ABSTRACT
Increase in prevalence of resistant microorganisms especially to synthetic drugs, has necessitated the need to search for new bioactive compounds having natural origin. Phytochemical investigation of Olea africana extracts afforded two triterpenoids namely erythrodiol and uvaol which were obtained through repeated column chromatography. The compounds were characterized using Nuclear Magnetic Resonance (NMR) spectroscopy and by comparison with literature values. The isolated triterpenoids exhibited moderate antibacterial activity whereas crude extracts exhibited relatively high antibacterial activity against Gram positive bacterial strains; methanol showed 12.4 mm zone of inhibition against Staphylococcus aureus, Erythrodiol exhibited higher antibacterial activity than uvaol against Gram positive bacteria S. aureus with zones of inhibition of 5.2 mm and 5.0 mm respectively. None of the pure compounds showed significant activity against Gram negative bacteria Escherichia coli. The results give a scientific validity and credence to the ethno-medicinal use of this medicinal plant as a chewing stick.
Key words: Olea africana, triterpenoids, antibacterial activity.
Abbreviation:
ATCC, American type culture collection; BCC, Belgian coordinated collection microorganisms; COSY, correlation spectroscopy; DCM, dichloromethane; HMBC, heteronuclear multiple-bond correlation spectroscopy; HSQC, Heteronuclear single quantum correlation spectroscopy; MH, Mueller Hinton; NMR, nuclear magnetic resonance; NOESY, nuclear overhauser effect spectroscopy; TLC, thin layer chromatograph; WHO, World Health Organization.
Medicinal plants have been identified and used throughout human history because they have the ability to synthesize a wide variety of chemical compounds (Bulunas et al., 2005). These compounds are used to perform important biological functions, and to defend the medicinal plants against attack from predators such as insects, fungus and herbivorous mammals (Fabricant et al., 2001). At least 12,000 such compounds have been isolated so far, a number estimated to be less than 10% of the total (Tapsell et al., 2006). According to WHO report, 80% of the world’s population, mostly from developing countries still rely on traditional medicine for the treatment of common ailments (Shagal et al., 2012).
Oral diseases, like most diseases, are unevenly distributed, with the greatest burden falling on needy and poor populations (Ogunbodede et al., 2015). Thus, lower socioeconomic status populations have higher rates of caries and periodontal diseases than their higher SES counterparts (Chidzonga et al., 2015). Elsewhere, there are also disparities in oral health between those from rural and urban areas of Africa (Ogunbodede et al., 2015). According to World Health Organization (2008),the main determinants of health in general are the social, economic, and environmental conditions. These factors influence the oral health of individuals and populations. Therefore proper oral hygiene for all implies use of cheaper and readily available alternatives.
Dental carries and periodontal diseases are among the most pursued global oral health problems. They are caused by plaque forming bacteria such as Myces, Actinobacillus, Streptococcus and Candida species which reside in the oral cavity (More et al., 2008). These bacteria have been reported to be unsusceptibility to antimicrobial agents compared with cultures grown in suspension (Sonkos and Gondson, 2011). It is therefore not surprising that bacteria growing in dental plaque, a natural occurring biofilm show increased resistance to antimicrobial agents.
They generate acids that eat away at a tooth or form dental plaque or biofilm. Due to the structure of these biofilms, physical removal is the most effective means of control. Regular and thorough removal of food deposits from teeth also plays a vital role in reducing dental and periodontal diseases especially if accompanied by the use of chewing sticks.
Chemical investigations on chewing sticks and other medicinal plants reported to have medicinal properties has also generated numerous purified compounds which have proven to be essential in the practice of modern medicine (Ncube et al., 2012). The use of teeth cleaning agents like tooth brush and tooth pastes or chewing sticks varies from country to country, from urban to rural areas and culture to culture. Its use has been documented in parts of Asia, Africa, the Middle East and South America (Verenne et al., 2006). Chewing stick has remained a common and acceptable teeth cleaning agent in different parts of the world especially in developing countries despite the widespread use of tooth brushes and tooth pastes (Al-Otaibi et al., 2004). Its regular use is based on factors such as availability, cost, therapeutic and social-cultural reasons.
Religious reasons have also contributed to the increasing popularity in the use of this form of teeth cleaning. It is often mentioned that the Islamic Prophet Mohammed recommended its use as he is quoted in various Hadith advising the use of siwak (Salvadora persica) (Almas, 2001; Hyson, 2003). Olive plant (Olea europea), Lime tree (Citrus aurantafolia), Neem (Azadirachta indica) and Orange tree (Citrus sineusia) are also used as a chewing stick.
In response to emerging trends of bacterial resistance to antibiotics there is continuous and urgent need to discover new antimicrobial compounds from natural products like chewing sticks with diverse chemical structures and novel mechanisms of the new emerging oral infectious diseases. The current research focused on the medicinal plant Olea africana that is used traditionally by local inhabitants of Kabianga in Kericho, Kenya, as a chewing stick, so as to isolate and test the antibacterial activity of the compounds extracted.
General experimental procedure
The samples were prepared for analysis by dissolving in 2 mL DCM in 5 mm NMR tubes. NMR spectra was recorded at room temperature on a 400 MHz variant UNITY INOVA spectrometer. 1H NMR spectra was referenced against the CHCl3 signal at δH 7.24 and 13C NMR spectra against the corresponding signal at δC 77.0. Coupling constants were given in Hz. solvent against an air background.
Plant material
The leaves and twigs of O. africana were collected in January, 2015 from University of Kabianga Botanical garden, Kericho County in Kenya. The plant was identified by a taxonomist, Mr. D. Maritim, University of Kabianga, Kenya and a voucher specimen (kemboi 01) was deposited in the Herbarium, University of Kabianga, Kericho County, Kenya.
Extraction and isolation
The air dried and ground plant materials of O. africana (1000 g leaves and 1000 g twigs) was sequentially extracted with organic solvents in the order of increasing polarities including; hexane, dichloromethane, ethyl acetate and methanol, using cold extraction for 24 h in each case. The extracts were then concentrated under a reduced pressure to a minimum volume using a Rotavapor. The yields obtained were hexane 30.0 g (leaves), 23.0 g (twigs); dichloromethane 24.1 g (leaves), 15.3 g (twigs); ethyl acetate 6.24 g (leaves), 5.22 g (twigs) and methanol 5.35 g (leaves), 4.35 g (twigs).
Phytochemical analysis
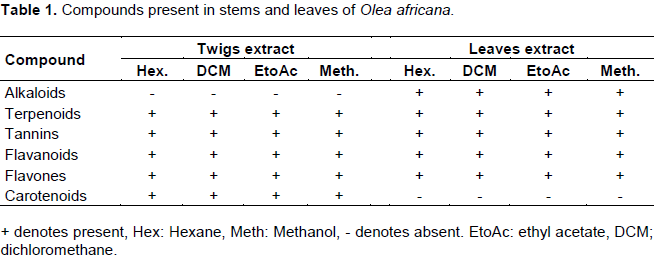
Qualitative tests for terpenoids, tannins, flavanoids, flavones, carotenoids and alkaloids was carried out by standard method as described by Edeoga et al. (2005). The tests were based on the visual observations of color change or formation of a precipitate after addition of specific reagent.
Isolation and purification of compounds 1 and 2
The DCM extracts of the leaves was separated by column chromatography using a step gradient of hexane: dichloromethane: ethyl acetate, starting with 100 % hexane stepped to 10, 20, 30, 50, 80 and 100% dichloromethane, followed by 20 and 30% ethyl acetate in dichloromethane. Twenty fractions of 100 mL each were collected in each step. Fractions 1 to 19 were combined and purified using 80% dichloromethane to produce erythrodiol (18 mg), fractions 34 to 36 were combined and separated with 20 and 30% ethyl acetate in DCM to afford Uvaol (12 mg).
Erythrodiol (1)
White amorphous solid, The 1H-NMR spectral data δH 5.17 (H-12), δH 3.54 (H-3), δH 3.20 (H-28), δH 1.22, 1.13, 0.96, 0.91, 0.89, 0.85, 0.76 (3H each). The 13C-NMR spectral data; δC 144.2 (C-13), δC 122.4 (C-12), δC 79.02 (C-3) and δC 69.65 (C-28).
Uvaol (2)
White amorphous solid, The 1H-NMR spectral data δH 5.17 (H-12),332 δH 3.50 (H-3), δH 3.14 (H-28), δH 1.35, 1.34, 1.34, 1.31, 1.32, 1.07, (3H each). The 13C-NMR spectral data; δC 138.7 (C-13), δC 122.3 (C-12), δC 79.0 (C-3) and δC 69.7 (C-28).
Biological studies
Antibacterial activities of the extracts were determined by micro-broth dilution assay as described by Buwa and Staden (2006). The four preserved strains of bacteria used were Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 87853, Staphylococcus aureus ATCC 25923 and Bacillus subtilis BCCM 1735. The bacterial strains were cultured for 18 h at 37°C and were standardized to a cell density of 1.5×108 cfu mgL-1 equivalent to 0.5 McFarland Standard. About 10 mL of standardized bacterial strains (E. coli ATCC 25922, B. subtilis BCCM 1735, P. aeruginosa ATCC 87853, and S. aureus ATCC 25923) were seeded in petri-plates containing MH agar by streaking entire MH media surface using sterilized cell spreader under luminous flame. The mixture was then allowed to dry for five minutes. This was followed by the application of plant extract discs which were placed over the numbered divided parts on culture media. Two-fold serial dilutions were used. Concentration was made in range of 1.0 to 0.3 mgL-1. Plates were covered to avoid contamination and were incubated for 24 h at 37°C. DMSO impregnated discs were used as the negative control. Zones of inhibitions of growth were measured in millimeters. The minimum inhibitory concentration (MIC) was described as the lowest concentration of the test compounds that completely inhibited the growth of microorganisms.
Phytochemical tests of the extracts revealed the presence of alkaloids, terpenoids, tannins and flavones (Table 1). Alkaloids were absent in all the twigs extract while carotenoids were absent in all leaves extracts, this was the main difference in the phytochemical profile of the twigs and leaves. Other researches have shown that quinolone group of alkaloids, cinchonine and cinchonidine were found to be present in the plant leaves of O. europea (Hansen et al., 2006) while studies on stems showed presence of tannins, alkaloids and flavones (Geissmann et al., 2005).
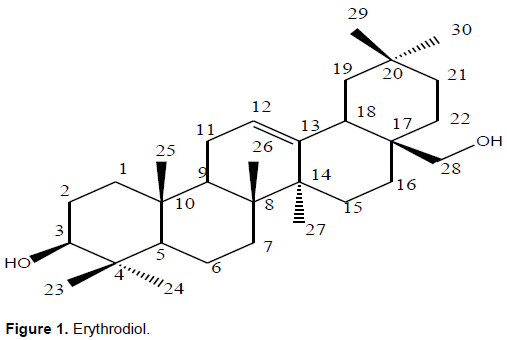
Phytochemical investigation of O. africana led to the isolation of two triterpenoids 1 and 2 which belongs to classes of oleanane and ursane respectively. Compound 1 (Figure 1) was obtained from DCM extracts as a white amorphous solid. The 1H-NMR spectrum compound showed characteristic peaks of a pentacyclic triterpenoid by correlation. Single olefinic proton signal (a triplet) was observed at δH 5.17 and was assigned to H-12, it also revealed two oxygenated protons resonating at δH 3.54 and δH 3.20. They were assigned to H-3 and H-28 respectively indicating the presence of hydroxyl moiety. Also present were seven methyl peaks at δH 1.22, 1.13, 0.96, 0.91, 0.89, 0.85 and 0.76 each 3H. The 13C-NMR spectra of compound exhibited the presence of 30 carbon signals (Table 2). The DEPT spectrum shows 7 methyl carbons, 11 Methylene, 5 Methine and 7 Quartenary carbon. The Sp2 signals at δC 144.2 and 122.4 were attributed to C-13 and C-12, respectively; characteristic of oleanane triterpenoids. Also present were two oxygenated carbon signals at δC 79.02 (C-3) and δC 69.65 (C-28) which correlated to the signals at δH 3.54 and δH 3.20 in the HSQC spectrum. The HMBC spectrum showed correlation of H-23 and H-24 with C-3, H-23 and H-24 with C-5, H-25 with C-9, H-29 with C-19, H-27 with C-8, C-14, H-11 with C-13, H- 18 with C-12 and C-15.The NOESY spectrum exhibited correlations of H-3 and H-28, indicating that they are both in the alpha position. Based on these spectral data, the compound was identified as a triterpenoid. The structure was proposed by comparison of data with those found in literature and was identified as the known erythrodiol (Marizeth et al., 2002). Erythrodiol has also been isolated from O. europea (Hansen et al., 2006).
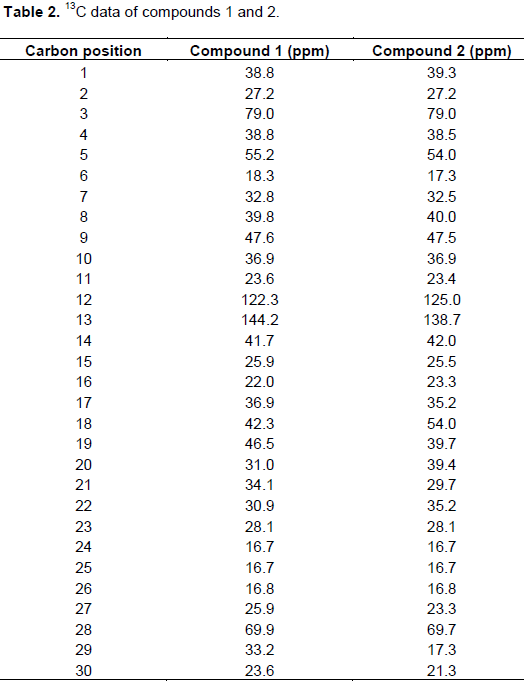
Compound 2 (Figure 2) was a white amorphous powder from dichlomethane extracts. The 1H NMR spectrum showed the presence of a single olefinic proton signal (a triplet) at δH 5.11 and was assigned to H-12. This proton correlated to methine carbon signal at δC 122.3 in the HSQC spectra The 1H NMR also revealed two oxygenated protons resonating at δH 3.50 and δH 3.14. They were assigned to H-3 and H-28 respectively indicating the presence of 3β-hydroxyl moiety. Methyl peaks were at δH 1.35, 1.34, 1.34, 1.32, 1.31, 1.31 and 1.07 each 3H. The 13C-NMR spectrum of compound 2 (Table 2) exhibited the presence of 30 carbon signals, the DEPT spectrum showed the presence of 6 methyl
.png)
carbons, 7 methine, 10 methylene and 7 quartenary carbon. The sp2 signals at δC 138.7 and δC 122.3 in the 13C NMR spectrum were attributed to C-13 and C-12 respectively, characteristic of ursane triterpenoids. Also present in the 13C NMR spectrum were two oxygenated carbon signals at δC 79.0 (C-3) and 69.7 (C-28). The HMBC spectrum showed correlation of H-18 and H-21 with C-29, H-30 and H-11 with C-9, H-9 with C-11. The position of the double bond at C-12, 13 was also evident from the HMBC correlations of olefinic proton with carbons at δc 138.87 (C-13), δc 125.30 (C-18). The NOESY spectrum exhibited correlations of H-3 and H-28. HSQC spectrum showed correlations between C-5 and H-5, C-9 and H-9, C-16 and H-16. Based on spectral data and literature values it was identified as the known uvaol (2) (Marizeth et al., 2002).
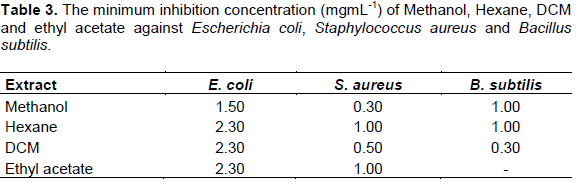
The MIC values (Table 3) recorded for the crude extracts and pure compounds suggested moderate antibacterial activity. The most active extracts were methanol with MIC value of 0.3 mg/mL against S. aureus and 1.0 mg/mL against B. subtilis. The least active extracts were from hexane with MIC values of 1.0 mg/ml against S. aureus and B. subtilis. This could be because plant-based constituents have also been reported to exhibit different modes of action against bacterial strains which range from interference with the phospholipoidal cell membranes, this has a consequence of increasing the permeability profile and loss of cellular constituents, damage of the enzymes involved in the production of cellular energy and synthesis of structural components and destruction or inactivation of genetic material (Kotzekidou et al., 2008). The study revealed that none of the pure compounds showed significant activity against Gram negative bacteria E. coli (Table 4). This could be due to the fact that E. coli is reported to be multiresistant to antibiotics (Cos et al., 2006). They have frequently been reported to have developed multi-drug resistance to many of the antibiotics currently available in the market. It is therefore not surprising to learn that E coli was the least responding bacteria strain to the tested plant extracts. Previous study using hot water extracts of O. europea leaves at concentration of 62.5 mg/mL were also found to be inactive against S. aureus and E. coli (Hansen et al., 2006). Erythrodiol (1) (Scheme 1)
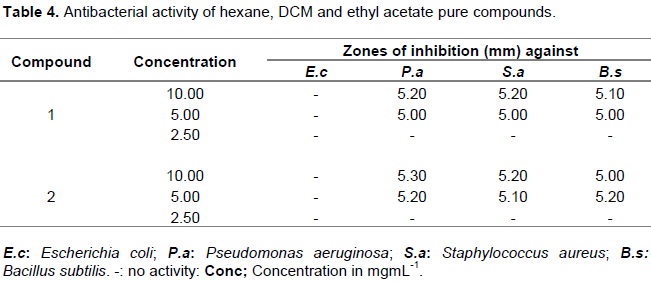
exhibited the higher antibacterial activity than uvaol (2) (Scheme 2) against Gram positive bacteria S. aureus. The results are comparable to findings by Waffo et al. (2000) on O. europea, a species from the same genus. Higher activity was exhibited by Gram positive bacteria than Gram negative bacteria. The difference in the sensitivity between them can be associated to the variance in morphological constitutes between these microorganisms. Gram negative bacteria have an outer phospholipidic membrane carrying the structural lipopolysaccharide components, this makes the cell wall impermeable to antimicrobial agents. The Gram positive bacteria on the other hand are more susceptible having only an outer peptidoglycan layer which is not an effective permeability barrier. Therefore, the cell wall of Gram negative organisms which are more complex than the Gram positive organisms act as a diffusional barrier and making them less susceptible to the antimicrobial agents than Gram positive (Nostro et al., 2000). Erythrodiol and uvaol have also been reported to possess unique biological properties such as antibacterial and wound healing effects. This may support the traditional use of this plant in healing open wounds (Marizeth et al., 2002).
O. africana is used in Kenyan traditional medicine as a chewing stick and for treatment of other periodontal diseases. Previous phytochemical studies have found presence of alkaloids, flavanoids, carotenoids and terpenoids among others in O. europea. Our study has revealed that the leaves crude extracts contains terpenoids, tannins, flavones and flavanoids while carotenoids were absent. Stem extracts tested positive for terpenoids, tannins, flavones and flavanoids while alkaloids tested negative. Purification of the leaves extracts afforded two triterpenoids, identified as erythrodiol and uvaol which belongs to oleanane and ursane classes respectively. They exhibited relatively good antibacterial activity against the bacterial strains used in this study. The triterpenoids have been reported to be used as antibacterial, antifungal, anti-inflammatory, anti-leishmanial and antimalarial. The plant could therefore be used in traditional medicine to treat the symptoms of inflammation and infections by bacteria and as a chewing stick upon further investigation.
The authors have not declared any conflict of interest.
The authors are thankful to the Division of Research, University of Kabianga for funding the research and Prof. Matasyo of Egerton University for allowing bioassay tests to be conducted in his research laboratory.
REFERENCES
|
Almas K (2001). The antimicrobial effects of seven different types of Asian chewing sticks. Odontostomatol. Trop. 24:17-20.
|
|
|
|
Al-Otaibi M, Al-Harthy M, Gustafsson A, Johansson A, Claesson R, Angmar B (2004). Sub-gingival plaque microbiota in Saudi Arabians after use of miswak chewing stick and toothbrush. J. Clin. Periodontal. 31:1048-1053.
Crossref
|
|
|
|
|
Bulunas J, Kerry N, Kinghorn D (2005). Drug discovery from medicinal plants. J. Life Sci. 78:431-444.
Crossref
|
|
|
|
|
Buwa V, Staden V (2006). Antibacterial and antifungal activity of traditional medicinal plants used against venereal diseases in South Africa. J. Ethnopharmacol. 130:139-143.
Crossref
|
|
|
|
|
Chidzonga M, Carneiro C, Kalyanyama M, Kwamin F, Oginni O (2015). Determinants of oral diseases in the African and Middle East region. Adv. Dental Resour. 27:26-31.
Crossref
|
|
|
|
|
Cos P, Vlentinck J, Vanden Berghe D, Maes L (2006). Anti-infective potential of Natural products. How to develop a stronger in-vitro proof of concept. J. Ethnopharmacol. 106:290-302.
Crossref
|
|
|
|
|
Edeoga O, Okwu E, Mbabie O (2005). Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotechnol. 4:685-688.
Crossref
|
|
|
|
|
Geissmann A, Dauter D, Victor S (2005). Anthocyanins, Flavones and related water soluble plants pigments. 'Modern Methods of Plants analyses'.Vol III, Paech K, Tracey MV (Editors). Springer Verley Gottingen. pp. 405-495.
|
|
|
|
|
Hansen K, Kompany R, Kamazawa P (2006). Isolation of an Angiotensin Converting Enzyme (ACE) inhibitor from Olea europaea and Olea lancea. Phytomedicne 2:319-325.
Crossref
|
|
|
|
|
Hyson M (2003). History of the toothbrush. J. Dental Health 51:73-80.
|
|
|
|
|
Kotzekidou P, Giannakidis P, Boulamatsis A (2008). Antimicrobial activity of some plant extracts and essential oils against foodborne pathogens in vitro and on the fate of inoculated pathogens in chocolate. Food Sci. Technol. 41:119-127.
Crossref
|
|
|
|
|
Marizeth L, Barreiros J, David M, Pedro A, Pereira P, Maria H, Guedes S, Jucen D (2002). Fatty acids Esters of Triterpenes from Erythroxylum passernum. J. Braz. Chem. Soc. 13:5669-5673.
|
|
|
|
|
More G, Tshikalangea E, Lall N, Botha F, Meyer M (2008). Antimicrobial activity of medicinal plants against oral microorganisms. J. Ethnopharmacol. 119:473-477.
Crossref
|
|
|
|
|
Ncube B, Finnie F, Van Staden J (2012). In vitro antimicrobial synergisms within plants extracts combinations from three South African medicinal bulbs. J. Ethnopharmacol. 139:87-89.
Crossref
|
|
|
|
|
Nostro A, Germano D, Angelo A, Marino A, Cannateli H (2000). Extraction methods and Bioutograph for evaluation of medicinal plant. Antimicrobial activity letters in application. Microbials 30:384-389.
|
|
|
|
|
Ogunbodede O, Kida A, Madjapa S, Amedari M, Ehizele A, Mutave R, Sodipo B, Temilola S, Okoye L (2015). Oral health inequalities between rural and urban populations of the African and Middle East region. Adv. Dental Resour. 27:18-25.
Crossref
|
|
|
|
|
Shagal H, Kubmarawa D, Alim H (2012). Preliminary phytochemical investigation and antimicrobial evaluation of roots, stem-bark and leaves extracts of Diospyros mespiliformis. Int. Res. J. Biochem. Bioinform. 2:11-15.
|
|
|
|
|
Sonkos N, Gondson M (2011). Photodynamic therapy in control of biofilms. Periodontology 55:143-166.
Crossref
|
|
|
|
|
Tapsell C, Hemphil I, Cobiac L (2006). Health benefits of herbs and spices; the past, present and future. Med. J. 185:54-64.
|
|
|
|
|
Verenne B, Petersen E, Quattaras S (2006). Oral health behavior of children and adults in urban and rural areas of Bukina Faso. Int. J. Dental Health 56:61-70.
Crossref
|
|
|
|
|
Waffo K, Azebaze A, Nkengfack E, Fomum T, Meyer M, Bodo B, Heerden R (2000). Olea europea and two isoflavonoids derivatives from the root bark of Olea europea. Phytochemistry 53:981-985.
Crossref
|
|
|
|
|
World Health Organization (WHO) (2008). Commission on Social Determinants of Health-final report. Closing the gap in a generation: health equity through action on the social determinants of health. Geneva (Switzerland): WHO.
|
|