ABSTRACT
Adverse drug reaction (ADR) is a noxious and undesirable reaction to drugs at dosage normally used in humans for diagnosis, treatment or prophylaxis of diseases or ailments. Spontaneous reporting is currently the major back bone for the detection of adverse drug reactions. The objective of the study was to assess the knowledge, attitude, and practice of health professionals towards ADR reporting in Boru Meda Hospital, North East Ethiopia. A quantitative cross-sectional study design was used. A self-administered questionnaire was used to collect data from the health professionals, and the collected data was analyzed using SPSS (version 16). A test of association for selected variables was done using Pearson chi–square. From a total of 57 respondents, 40 (70.1%) were able to differentiate ADR from side effects. Thirty six (63.2%) and 34 (59.6%) respondents knew the availability of national reporting system and ADR reporting form in Ethiopia, respectively. Majority, 46 (80.7%), of the respondents said that ADR should be reported only when they are serious and life threatening. Out of 12 respondents who encountered ADR in the past 12 months in their clinical activities, 10 reported to responsible body. Health professionals working in Boru Meda Hospital have good attitude towards ADR reporting and good reporting culture of encountered ADRs, but insufficient knowledge about ADRs. The unavailability of ADR reporting forms take the lions part in significantly discouraging them to detect and report ADRs.
Key words: Adverse drug reactions, knowledge, attitude, practices, health professionals, pharmacovigilance, Boru Meda Hospital.
Nowadays drugs have changed the way in which diseases are treated. Despite all their advantages, adverse reactions to medicines are a common but preventable, cause of illness, disability and even death (WHO, 1972). An adverse drug reaction (ADR) is defined as noxious, unintended and which occurs at dosages normally used in human beings for prophylaxis, diagnosis or therapy of disease or for the modification of physiological function (Parker, 1983). Basically, ADRs are defined as type A, type B, type C and type D. Type A reaction (predictable) is related to dosage and is an extension to the normal pharmacology of the medication; type B reaction (unpredictable) is unrelated to normal pharmacology; type C reactions are associated with prolonged therapy; type D reactions are delayed reactions (Rawlings and Thompson,1977; Naranjo et al., 1981).
The history of pharmaco vigilance (ADRmonitoring) dates back as much as thirty years when the 20th world’s gathering adopted a mechanism to start a task on the possibility of international system of monitoring adverse reactions of drugs. This was based on Thalidomide disaster that caused death of thousands of children, creating the basis of the World Health Organization (WHO) program on worldwide system for drug monitoring. As a result international system for monitoring ADR was proposed (WHO, 2000, 2001).
ADRs have been regarded as a major public health problem since they result in a measurable percentage of hospital admissions and an economic burden (Lundkvist and Jonsson, 2004). Hence, ADRs reduce patients’ quality of life and impose a significant financial burden on the health care systems. The commitment of health care providers to ADRs databases is massively significant and has energized continuous ascertainment of the risk-benefit ratio of few drugs and in addition, added to signal detection of unsuspected and unusual ADRs (Wysowski and Swarlz, 2005). The part of healthcare providers is crucial in recording and reporting suspected ADRs in order to caution regulatory agencies about rising safety concerns and thereby calls for immediate and appropriate action. All health care providers ought to be urged to report all suspected adverse reactions resulting from medicines, especially when the reaction is not expected and potentially serious or clinically significant (WHO, 2006).
Once a drug is available to the public, making a determination about its safety is the shared responsibility of all healthcare providers (Zolezzi and Parsotam, 2005). Pharmacovigilance programs encourage ADR detection, enable ADR documentation, promote the reporting of ADRs and elucidate a mechanism for monitoring the safety of drug use in high risk patient population. A complete progressing ADR system ought to incorporate components for observing, distinguishing, assessing, recording, and reporting ADRs and in addition, mediating and giving instructive criticism to prescribers, other health care providers and patients (Kohn et al., 2000). So far, there is no study conducted in Boru Meda Hospital to assess ADR reporting. This study investigated the knowledge, attitudes and practices of healthcare professionals towards ADR reporting in Boru Meda Hospital.
A cross sectional study was conducted among the healthcare providers (Nurses, Doctors, Pharmacy Personnel, Midwifery and Health Officers) working at Boru Meda Hospital, North East Ethiopia from March 2014 to June 2014. The hospital is located 411 km North East of Addis Ababa, Ethiopia. It serves Dessie town and the surrounding population, which is about 2.1 million. The hospital has different units: internal medicine, pediatrics, gynecology/obstetrics, surgery, emergency, psychiatry, ophthalmology, hospital pharmacy, dermatology, orthopedics and multidrug resistant tuberculosis treatment unit. The dependent variables were knowledge, attitude and practice of ADR reporting whereas the independent variables were age, sex, profession, and years of experience. A structured questionnaire, adapted from other similar studies (Kamtane and Jayawardhin, 2015; Santosh et al., 2013) on knowledge, attitude, and practice of the health professionals on ADR reporting with a little modification to suit the hospital setting, was used to collect data. The questionnaire was distributed to the healthcare professionals who were willing to participate in the study. The completed questionnaires were collected from the participants within one week. Questionnaires included socio-demographic variables, variables used to measure knowledge, attitude and practice about ADR reporting. Knowledge about ADR reporting was determined using fourteen multiple choice questions. Participants who scored seven and above out of fourteen were considered to have adequate knowledge, otherwise they were considered as having inadequate knowledge. Attitude was evaluated utilizing twelve questions rated on a three point Likert scale. Based on the cumulated score, respondents who scored greater than or equal to 50% and less than 50% of the total were considered as having favorable attitude and unfavorable attitude, respectively. Regarding practice about ADR reporting, those who record and report at least one of the encountered ADRs are considered to have good practice while those who are unable to do this are regarded as having poor practice.
The collected data was checked for completeness, categorized and the results were analyzed using SPSS version 16, interpreted and presented using tables and charts. The chi-square test was used to find out the association between the outcome and independent variables and statistical significance was set at p<0.05.
Ethical clearance was obtained from College of Medicine and Health Sciences, Wollo University, and permission was sought from Boru Meda Hospital.
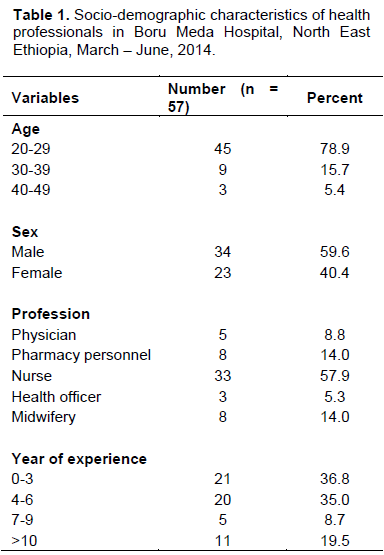
Demographics
Out of 62 questionnaires distributed, only 57 were filled and returned within the stipulated time frame giving a response rate of about 92%. From 57 health professionals, majority (57.9%) were nurses. Most of the participants 45 (78.9%) were in the age range of 20-29 years and 34 (59.6%) were males as shown in Table 1.
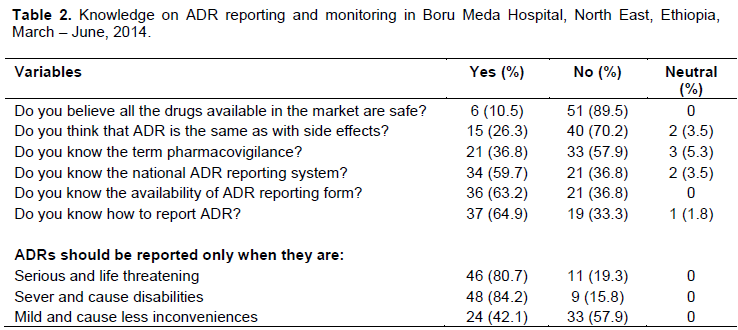
Knowledge
Forty (70.2%) of respondents could differentiate ADR from side effects. Only 21 (36.8%) respondents knew the term pharmacovigilance. Likewise, 36 (63.2%) and 34 (59.6%) respondents were aware of the availability of national reporting system and ADR reporting form in Ethiopia, respectively. Out of the total study participants, 46 (80.7%) and 48 (84.2%) health professionals said that ADRs should be reported only when they are life threatening and cause disability, respectively as shown in Table 2.
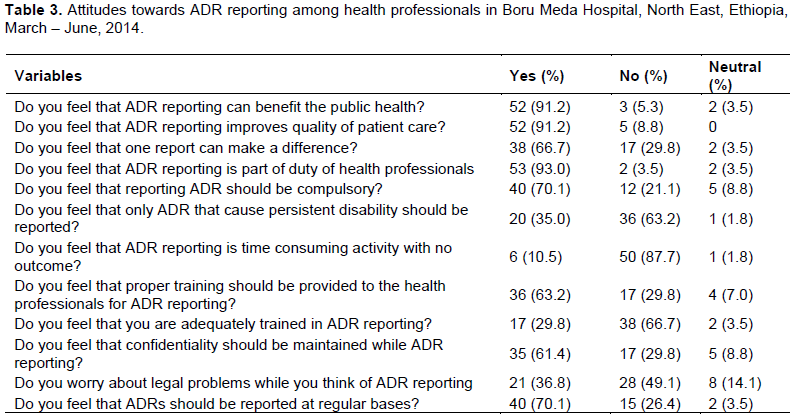
Attitudes
The study showed that 53 (93.0%) respondents established that ADR reporting should be part of their duty and 40 (70.1%) supported that ADR reporting should be mandatory. Six (10.5%) of health professionals believed that ADR reporting is time consuming activity with no outcome. The results also showed that, 38 (66.7%) health professionals agreed that they were not adequately trained in ADR reporting as shown in Table 3.
Practices
From these 57 study participants, only 12 (21.1%) met patients with ADR in their clinical practice in the past 12 months, among which 10 (83.3%) recorded the encountered ADR in patient follow up card and also reported to responsible body as shown in Table 4.
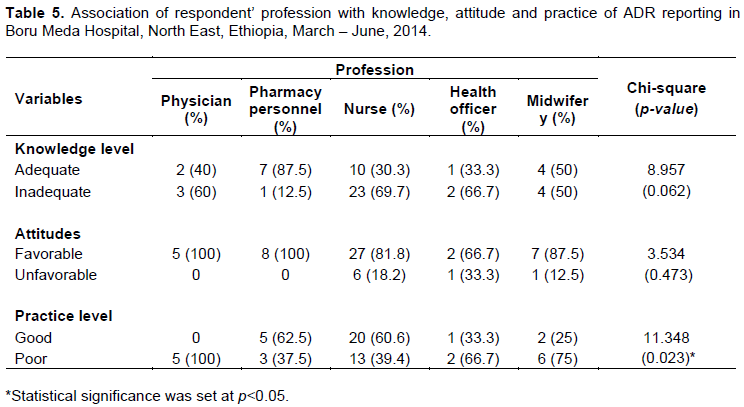
Association between the respondents’ profession and the outcome variables
From a total of 57 health care providers, 24 (42.1%) have adequate knowledge (more than 50% right response), the rest have inadequate knowledge (less than or equal to 50% right response) of the total 14 questions used to assess their knowledge as shown in Tables 2 and 5. With respect to overall level of attitude towards ADR reporting, the majority 49 (86.0%) have favorable attitude towards ADR reporting based on a three point Likert scale as shown in Tables 3 and 5. Table 5 also, shows the association between respondents’ profession and knowledge, attitude and practice of ADR reporting in the study area. There is a significant (X2 = 11.348; p = 0.023) association between respondents’ profession and their practice on ADR reporting. On the contrary, there is no significant association between respondents’ profession and knowledge and attitude towards ADR reporting.
Adverse drug reaction monitoring, is an area of pharmaceutical consideration which bargains principally with the recognition, management and reporting of ADRs which may result from drugs that are taken in normal dose for prophylaxis or treatment of diseases. These ADRs may vary from simple reactions to permanent disability and death (Lazarou et al., 1998; Wiffen et al., 2002). The knowledge, attitude and practice of health professionals on ADR reporting is closely associated with their professional roles and can alleviate problems associated with under reporting ADRs.
In the present study out of the total 57 respondents, 15 (26.3%) of them are unable to differentiate ADRs from side effects. This might be due to lack of sufficient information regarding ADR in the courses and/or trainings. However, WHO recommend that in order to avoid increasing the figures of drug induced problems; it is helpful to hold the term side effect for minor effects which are related to the pharmacological properties of the drug (Ernst and Grizzle, 2001). Among the 57 participants, 34 (59.7%) and 36 (63.2%) health care providers were aware of the availability of national ADR reporting system and reporting form in Ethiopia. But a similar study in Jimma Zone showed that 23.17% and 25.61% health professionals knew the availability of national ADR reporting system and reporting form in Ethiopia (Angamo and Wabe, 2012). Knowledge on the availability of ADR reporting system and reporting forms can foster the practice of ADR reporting and hence reduce the risk associated with ADRs.
Knowledge of the term pharmacovigilance and its roles is one of the components used to assess the overall knowledge of the study participants on ADR reporting. Accordingly, among the total of 57 respondents, only 21(36.8%) of the respondents (6 pharmacy personnels, 3 physicians, 10 nurses and 2 health officers) knew pharmacovigilance and its roles. According to a study done in Nigeria on attitude of doctors to ADR reporting showed that 40.4% of the respondent were aware of the existence of National Pharmacovigilance Center in their country (Rehabs and Vasudeuk, 2002; Kazeam and Jacob, 2009) and this shows that they have more awareness towards ADR monitoring than professionals in this study. On the contrary, a study in Jimma Zone (Angamo and Wabe, 2012) showed that 19.5% of the participants were aware of the term pharmacovigilance and its roles, showing that they have less awareness towards ADR monitoring than professionals in this study.
Attitudes are potentially modiï¬able variables exerting a strong influence on ADRs reporting, the greater the patient attitude the more positive influence on the overall ADRs reporting rate (Herdeiro et al., 2006). In this study, fifty three (93.0%) respondents felt that ADR reporting should be part of their duty and 40 (70.1%) supported that ADR reporting should be mandatory. On the
contrary, 17 (29.8%) did not believe that one report of ADR makes a difference. Besides, most respondents 52 (91.2%), agreed that reporting ADR is important for the public and improves quality of patient care respectively. Educational program can signiï¬cantly modify health professionals’ reporting-related attitudes and influence the ADRs reporting behavior in a positive manner.
Reporting the occurrence of ADRs is important to prevent morbidity and mortality associated with the specific drug that caused the adverse effect. From a total 57 respondents, only 12 (21.1%) met patients with ADRs and ten of them recorded and reported it to the concerned body. A study done in Turkish showed that 65% of the health care providers met patients with ADRs and 7% of them reported ADR to their National Pharmacovigilance Center (Toklu and Uysal, 2008). ADR detection and reporting requires an appropriate knowledge regarding the outcome of ADRs. So there should be awareness raising programmes and trainings regarding ADRs in order to encourage health professionals to detect and report ADRs.
The overall knowledge level of health professionals in this study is below the average, and hence, this might affect their experience in recording and reporting of ADR, despite their higher level of perceptions towards ADR reporting. It is important to note the lack of association between respondents profession with the knowledge and attitude level in this study. Not with standing, one needs to look into consideration several confounding factors that affect the validity of such comparisons, such as the use of different instrument to measure the outcome variable.
Limitations
The self-reporting nature of the study depends on the exactness and trustworthiness of the respondents. So results may stray from what really happens in practice. The small sample size may make it hard to extrapolate conclusions from this study; however, it can provide an indication of perspectives and experiences of these groups of health care providers.
This study showed that health professionals working in Boru Meda Hospital have favorable attitude about ADR reporting, and there is a good reporting culture of encountered ADRs, but insufficient knowledge about ADRs and unavailability of ADR reporting forms take the lions part in significantly discouraging them to detect and report ADRs. However, encouraging all health professionals to report and also provide trainings that would significantly improve ADR reporting. Our study emphatically proposes that there is an awesome need to make awareness and to advance the reporting of ADRs amongst health care providers, which will establish a strong framework for health care providers to be steadily included in quality pharmacovigilance and unconstrained reporting in their future practices.
The authors have not declared any conflict of interests.
REFERENCES
|
Angamo MT, Wabe NT (2012). Knowledge, Attitude and Practice of Adverse Drug Reaction reporting among health professionals in South West Ethiopia. TAF. Prev. Med. Bull. 11(4):397-406
Crossref
|
|
|
|
Ernst FR, Grizzle AJ (2001). Drug related Morbidity and Mortality: Updating the cost of illness Model. J. Am. Pharm. Assoc. (Wash). 41(2):192-199.
Crossref
|
|
|
|
|
Herdeiro MT, Figueiras A, Polonia J, Gestal-Otero JJ (2006). Influence of pharmacists' attitudes on adverse drug reaction reporting: a case-control study in Portugal. Drug Saf. 29(4):331-340.
Crossref
|
|
|
|
|
Kamtane RA, Jayawardhani V (2012). Knowledge, attitude and perception of physicians towards adverse drug reaction (ADR) reporting: A pharmacoepidemiological study. Asian J. Pharm. Clin. Res. 5(3):210-214.
|
|
|
|
|
Kazeam A, Jacob O (2009). Perceptions of Doctors to Adverse Drug Reaction reporting in a teaching Hospital in Lagos, Nigeria. BMC Clin. Pharmacol. 9:14.
Crossref
|
|
|
|
|
Kohn LT, Corrigan JM, Donaldson MS (2000). To err is human: building a safer health system (Vol. 6). National Academies Press.
|
|
|
|
|
Lazarou J, Pomeranz BH, Corey PN (1998). Incidence of Adverse Drug Events in Hospitalized Patients: A Meta-analysis of prospective studies. JAMA 279(15):1200-1205.
Crossref
|
|
|
|
|
Lundkvist J, Jönsson B. (2004). Pharmacoeconomics of adverse drug reactions. Fundam. Clin. Pharmacol. 18(3):275-280.
Crossref
|
|
|
|
|
Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ (1981). A method for estimating probability of adverse drug reactions. Clin. Pharmacol. Ther. 30(2):239-245.
Crossref
|
|
|
|
|
Parker CW (1983). Allergic reaction in man. Pharmacol. Rev. 34:85-194.
|
|
|
|
|
Rawlings MD, Thompson JP (1977). Pathogenesis of adverse drug reactions. Textbook of adverse drug reactions. Oxford University Press, Oxford. 44p.
|
|
|
|
|
Rehans H, Vasudeuk T (2002). Knowledge, attitude and practices of medical students and prescribers on adverse drug reaction monitoring. Natl. Med. J. India 15(1):24-26.
|
|
|
|
|
Santosh KC, Pramote T, Sarun G, Ralph E (2013). Attitudes among health care professionals to the reporting of adverse drug reaction in Nepal. BMC Pharmacol. Toxicol.14:16.
Crossref
|
|
|
|
|
Toklu HZ, Uysal MK (2008). Knowledge and Attitude of Turkish community pharmacists towards Pharmacovigilance in the Kadikoy district of Istanbul. Pharm World Sci. 30(5):556-562.
Crossref
|
|
|
|
|
Wiffen P, Gill M, Edwards J, Moore A (2002). Adverse drug reactions in hospital patients: a systematic review of the prospective and retrospective studies. Bandolier Extra 101(4):1-15.
|
|
|
|
|
World Health Organization (1972). International drug monitoring: The role of national centers. WHO technical report. Geneva: Switzerland.
View
|
|
|
|
|
World Health Organization (2000). Safety monitoring of medicinal products: guidelines for setting up and running a pharmacovigilance centre. Uppsala: UppsalaMonitoring Centre.
View
|
|
|
|
|
World Health Organization (2001). A Guide line to detecting and reporting adverse drug reaction. WHO report Geneva: Switzerland.
|
|
|
|
|
World Health Organization (2006). The Safety of Medicines in Public Health Programmes: Pharmacovigilance and essential tool. Geneva: Switzerland.
View
|
|
|
|
|
Wysowski DK, Swartz L. (2005). Adverse drug event surveillance and drug withdrawls in United states, 1969-2002. Importance of reporting suspected reactions. Arch. Intern. Med. 165(12):1363-1369.
Crossref
|
|
|
|
|
Zolezzi M, Parsotam N (2005). Adverse drug reporting in New Zeeland: Implications for Pharmacists. Ther. Clin. Risk. Manage. 1(3):181-188
|
|