ABSTRACT
Indian Pharmaceutical Industry is the third largest in the world in terms of volume and this industry has a business of about USD 30 billion annually. Availability of quality medical products, especially drugs is important from the perspective of health of the consumers. The manufacture and sale of drugs in India is a licensed activity under Drugs and Cosmetics Act, 1940 and the Drugs and Cosmetics Rules, 1945. The use of ineffective, poor quality, harmful medicines can result in therapeutic failure, exacerbation of disease, resistance to medicines and sometimes death. Proper and effective enforcement of the Act is mandatory for curbing the sale, distribution and consumption of spurious which is not of standard quality medicines in any country, there by safeguarding the public health. In this paper, we report a post market quality survey of 3925 drug samples over a period of 30 months. Drugs Control Laboratory, Vijayawada, India during the said period (Jan, 2015 to June, 2017) 3925 samples were analyzed. It was found that, in the year 2015, 2016 and 2017 (up to June) the percentage of NSQ drugs was 5.70, 1.92 and 2.27%, respectively. The effective enforcement of Drugs and Cosmetics Act, 1940 is necessary at the field level to ensure that, safe medicines are made available to general public by and large.
Key words: Public health, spurious drug, not of standard quality drug, D & C Act 1940, Andhra Pradesh.
The Indian Pharmaceutical Industry is presently the third largest in the world (volume wise). In terms of value, the size of the industry is approximately Rs. 2,00,000 Crores (USD 30 billion), out of which more than half is exported to different countries across the globe. The Indian Pharmaceutical sector has shown an exponential growth over the last 10 years. The growth of the sector over a short period of time has thrown up challenges for, up scaling the regulatory structure in the country. Availability of quality medical products, especially drugs is important from the perspective of health of the consumers. NSQ drugs may lead to not only sub-therapeutic dosage, but also give rise to anti-microbial resistance which is a major health concern. With such a large domestic pharmaceutical market, circulation of Spurious and Not of Standard Quality Drugs (NSQ) can lead to grave and adverse consequences for both consumers and Drug manufacturers. Spurious and NSQ Drugs is a patient safety issue as it can challenge quality of treatment, lead to emergence of drug resistance,spread of diseases and cause economic burden on the society. It is a shared responsibility of all stakeholders to tackle the challenges of Spurious and NSQ Drugs. The manufacture and sale of drugs in India is a licensed activity under Drugs and Cosmetics Act, 1940 and Drugs and Cosmetics Rules, 1945. The licensees are required to comply with the provisions of the Act, Rules and the condition of the license granted to them by the licensing authorities for manufacture and sale of drugs.
Drugs and Cosmetics Act, 1940 have elaborate provisions to check the production of spurious and substandard drugs in the country. The Act provides elaborate definitions of the terms spurious, adulterated and misbranded drugs for the purpose of taking penal actions against the offenders. The use of ineffective, poor quality, harmful medicines can result in therapeutic failure, exacerbation of disease, resistance to medicines and sometimes death. It also undermines confidence in health systems, health professionals, pharmaceutical manufacturers and distributors. Medicine are lifesaving entities and thus more essential for the treatment which account for 20 to 60% of care cost while 50 to 90% of this cost is being paid by the patient, particularly in low and middle income countries (WHO, 2004). India is a developing country where more than 40% of the population survives on less than US $1 a day (Bate et al., 2011) and if a patient needs medicines he has to pay more than half of this. In India, as per Drug and Cosmetic (D and C) act, 1940, under section 17, 17A and 17B poor quality drug comprises of misbranded, spurious and adulterated drugs, respectively (Government of India, 2005). This article briefly present a glimpse of spurious and not of standard quality drugs in the state of Andhra Pradesh, India over a period of 30 months (from January, 2015 to June, 2017).
Collection of samples
Under section 22.1.b (i) and (ii) of the Drugs and Cosmetics Act, 1940 (D and C Act) of India, about 3925 drug samples falling into 23 therapeutic categories were lifted and sent by 59 Drugs Inspectors of Andhra Pradesh state received in Drugs Control Laboratory (DCL), Vijayawada, India.
Period of sampling
The period of sampling in this context is January, 2015 to June, 2017.
Analysis of drugs and cosmetics
The samples received in DCL were assigned a unique number and analyzed as per the established monographs in standard pharmacopoeias like IP, BP, USP etc. In case of non-pharmacopeial patent and proprietary formulations, Standard test procedures were adopted for the analysis of such samples.
Reporting of analysis result
After the completion of the analysis of drug samples, the report was generated in Form-13 as prescribed in D and C Act, 1940 and opinion was declared as Standard Quality (SQ) or not of Standard Quality (NSQ) sample by the Government Analyst as per the powers delegated to him under section 20 of the D and C Act, 1940 and Rules under, 1945.
Received samples
During the sampling period from January, 2015 to June, 2017 drugs and cosmetics samples in 3925 numbers were analyzed in DCL, Vijayawada belonging to 23 different therapeutic categories as part of enforcement of Drugs and Cosmetics Act in the state of Andhra Pradesh in post market survey (Table 1). Among the 3925 samples, highest number of samples was from anti-microbial group (1193 samples) which included anti-bacterials, anti-fungals, anti-virals, anti-helminths and anti-protozoals etc, following highest number of samples received belonged to the therapeutic category of analgesics which is 656 in number. Drugs acting on gastro-intestinal and respiratory tract were also high in number with 414 and 343 samples, respectively. Lowest samples belonged to Antivirals class which was only 07 for a period of 30 months.
Analysis of received samples
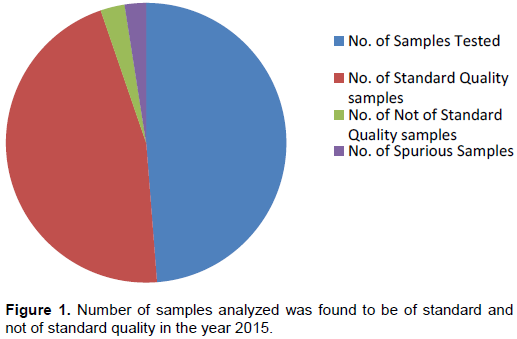
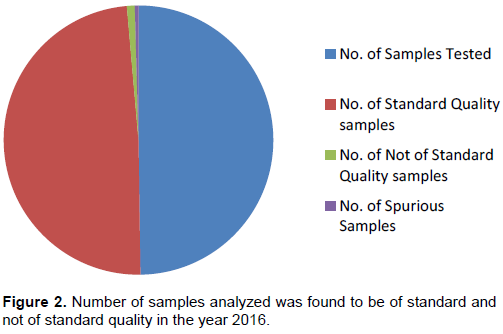
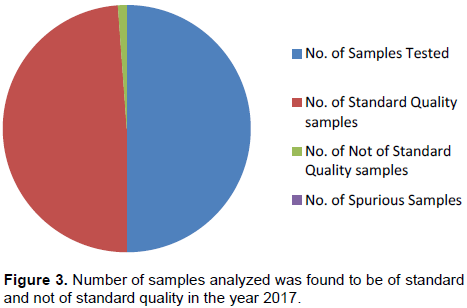
During the period of 30 months the analysis of received samples revealed that in the year 2015, out of 1524 samples analyzed 1437 samples were found to be of standard quality while 87 samples were not. Also, out of 87 NSQs reported, 77 samples were demarcated as ‘Spurious’ due to the absence of active pharmaceutical ingredient. The percentage of NSQs in the year 2015 was 5.70% (Figure 1).Totally, 1483 samples were analyzed in 2016 out of which 1455 were SQ samples and 28 were NSQ samples. Among 28 NSQs, 13 were identified as ‘Spurious’. In the calendar year 2016 the percentage of NSQs was 1.92% (Figure 2). Similarly, in the year 2017 from January to June, about 925 samples were received and analyzed and 904 samples were marked as SQ samples while 21 were found to be NSQ samples. No spurious samples were identified in the same year and the percentage of NSQs was found to be 2.27% (Figure 3).
Test parameters Vs NSQs
It was found that, of the total NSQs in the year 2015 and 2016, highest NSQs were with reference to failing in Identification test for active ingredients which were 77 and 14 in number, respectively. In these two years, samples which failed in dissolution test were the lowest with only two samples each year (Table 2). In the year 2017, interestingly highest number of samples failed in dissolution test is 13 and lowest number of samples failed in uniformity of weight was only 2 in a span of six months (Table 3).
Poor quality drug or substandard product encounters a major stringent issue for the global health system (Bate et al., 2011) which cannot be ignored. In most streamlined regions of the globe like Japan, Canada, Australia, New Zealand, the United States of America and most of the European Union, hardly 1% of the market value products are counterfeit, developing countries like Africa, Latin America and many parts of Asia may markedly be the seller and producer of spurious/falsely-labeled/falsified/counterfeit (SFFC) drugs medicines (WHO Report, 2012). In the current study, it was found that over a period of 30 months the percentage of NSQs ranged between 1.92 to 5.70%. In the year 2015 out of 1524 samples analyzed, 1437 samples were declared as of standard quality and 87 samples were not. The reason for such high percentage of NSQs in the year 2015 could be due to declaring 77 samples as NSQs due to their spurious nature (Figure 1), wherein active pharmaceutical ingredient was completely absent. In the year 2016, the number of NSQs declared was restricted to only 28, of which 14 were found to be spurious, which failed to meet identification test of labeled drug or had zero active ingredient. The overall percentage of spurious drugs was about 2.3% for a period of 30 months (Figures 1 and 2). The high percentage of encountering spurious drugs in the years 2015 and 2016, belonged to only one manufacturer namely, M/s Avya Health Care, with its fake manufacturing unit in Himachal Pradesh state. The spurious drug samples declared as NSQ belonged to therapeutic categories which consists of Antibiotics, Analgesics, Anti-emetics, Anti-cough and cold, Anti-protozoal, Iron and Vitamin and Anti-ulcers in the form of tablets, capsules and syrups/suspensions. Substandard drugs receive a lot less attention than spurious drugs. According to official data, substandard drugs outnumber spurious drugs amongst poor quality drugs (Maulik et al., 2015).
In the present study it was found that, 88.5% of samples in the year 2015 were NSQs with respect to Identification test (spurious samples) and the other tests accounted for 11.5% of NSQs. Majority of the samples tested in the year 2015 were NSQs with respect to one category of drug i.e. anti-microbials and hence spurious. The spurious nature of antibiotics could result in increased anti-microbial resistance, treatment failure and side effects (Okeke et al., 1999; Syhakhang et al., 2004) whereas, in the year 2016 spurious samples accounted for 46.4% of total NSQs. In contrary, in the year 2017, highest number of NSQs was declared with respect to dissolution test which accounted for about 61.9% of total 26 NSQs and next were in assay for active ingredients with 38.09%. A similar trend of NSQs was reported with highest number of samples failing in dissolution and assay in a recent world’s largest drugs quality survey conducted by National Institute of Biologicals, India (NIB Drug Survey, 2014 to 2016). The reason for such higher number of samples failing in dissolution test could be due to the insoluble nature of binding matrix or changes in formulation constitution etc which does not permit to release the drug from dosage form in stipulated time and thereby poor pharmacological action. Another reason for failure in dissolution test of tablets could be due to storage conditions particularly in tropical country like India (Johnston and Holt, 2013). In a nutshell, it is estimated that, the post-market survey (for a period of 30 months) under the Drugs and Cosmetics Act, 1940 in the state of Andhra Pradesh revealed that, the percentage which is not of standard quality drugs in the state of Andhra Pradesh on an average, was 3.95%. This percentage is fairly higher than the national average of 3.16% during 2014 to 2016. It is further understood that, manufacturers have to follow strict cGMP guidelines to see that there is a reduction in number of NSQs which can take care of the supply chain management for better receipt of drugs to the consumers in a form it intended to be. Government drugs procurement agencies must also check the medicines procured by them for meeting the standards. Drug regulation through stringent law enforcement in state and central regulators is also an effective step to curb the movement of spurious and sub-standard drugs in the market.
In the absence of effective medicines regulation, increased globalization of the pharmaceutical trade can lead to the proliferation of harmful, ineffective, substandard and counterfeit medicines on national and international markets. With the current study it is evident that, the movement and market of spurious and NSQ drugs is near to national average in the state of Andhra Pradesh. Substandard/counterfeit antimicrobial drugs represent an expanding problem throughout developing countries with considerable consequences for global public health. Well-designed studies are needed to determine the magnitude of this problem. A substandard concentration of the active ingredient was the main reason for low quality, which can lead to increased morbidity and mortality and emergence of antimicrobial resistance.Further, in order to curb the movement of such sub-standard medicines further effective enforcement is required at the field level to ensure that, safe medicines are made available to general public by and large in the interest of the state and nation.
The authors have not declared any conflict of interests.
REFERENCES
|
Bate R, Zhe Jin G, Mathur A (2011). Does price reveal poor-quality drugs? Evidence from 17 countries. J. Health Econ. 30(6):1150-1163.
Crossref
|
|
|
|
Government of India (2005). The Drugs and Cosmetics Act and Rules (Amendment). Ministry of Health and Family Welfare. Available at:
View
|
|
|
|
|
Johnston A, Holt DW (2013). Substandard drugs: A potential crisis for public health. Br. J. Clin. Pharmacol. 78(2):218-243.
Crossref
|
|
|
|
|
Maulik C, Mongia R, Wattal V (2015). Drug Regulatory Reforms in India. (Drugs Quality and Safety Issues in India). Policy Brief#2. Available at:
View
|
|
|
|
|
NIB Drug Survey (2014-2016). Report.
View. Accessed on 25.10.2017.
|
|
|
|
|
Okeke IN, Lamikanra A, Edelman R (1999). Socioeconomic and behavioral factors leading to acquired bacterial resistance to antibiotics in developing countries. Emerg. Infect. Dis. 5:18-27.
Crossref
|
|
|
|
|
Syhakhang L, Lundborg CS, Lindgren B, Tomson G (2004). The quality of drugs in private pharmacies in Lao PDR: a repeat study in 1997 and 1999. Pharm. World Sci. 26:333–338.
Crossref
|
|
|
|
|
World Health Organization (WHO) (2004). The World Medicines Situation.
|
|
|
|
|
World Health Organization (WHO) Medicines (2012). Spurious/falsely-labeled/falsified/counterfeit (SFFC) medicines. Geneva. Available at:
View. Accessed on 05.10.2017.
|
|