ABSTRACT
Artemisia parviflora Roxb.(Asteraceae) has medicinal properties. This study evaluates the gastroprotective potential of A. parviflora seeds against aspirin induced ulcers in albino rabbits.Thirty-six rabbits were randomly divided into 6 equal groups (n = 6). Group 1 was control; group 2 received aspirin for 14 days; group 3 received omeprazole + aspirin for 14 days; groups 4, 5, and 6 received A. parviflora seed powder 250, 500, and 750 mg/kg, respectively along with aspirin, for 14 days. Total oxidant status (TOS), totalantioxidant capacity (TAC), malondialdehyde (MDA), and catalase (CAT) were determined to check the gastric damage. Ulcer score, gastric volume, gastric pH, and total acid output was also measured to determine gastroprotective potential of A. parviflora. A. parviflora seed powder exhibit gastroprotective potential with significant reduction in the ulcer score, acid output, and gastric volume while the pH of gastric mucosa increases significantly at the dose of 750 mg/kg when compared to aspirin treated group. Biochemical analysis showed a significant increase in TAC and CAT activity while it showed significant decrease in the levels of TOS and MDA which indicate reduction in gastric damage.A. parviflora seed powder proved to be gastroprotective at 250, 500 and 750 mg/kg with gastric protection of 47.5, 58.1, and 73.5%, respectively. It also has potent antioxidant properties.
Key words: Antiulcer, ulcer score, gastric volume, gastric pH.
Stomach stores food, possesses antibacterial action, and secretes gastric juices (Baumgart and Sandborm, 2007) while gastric ulcer is a rupture in the normal gastric mucosa that extends throughout the muscularis mucosa. Gastric ulcer could be divided into two common types according to location, ulcerative colitis (lower) and peptic ulcer (upper). Peptic ulcer develops when aggressive and protective factors are imbalanced. Helicobacter pylori, non-steroidal anti-inflammatory drugs (NSAIDs), pepsins, hydrochloric acid (HCl) and bile acid are the aggressive factors (Malairajan et al., 2008). NSAIDs weaken the protective mucous layer of the stomach wall and increase the secretion of HCl (Awaad et al., 2013). Epigastric pain is the predominant symptom of uncomplicated gastric ulcer along with more dyspeptic symptoms such as early satiety, bloating, nausea and fullness.
In duodenal ulcer patient, epigastric ache occurs frequently during the night or the state of fasting and is usually relieved by acid-neutralising agents or food intake. Heartburn and erosive esophagitis are also reported in these patients (Gisbert and Pajares, 2003). The long term use of NSAIDs (aspirin) mainly inhibit the prostaglandin (PG) and cyclooxygenase (COX) enzyme which causes damage in gastric mucosa with production of free redicals (Baigent et al., 2009). The amount of serum lipid peroxidation (LPO) improved during the ulcer formation while catalase (CAT) and superoxide dismutase (SOD) levels decreased. There is an affirmative relationship that exists between the oxidative stress produced by free radicals, gastric ulcer and gastric carcinoma (Tandon et al., 2004). Most of the existing gastroprotective drugs operate on the offensive aspects neutralizing acid discharge like antacids, H2 receptor blockers like ranitidine, anticholinergics like pirenzepine and proton pump inhibitors like omeprazole that interfere with acid secretion (Ahmad et al., 2006).
Proton pump inhibitors are the main synthetic antiulcer class of drugs that reduce the production of acid. Alternative therapies have been developed for the management of GI ulcers and herbal medicines are most important source for GI ulcer management. Artemisia parviflora Roxb. is an aromatic shrub, leaves are sessile, about 40-100 cm in height and found throughout Pakistan at high elevations. Although phytoconstituents of A. parviflora have medicinal importance, its gastroprotective effect has not been studied. A. parviflorais commonly used for skin diseases, cuts and wounds this could be due to the different phytochemical constituents of A. parviflora. These constituents have inhibitory effect on carcinogenesis in humans and also shows antioxidant activity (Ahuja et al., 2011). Vital oils of Artemisia species are extensively utilized for a variety of medicinal uses such as antibacterial, fungicidal, antiviral,nematicidal and antimalarial (Ahmad et al., 2006).In present study antiulcer effect of A. parviflora was evaluated against aspirin induced ulcers in albino rabbits.
Experimental animals
Thirty-six male adult albino rabbits weighing 1.63 ± 0.25 kg were selected for the current studyand divided into six equal groups (n = 6). Animals were housed in the experimental animal room, Department of Physiology and Pharmacology, University of Agriculture, Faisalabad (UAF), Pakistan at temperature 22 ± 2ºC, humidity 65 to 70% and a 12 h light/dark cycles for a week before the start of the experiment and during the experiment. Animals were provided with standard feed and water ad libitum. Further, the institutional ethical committee of UAF, Pakistan approved all procedures adopted in this study.
Plant materials
A. parviflora seeds were purchased from the herbal dealers of Faisalabad, Pakistan. The plant material was authenticated by Dr.Mansoor Hameed, Department of Botany, Faculty of Sciences, UAF, Pakistan. A voucher specimen of A. parviflora seeds have been deposited in the Herbarium maintained by Department of Botany, UAF, Pakistan. The samples were preserved in the Pharmacology laboratory of theDepartment of Physiology and Pharmacology, UAF, Pakistan. The material was finely powdered with the help of a special electrical grinder. This process was done withthe precaution that the temperature did not rise up to 40ºC. This powder was passed through mesh sieve no. 200 and then stored in an airtight container for further experimental use.
Treatment protocols
All the rabbits were divided into six equal groups (n = 6). Group 1 served as untreated control and received normal diet throughout the experiment, group 2 received aspirin (Disprin®; Reckitt Benckiser(Pakistan) Ltd. Karachi, 150 mg/kg orally for 14 days (Herbert et al., 2011). Group 3 received omeprazole (Omega®; Ferozsons Laboratoies Ltd. Karachi, 20 mg/kg) + aspirin (150 mg/kg) for 14 days (Maity et al., 2003). Groups 4, 5, and 6 received A. parviflora seed powder 250, 500, and 750 mg/kg, respectively along with aspirin 150 mg/kg for 14 days. Five ml distilled water was used to dilute the A. parviflora seeds powder before administration to the rabbits.
Surgical procedures
The animals were fasted for at least 24 h before the surgical procedure. On the 14th day of theexperiment these animals were sacrificed by decapitation. After this, the stomach was dissected out and transferred in to small tubes. The stomach was cut along the greater curvature and the contents were collected into small tubes for biochemical parameters. These gastric contents were subsequently centrifuged at 3000 rpm for 5 min. The supernatant was separated and its volume was expressed as ml/100 g body weight.
Blood sampling
Blood samples were collected at 14th day of experimental treatments. The samples were allowed to clot for 20 min at refrigeration temperature and then centrifuged for 5 min at 4000 rpm to separate serum. Serum was stored at -4ºC till the estimation of different antioxidant parameters.
Acid output
The acid output was calculated by titrating the supernatant fluid collected from the stomach with 0.05N NaOH. Acidity was expressed as molEq/l/100 g of body weight (Maity et al., 2003).
Ulcer index
The number of ulcers was noted and the severity was determined with the following scores: Normal coloration (0.0), Red coloration (0.5), Spot ulcer (1.0), Hemorrhagic stress (1.5), Deep ulcers (2.0), Perforation (3.0). Ulcer index (UI) was calculated using the formula (Vogel, 2002).
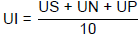
Where, US = mean severity of ulcer score. UN = average number of ulcers per animal. UP = percentage of animals with ulcer incidence.
Curative ratio
The curative ratio from the ulcer was calculated for the treated groups by using the following equation.
Where, CUI = ulcer index of control groups. TUI = ulcer index of treated groups.
Biochemical examination
Malondialdehyde (MDA) was determined according to the method developed by Ohkawa et al. (1979). Enzymatic activity of enzyme catalase (CAT) was measured by method of Goth (1991). The total oxidant status (TOS) and the total antioxidant capacity (TAC) in serum was measured by methods developed by Erel (2004, 2005) using spectrophotometer.
Statistical analysis
The values were expressed as mean ± SE. Statistical analysis was performed by one way analysis of variance (ANOVA) at 5% level of significance (Steel et al., 1997).
The seeds powder of A. parviflora 250 to 750 mg/kg, given orally once daily for fourteen days showed dose dependent protective effect against gastric ulcer induced by aspirin. A. parviflora at the dose rate of 750 mg/kg significantly protected the animal and healed ulcer after fourteen days of treatment. Mean ulcer score of group 2 was 2.35 that increased significantly (p<0.05) after administration of aspirin, while it significantly decreased for group 3 as 0.67 which was treated with omeprazole + aspirin and also it was decreased for group 6 which was treated with the highest dose of test plant, having value of mean ulcer score 0.54. Ulcer index also showed the similar pattern of results as that of ulcer score. After fourteen days of the treatment ulcer index for groups 1, 2, 3, 4, 5, and 6 was 0.00, 13.9, 3.25, 7.29, 5.82, and 3.67, respectively. Group 3 (Omeprazole + aspirin) and highest plant dose in group 6 showed the significant (p < 0.05) reduction in ulcer index. The percent curative ratio of A. parviflora seed powder at 250, 500, and 750 mg/kg was 47.5, 58.1, and 73.5%, respectively as shown in Table 1.
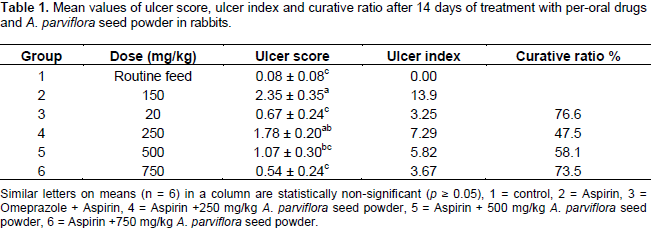
Total acid output (molEq/l/100 g body weight) of rabbits in after 14 days are shown in Table 2. The mean values for acid output showed that the aspirin increased the acidity in group 2 having mean value 39.7 molEq/l/100 g as compared to the group 1 which has mean value for acidity 26.5 molEq/l/100 g, while the groups treated with A. parviflora seed powder showed significant results at dose 500 and 750 mg/kg as 31.7 and 29.4 molEq/l/100 g, respectively.It was also observed that pH was decreased markedlyin aspirin (group 2) treated rabbits compared to group 1, from 2.05 to 0.87. Omeprazole (group 3) enhanced pH (4.47) of gastric mucosa. Our test plant also increased the pH of gastric mucosa which was 4.10 at the dose of 750 mg/kg. It was observed from the result that gastric volume also increased after the administration of aspirin (35.1) while administration of omeprazole and A. parviflora (750 mg/kg) significantly decreased mean gastric volume 24.9 and 25.6, respectively presented in Table 2.
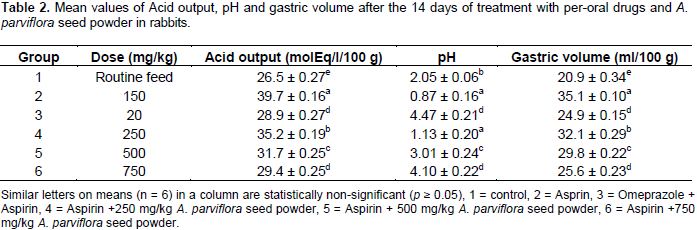
Results of the study showed that mean values of TAC for the group 1 was 3.06 mmol/l. TAC decreased (0.93 mmol/l) with ulcer (group 2) production in stomach by the use of aspirin while it significantly (p< 0.05) increases upto normal values for groups 3 and 6 as 2.98 and 2.78 mmol/l, respectively presented in Table 3. The mean values of TOS of group 1 was 3.69 μmol/l. TOS increased (7.03 μmol/l) with ulcer production in stomach by the use of aspirin while it significantly (p< 0.05) decrease up to normal values for groups 3 and 6 as 3.65 and 3.63 μmol/l, respectively when compared with aspirin treated group presented in Table 3. Results of the current study also demonstrated that the mean values of MDA activity were increased (from 3.34 to 7.97 nmol/l) when aspirin was used alone for the production ofan ulcer in the stomach.
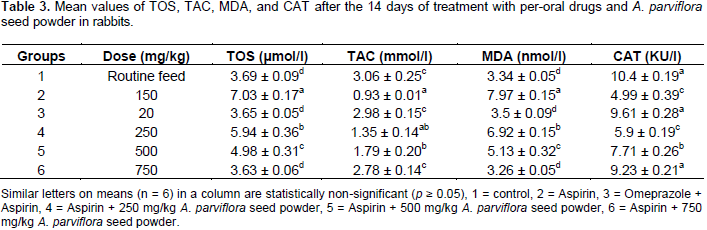
The mean values decrease significantly (p< 0.05) for groups 3, 4, 5, and 6 as 3.5, 6.92, 5.13, and 3.26, respectively provided in Table 3. The mean values of CAT activity decreased up to 4.99 KU/l when aspirin was used alone for the production of anulcer inthe stomach. This value is significantly (p < 0.05) decreased froma normal value, that is, 10.4 KU/l and its value seems to increase significantly when treated with omeprazole (9.61 KU/l). It also had a normal range of values for CAT activity whenthe plant was used atthe highest dose as in group 6 (9.23 KU/l) which presented in Table 3.
Gastric ulcer is a break in the normal gastric mucosa of the stomach that extends throughout the muscularis mucosa into the submucosa or deeper. In ulcer condition erosions are formed and superficial epithelium of mucosa is loosened. In the alimentary tract ulcer may occur everywhere. PGs play a significantprotective role in the stomach by stimulating the synthesis and secretion of mucus and bicarbonate, increasing mucosal blood flow and promoting epithelial proliferation. The major mechanism via NSAIDs cause ulcers is the inhibition of PGs by the inhibition of COX, which isa key enzyme in the bio-synthesis of PGs (Hamid et al., 2012). So to avoid all these adverse reactions of the drugs, herbal remedies should be given to the ulcer patient to cure the ulcer. Many natural products in plants have multifunctional molecules that protect them from infections of bacteria, viruses and other microorganisms. For this reason, we evaluated the antiulcer activity ofthe graded dose ofA. parviflora seed powder in albino rabbits.
In the present study, results demonstrated that ulcer scores were significantly increased in animals treated with aspirin. Administration of synthetic antiulcer drug, omeprazole at dosea of 20 mg/kg along with aspirin significantly reduced the ulcer scores in comparison with aspirin treated rabbits. Concomitant administration of A.parviflora seed powder atthe dose rate of 250, 500, and 750 mg/kg along with aspirin significantly reduced the ulcer scores. The mean value of A. parviflora at dose rate of 750 mg/kg was not significantly different from omeprazole. The results of our study conicide with other studies (Aslam et al., 2013; Begum et al., 2014). pH of gastric secretions was measured in the current study. Administration of aspirin significantly reduced the pH of gastric mucosa as described in previous studies (Aslam et al., 2013). The administration of omeprazole along with aspirin significantly enhanced the pH of gastric mucosa. Concomitant administration of A. parviflora seed powder with aspirin significantly enhanced the pH of gastric secretions at three different doses 250, 500, and 750 mg/kg.
A. parviflora ata dose rate of 750 mg/kg significantly enhanced the pH and it produced similar results as synthetic antiulcer drug omeprazole. Aspirin causes the gastric damage by making the stomach pH more acidic which increases the acidity of the gastric mucosa by enhancing the concentration of hydrogen ions. These results are parallel as described in previous studies (Alsabri et al., 2013; Aslam et al., 2015). The gastric volume was significantly increased in groups 2. Aspirin enhances the acid secretions in gastric mucosa due to its acidic nature which enhances the volume of gastric secretions. Administration of omeprazole along with aspirin significantly reduces the gastric volume. Concomitant administration of A. parviflora along with aspirin significantly reduced the gastric volume at 250, 500, and 750 mg/kg. A. parviflora at a dose of 750 mg/kg significantly reduced gastric volume and its results were statistically similar with synthetic antiulcer drug omeprazole. The above mentioned results are consistent with previous research studies on aspirin induced gastric ulcer (Goswami et al., 2011).
Pretreatment with an oral dose of A. parviflora could partially decrease the ulcer index and permit the healing of gastric lesions induced by the administration of aspirin.The antioxidant (TAC, TOS, MDA, and CAT) activity of A. parviflora was also determined. Phytochemical screening showed a positive result for the steroids, terpenoids, alkaloids, di- and triterpenoids, phenols, flavonoids, tannins and volatile oils. These constituents have different activities which are helpful in healing ulcer. Flavonoids have free radical sacvenging and antioxidant activity. The important derivative of flavonoids is quercetin. Flavonoid increases mucus production and also have antihistaminic properties which reduces the histamine production and reduction of mast cells which are produced by the aspirin.
The main mechanism of action for the gastroprotective effects of this flavonol are its proton pump inhibitor and antioxidant properties. Oral administration of NSAIDs, such as aspirin, indomethacin have several undesirable effects on the gastrointestinal tract and increase the likelihood of myocardial infarction. Flavonoids also have anti-inflammatory properties without any ulcerogenic action as a side effect and thus show a great advantage in the treatment of peptic ulcers (Kelly et al., 2009). Phenolic substances e.g. phenolic acid and tannins contribute directly to antioxidant activity. Tannins are important constituents of A. parviflora. These are poly phenols and water soluble compound present in plants. They have astringent properties so they are used primarily in medicine. Tannins precipitate micro proteins to the peptic ulcer location. Tannins form an impermeable layer over the coating that prevents the gut secretions and protects the basic mucosa from toxins and other irritants. Tannins endorse resistance to the achievement of proteolytic enzymes, a related activity against Helicobacter pylori. They also inhibit gastric acid secretion (Wallace et al., 2000).
This study gives the suggestion that in case of gastric ulcer the oxidative stress is increased then the MDA activity and levels of TOS are further increased in ulcerated group, while the levels of TAC and CAT are decreased for the same group. Antioxidants have defensive properties against gastric ulcer and many other ailments (Saravanan, 2011). The oxidative alteration in the cellular membrane or intracellular molecules occurs by the imbalance between ROS and antioxidant defense mechanism. LPO causes loss of membrane fluidity, impaired ion transport and membrane integrity (Tandon et al., 2004). It has been concluded from the results that when aspirin was given at a dose rate of 20 mg/kg, TOS and MDA were enhanced significantly while TAC and CAT activity were reduced significantly. Aspirin encourages the reactive oxygen metabolites that may play a part in gastric damage.
These reactive species cause damage to the biochemical markers e.g. lipid and increased the production of free radicals that increased MDA production and decreased CAT and these free radicals production also because of impairment of cellular enzyme that involve in defensive mechanism of gastric ulcer such as total antioxidant capacity and CAT activity (Filho et al., 2012). The curative ratio for ulcer of treated groups was computed. A. parviflora decreased gastric lesions dose dependently. A. parviflora significantly decreased the gastric MDA content while it increased CAT activity compares to aspirin. This showed that the antiulcer activity of the A. parviflora might be recognized by these tests.
In the present study, A. parviflora seeds extract proved to be gastroprotective. It also significantly enhanced the TAC and CAT activity comparable to synthetic antiulcer drug while it caused a significant reduction in TOS and MDA levels which indicates it as an antioxidant. Overall study revealed that extract of A. parviflora at 250, 500, and 750 mg/kg showed gastric protection of 47.5, 58.1, and 73.5%, respectively.
The authors have not declared any conflict of interests.
REFERENCES
|
Ahmad S, Ali A, Beg H, Dasti AA, Shinwari ZK (2006). Phytochemical screening of aerial parts of Artemisia parviflora. Pak. J. Weed Sci. Res. 12:183-190.
|
|
|
|
Ahuja J, Suresh J, Deep A, Madhuri, Pratyusha R (2011). Phytochemical screening of aerial parts of Artemisia parviflora: A medicinal plant. Der. Pharm. Lett. 3:116-124.
|
|
|
|
|
Alsabri SG, Rmeli NB, Zetrini AA, Mohamed SB, Meshri MI,Aburas KM, Bensaber SM, Mrema IA, Mosbah AA, Allahresh KA, Hermannand A,Gbaj A (2013). Phytochemical, anti-oxidant, anti-microbial, anti-inflammatory and anti-ulcer properties of Helianthemum lippii. J. Pharmacogn. Phytochem. 2:86-97.
|
|
|
|
|
Aslam B, Awan T, Javed I, Khaliq T, Khan JA, Raza A (2015). Gastroprotective and antioxidant potential of Glycyrrhizaglabraon experimentally induced gastric ulcers in albino mice. Int. J. Curr. Microbiol. Appl. Sci. 4(2):451-460.
|
|
|
|
|
Aslam B, Majeed W, Javed I, Muhammad F, Khaliq T, Khan JA, Ali A, Sindhu ZD (2013). Gastroprotective effect of Berberis vulgaris (zereshk) seeds against gastric ulcer induced by aspirin in male adult albino mice. Indo. Am. J. Pharm. Res. 3:4518-4527.
|
|
|
|
|
Awaad AS, Meligy RME, Soliman GA (2013). Natural products in treatment of ulcerativecolitis and peptic ulcer. J. Saudi Chem. Soci. 17:101-124.
Crossref
|
|
|
|
|
Baigent C, Blackwell L, Collins R (2009). Aspirin in the primary and secondary prevention of vascular disease. Collaborative meta analysis of individual participant data from randomized trials. Lancet 373:1849-1860.
Crossref
|
|
|
|
|
Baumgart DC, Sandborm WJ (2007). Inflammatory bowel disease: Clinical aspects and established and evolving therapies. Lancet 369:1641-1657.
Crossref
|
|
|
|
|
Begum R, Bilal A, Ijaz J, Tanweer K, Faqir M, Ahmad R (2014). Gastro protective and antioxidant effect of Euphorbia prostrata against indomethacin induced gastric ulcers in healthy adult male albino rabbits. Int. Res. J. Pharm. 5:846-850.
Crossref
|
|
|
|
|
Erel O (2004). A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 37:277-285.
Crossref
|
|
|
|
|
Erel O (2005). A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 38:1103-1111.
Crossref
|
|
|
|
|
Filho TP, Olaitan SB, Almedia DA, Lima JC, Ascencio PGM, Ascencio DS (2012). Evaluation of antiulcer activity and mechanism of action of methanol stem bark extract of Lafoensia pacari A. St.-Hil. (Lytraceae) in experimental animals. J. Ethnopharmacol. 144:497-405.
Crossref
|
|
|
|
|
Gisbert JP, Pajares JM (2003). Helicobacter pyloriinfection and perforated peptic ulcerprevalence of the infection and role of antimicrobial treatment. Helicobacter 8:159-167.
Crossref
|
|
|
|
|
Goswami M, Kulshreshtha M, Chandana VR, Yadav S (2011). Anti-ulcer potential of Lawsonia inermis leaves against gastri ulcer in rats. J. Appl. Pharm. Sci. 1:69-72.
|
|
|
|
|
Goth L (1991). A simple method for determination of serum catalase and reversion of reference range. Clin. Chem. Acta. 2:143-51.
Crossref
|
|
|
|
|
Hamid AR, Foong CP, Ahmad Z, Hussain MK (2012). Antinociceptive and anti-ulcerogenic activities of the ethanolic extract of Annona muricataleaf. Braz. J. Pharmacogn. 22:630-41.
Crossref
|
|
|
|
|
Herbert M, Jackson C, Ekpo M, Okopedi E, Anah V (2011). Gastroprotective effects of ethanolic leaf extract of Musa paradisiaca in rats. J. Chem. Pharm. Res. 3:322-27.
|
|
|
|
|
Kelly SLM, Dias GEN, Pinto MEF, Ferreira AL, Brito AMS, Lima CAH, Filho JMB, Batista LM (2009). Flavonoids with gastroprotective activity. Molecules 14:979-1012 .
Crossref
|
|
|
|
|
Maity S, Chaudhri T, Vedasiromoni JR, Ganguly DK (2003). Cytoprotection mediated antiulcer effect of tea root extract. Ind. Institute Chem. Biol. 35:213-219.
|
|
|
|
|
Malairajan P, Geethav, Narasimhan S, Veni KJK (2008). Evaluation of antiulcer activity of Polyalthia longifolia(sonn). Thwaites in experimental animals. Indian J. Pharmacol. 40:126-138.
Crossref
|
|
|
|
|
Ohkawa H, Ohishi N, Yagi K (1979). Assay for lipid peroxide in animal tissue by thiobarbituric acid reaction. Anal. Biochem. 95:351-358.
Crossref
|
|
|
|
|
Saravanan J, Venkatesh P, Soumya V, Hariprasath K, Prasad RH, Kumar KTM (2011). Antiulcer and antioxidant activity of methanolic leaf extract of Soymida febrifuga. J. Pharm. Res. 5:528-531.
|
|
|
|
|
Steel RGD, Torrie GH, Dickey DA (1997). Principles and procedures of statistics. 3rd Ed. McGraw Hill, New York. pp. 1746-1762.
|
|
|
|
|
Tandon R, Khanna HD, Dorababu M, Goel RK (2004). Oxidative stress and antioxidant status in peptic ulcer and gastric carcinoma. Indian J. Physiol. Pharmacol. 48:115-118.
|
|
|
|
|
Vogel HG (2002). Drug discovery and evaluation, pharmacological assay. 2nd Ed, Springer, Germany. pp. 759-767.
|
|
|
|
|
Wallace JL, McKnight W, Reuter BK, Vergnolle N (2000). NSAID-induced gastric damage in rats, requirement for inhibition of both cyclooxygenase 1 and 2. Gastroenterology 119:706-714.
Crossref
|
|