following the 2 or 4 hr incubation period DMEM-5 medium containing extract (10 µg/ml) or Ribavirin (3 µg/ml) (positive control) or 0.5% DMSO in DMEM-5 (negative control). After 48 h incubation, the numbers of plaque forming units (pfu) in drug treated and untreated HEp-2 cells were determined by fluorescence microscopy where localized green cells harboring rgRSV were counted as viral plaques. Percentage viral plaques reduction relative to control were then calculated and established.
Protection from viral-induced cytopathic assay
HEp-2 cells were incubated overnight in 96 well plates (6000 cells/well) until cell cultures is confluent. Next culture medium was removed from the confluent monolayer cells and virus suspension containing virus at MOI of 3 together with 100 µl of DMEM-5 medium containing the appropriate concentrations of the test compounds were added. As the virus control, similar virus suspensions in medium without compounds were added. The plates were incubated at 37°C in a humidified CO2 atmosphere (5% CO2), for 5 to 6 days. After that, cytopathic effect (CPE) was observed. The virus induced CPE was determined by MTT assay as described above in cytotoxicity assay. The reduction of virus multiplication was calculated as % of virus control:
Percentage (%) CPE=(OD Mock – OD herb+virus / OD Mock – OD virus control) × 100
[where OD=Optical density]
The concentration reducing CPE by 50% in respect to virus control was estimated from graphic plots using the regression statistic, and was defined as 50% inhibited or protective concentration (EC50).
Statistical analysis
Antiviral activity was calculated based on the standard error of mean (±SEM) derived for percent values of triplicate experiments. All calculations and plots were performed using the GraphPad Prism software (Graphpad Software Inc., La Jolla, CA).
Activity against rgRSV
The Preliminary data showed anti-RSV activities with IC50 = 5.64 ± 1.16 and 75.62 ± 3.38 µg/ml (Linear regression r2= 0.9898 and 0.9070) for AC and AF, respectively when tested using the recombinant strain rgRSV strain (Table 1 and Figure 1).
Cytotoxicity screening
The Preliminary data for corresponding assays for the cytotoxic effect of extracts against utilized HEp-2 cell line recorded TC
50 = 103.14 ± 3.98 and 333.82 ± 6.32 µg/ml (Linear regression r
2= 0.9614 and 0.9104) for AC and AF,
respectively . Figure 2 showed the pattern of HEp-2 cells viability status in the presence of the extracts.
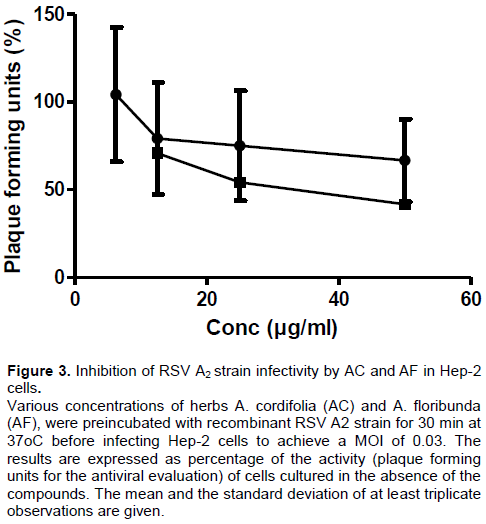
Activity against RSV A2 strain
As observed in Figure 3 the incubation of various concentrations of herbs AC and AF with the RSV A2 strain inhibition of viral infectivity outcome showed anti-RSV activities with IC50 = 69.12 ± 0.67 and 79.89 ± 1.20 µg/ml (Linear regression r2 = 0.6727 and 0.9276) for AC and AF, respectively.
Cell protective effect
AC and AF both demonstrated some cell protective effect from RSV-induced cytopathology in HEp-2 cells. Table 2 showed these outcomes with some demonstrable protection by AC and AF. The evaluation for herb-induced protection from viral-induced cell cytopathic effects assay was done. The results showed cell protection capacities with EC50 value of 51.76 ± 5.22 µg/ml for AF. A much better protective efficacy was recorded for AC at 4.63 ± 2.81 µg/ml.
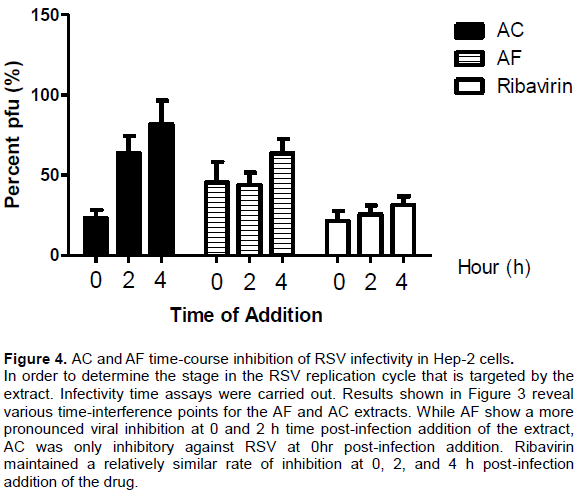
Time-of-addition mechanistic assay
From Figure 4, it could be seen that AC and AF inhibit RSV at different time points of the viral replication cycle.
Search for novel anti-RSV compounds with better therapeutic efficacy and safety margins than ribavirin is currently a hotbed of research focus in the RSV therapeutics field. This is largely predicated on well-known precedents that potent anti-viral agents have previously been harnessed from medicinal plants (Semple et al., 1998;
Kott et al., 1999; Sindambiwe et al., 1999 ). As a result of these ongoing efforts a plethora of potential anti-respiratory syncytial virus (anti-RSV) substances have been uncovered in various investigations; however some are too cytotoxic to develop as clinically useful agents (Baker et al., 1995; Golankiewicz et al., 1995; Odimegwu et al., 2011). Given the consistency in reports of anti-RSV substances with such recorded tight therapeutic window, the continued screening of existing medicinal plants for promise of therapeutically useful anti-RSV compounds remains strongly imperative. Therefore, taking cue from this background we embarked on reporting the outcome of our investigations of extracts of two useful medicinal plants as sources of potential anti-RSV compounds. Our investigations of the two plant extracts sourced from Nigeria in West Africa, AC and AF for anti-RSV activity resulted in promising antiviral profiling (Figures 1 and 2) against RSV. Their
aqueous methanolic extracts were studied to detect antiviral activity against RSV. Our interpretation is that extracts of both AC and AF have shown demonstrable antiviral activities against RSV following their recorded TC
50, IC
50 values and selective indices (SI) also displayed in Table 1. Derived IC
50 values of 5.64 and 75.62 µg/ml for AC and AF, respectively represent the inhibitory concentrations of both extracts effective in inhibiting growth of recombinant strain of RSV (rgRSV) expressing the green fluorescent protein by 50% in culture of Human Larynx Epidermoid cell (HEp-2 cells).
The rgRSV strain harbours the recombinant ribogreen protein which enables the enumeration of unfixed viable virus under microscopy.
Thus, in determining the 50% inhibition of RSV infectivity, only infected HEp-2 cells displaying fluorescence green when observed under the fluorescent Microscope would be enumerated When these determined inhibitory concentration values were matched with their corresponding assay results for the cytotoxic effect of the extracts against utilized cell lines TC
50 values (103.14 and 333.82 µg/ml, respectively) also shown in Table 1, their overall anti-RSV utility can therefore be better adequately envisioned. Recall that medicinal plants screening exercises for drug discovery of suitable anti-RSV candidates has persisted. And as a result of these ongoing efforts a plethora of potential anti-respiratory syncytial virus (anti-RSV) substances have been uncovered in various investigations albeit with several being too cytotoxic (Baker et al., 1995; Golankiewicz et al., 1995; Odimegwu et al., 2011). Thus, a grossly diminished cytotoxicity score represents a strong positive scorecard for any desirable antiviral anti-RSV substance, which in the case of AC and AF has shown promising values.
Another closer observation could show that with a lower IC
50 (5.64 µg/ml) AC show a preferentially remarkable tenfold antiviral effect against RSV in comparison to AF. This observation perhaps do not come as a surprise given that AC has previously been cited for utility in various capacities as anti-inflammatory, antifungal and
antibacterial agent (Noundou et al., 2016), immunomodulatory and anti-oxidant activities (Okoye et al., 2015), and could now perhaps for the first time be considered for provision of molecular targets against RSV. Arising from this, folkloric usage of AC is no strange phenomenon and may thus have covered some safety considerations gaining a longer stride towards systematic utility as treatment for RSV infection in the future. What may additionally be needed here is the pursuit of future investigations towards the possibility of further enlarging the selectivity index (SI) which is the ratio of cytotoxicity (TC
50) and antiviral activity (IC
50) (SI=TC
50/IC
50). This could be achievable with either a later further fractionation of the extract of AC and AF or other chemical modification procedures. In our bid to further validate these promising earlier results, we proceeded to evaluate the anti-RSV activity of both AC and AF by testing against another RSV A2 strain, which is the circulating clinical RSV strain among human population. Outcome of this screening against RSV A2 strain (Figure 3) slightly unlike the previous recorded elevated IC
50 values were nevertheless still in agreement with the promising antiviral utility of the extracts and their possible clinical utility in human population settings.
Since in the prognosis of RSV-induced disease, RSV-induced cell cytopathy represents a major arm of respiratory disease associated with RSV infection. Therefore, cell protection from the lethal effect of virus presence and propagation within the cellular compartment would ameliorate the disease impact and progression. Accordingly, the ability of any substance including medicinal extract to protect cell form the pathological effect of virus infection is desired and could be measured as a function of cell viability status following infection of the cells by virus inoculum (Wang et al., 2008). Thus, our evaluation for extract-induced protection from viral-induced cell cytopathic effects assay revealed that both AC and AF were capable of protecting viral-infected cells from the virus-induced cytopathy at EC50 values of 4.63 and 51.76 µg/ml, respectively. It is therefore deducible that apart from providing direct antiviral activity against RSV, these naturally derived agents may promote the profound recovery of RSV-infected cells from the pathological effects of the RSV infection. Morever, given the lower effective concentration (EC50) value (4.63 µg/ml) AC may better protect RSV-infected cells from the pathologic consequences of infection thereby promoting cell viability and recovery. In order to determine the stage in the RSV replication cycle that is targeted by the extracts, infectivity time assays were carried out. This infectivity time assay allows for the relatively clear observance of viral infectivity when the medicinal extracts are added post-infection to cell culture containing the virus (Lai et al., 2013). Results as shown in Figure 4 reveal various time interference points for the medicinal extract. While AC shows inhibition only at post-infection time of 0, AF shows interference at 0, 2, and 4 hrs post-infection. Considerable information can be inferred from this observation; it is possible that while the mechanism of anti-RSV activity of AC may be limited to only early phase events associated with interference with RSV structural proteins G (attachment protein), F (fusion protein), and SH (small hydrophobic protein), the mechanism of anti-RSV activity of AF appears to transcend early phase events going through late phase events associated with viral replication including possible interference with the viral nucleic acid. Thus AC may simply limit RSV infection of cells while AF appears to signify ability to modulate both infection and propagation of virus.
In the current study the anti-RSV activities of extracts from AC and AF have been evaluated for anti-RSV activities. The findings revealed the potential utility of both plant extracts as future sources of antiviral agents against the respiratory syncytial virus.
The authors have not declared any conflict of interests.
The DAAD doctoral scholarship award to DCO is gratefully appreciated