There are reports of the presence of vasodilatory alkaloids in Heimia salicifolia extracts. The action mechanisms of the potential antihypertensive effect of such alkaloids have not however, been elucidated. The objective of the present study was to corroborate the antihypertensive and vasorelaxant activities of the isolated alkaloids from H. salicifolia, and to characterize their vasorelaxant actions. A chloroform extract (HSCE) was obtained from H. salicifolia. The alkaloids were separated by thin layer chromatography, extracted from silica gel, and evaluated using spectroscopy. The alkaloid with the most potent vasorelaxant activity was identified as lythrine. The antihypertensive effect of the HSCE was corroborated on normotensive rats and on animals with Nω-nitro-L-arginine-methyl ester (L-NAME) induced hypertension. The action mechanisms of the HSCE and lythrine were studied on isolated and perfused rat mesenteric vascular bed (MVB) preparations. HSCE administration produced a concentration-dependent relaxation, but in preparations without vascular endothelium, the relaxant response to HSCE was significantly diminished. In MVB preparations with intact vascular endothelium, pre-contracted with phenylephrine or L-NAME, perfusion with lythrine produced concentration-dependent relaxation; a similar effect was produced by acetylcholine. Wortmannin (phosphatidylinositol 3-kinase inhibitor) and methylene blue (guanylate cyclase inhibitor) decreased the relaxant effect of lythrine. These data suggest that the vascular relaxation induced by alkaloids isolated from H. salicifolia is dependent on the activation of the nitric oxide/guanylate cyclase pathway.
Hypertension can be defined as chronic elevation of systolic and/or diastolic blood pressure above 140/90 mm Hg. It is a major risk factor for cardiovascular diseases such as congestive heart failure, coronary artery disease, stroke, and renal disease. Despite progress in prevention, detection, treatment, and control of high blood pressure, hypertension remains an important public health problem (Go et al., 2014), and its prevalence has been gradually increasing worldwide. The World Health Organization (WHO) reported in 2013 that hypertension is a major public health issue and it is the cause one in every eight deaths. Globally, cardiovascular diseases account for approximately 17 million deaths/year, and complications of hypertension account for 9.4 million deaths/year worldwide (World Health Organization, 2013). In Mexico, hypertension is considered a national health problem (Balam-Ortiz et al., 2014). Hypertension prevalence in Mexican adults ≥ 20 years was 23.8% in 1993, 30.8% in 2006, and 31.5% in 2012 (Campos-Nonato et al., 2013). Many synthetic antihypertensive drugs have been commonly used for hypertension control in developed countries. Some of these drugs have many side effects.
Complementary and alternative medicine may have the potential for hypertension treatment (Magos et al., 2017; Baharvand et al., 2017). Herbal medicines still remain the most popular choice in certain developing countries. The extensive use of herbal remedies in such nations has led to extensive research in this area to determine their potential efficacy. Several modern cardiovascular drugs are now available as natural/herbal products (Tirapelli et al., 2010; Luna et al., 2013). There are several regions in Mexico with many plants used as herbal remedies that may represent an opportunity for the discovery of new antihypertensive drugs (Hernández et al., 2013). Also, it has been demonstrated that a partial separation of H. salicifolia alkaloids produced antihypertensive effects on angiotensin II-caused acute hypertension. Nevertheless, the pharmacological properties of H. salicifolia have not yet been widely studied. The aim of the present study was to investigate the antihypertensive and vasorelaxant activity of the isolated alkaloids from H. salicifolia and to characterize its possible vasorelaxant action.
Study species
Heimia salicifolia (H.B&K) Link & Otto (Lythraceae family) is a wild flowering shrub distributed over Mexico, Western Texas, El Salvador, Jamaica, Uruguay, and Argentina (Malone and Rother, 1994). This plant is known as sun opener (Graham, 1997). In Mexico, the most common name is sinicuichi (Guzman et al., 2006). H. salicifolia has been used in folk medicine in several countries for different indications, as an emetic, haemostatic, tonic laxative, diuretic, anti-inflammatory, and to treat some syphilis symptoms (Malone and Rother, 1994; Aguilar et al., 1994). Due to its psychotomimetic activity, native people in Central America and Mexico have used the plant for shamanic purposes (Graham, 1997). After the administration of alcohol decoction or plant juice, the subjects experiment a variety of effects such as yellow-colored vision and auditory distortion in which bells or voices sound as if they were originated in farther distances (Malone and Rother, 1994). Phytochemical analyses have shown that H. salicifolia contain biphenylquinolizidine lactone alkaloids (Malone and Rother, 1994). More than 20 of these alkaloids such as vertine, sinicuichine, lythrine, nesodine, lyfoline, dehydrocodeine, and demethyllasubine I, have been isolated (Blomster et al., 1964; Malone and Rother, 1994).
Vertine, lyfoline, lythrine, and nesodine seem to be the primary source of the traditional effects of H. salicifolia. It also has been reported that these alkaloids possess cardiovascular effects; nesodine produced a fall in blood pressure (Malone and Rother, 1994; Kaplan and Malone, 1966). Another reported effect for cryogenine and nesodine is the inhibition of prostaglandin synthase, which may support the folklore anti-inflammatory usage of H. salicifolia. Cryogenine has been reported to have anti-inflammatory activity similar to that of aspirin (Lema et al., 1986). Rumalla et al. (2008) isolated two more alkaloids from H. salicifolia and elucidated its chemical structure using spectroscopic techniques. The new alkaloids showed antimalarial activity. There are no ethnopharmacologicalycal reports of H. salicifolia being used as an antihypertensive drug. It has been found that the aqueous extract from H. salicifolia leaves decreased the systolic blood pressure in anaesthetized normotensive rats.
Chemicals and drugs
Phenylephrine bitartrate (Phen), acetylcholine chloride, N-nitro-L-arginine methyl ester (L-NAME), atropine, methylene blue, wortmannin and diphenhydramine were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). All other reagents were analytical grade from Merck, Germany.
Experimental animals
The experiments were performed in male Wistar rats (300-350 g) breed in the Animal Facility of the Facultad de Estudios Superiores Iztacala (FESI), Universidad Nacional Autónoma de México (UNAM). Female animals were not studied to avoid physiological changes due to oestrus cycle. Animals were housed under conditions of controlled temperature (24±0.5ºC) and with 12 h light/12 h dark photoperiod. They were fed rat chow (Ralston-Purina) and water ad libitum. All animal procedures and protocols were conducted in accordance with the Federal Regulations for Animal Experimentation and Care (NOM-062-ZOO-1999, Ministry of Agriculture, Mexico) on care and use of laboratory animals, and approved by the institutional ethics review board.
Plant material
Dried leaves of H. salicifolia were obtained from the Sonora Market, a medical plants selling place in Mexico City. The plant’s botanical identity was verified at the Izta Herbarium of the Botanic Department, Facultad de Estudios Superiores Iztacala and a voucher specimen numbered 41653 was deposited at the same herbarium for reference.
Preparation of chloroform extract and alkaloids isolation
A published method for chloroform extract preparation and alkaloids isolation from H. salicifolia was used (Blomster et al., 1964). Briefly, 5 kg of H. salicifolia leaves were dried at 45ºC and powdered. Afterwards, to remove fat, 4 L of petroleum ether (J.T. Baker) was added; then the plant material was macerated in 5 L of methanol (J.T. Baker) during 24 h, and concentrated in a rotary evaporator (Buchi Rotavapor model Mp60) under reduced pressure to get a final volume of 50 mL. Alkaloids detection was carried out with Dragendorff reagent. Methanol concentrate was dried at 50ºC for 24 h, in addition to 100 mL distilled water and acidified to pH 2.0 with 10% hydrochloric acid solution (Tec. Chem.), and filtered through celite (Sigma Chem.); the precipitate was washed with distilled water. The aqueous acidic filtrate was further defatted in a continuous extractor with 500 mL ethyl ether. The pH was adjusted to 9 with 28% ammonium hydroxide solution, continuously extracted with 200 mL chloroform. This H. salicifolia chloroform extract (HSCE) was dried in vacuum at 40ºC with a yield of 15 g (approximately 0.3%). HSCE was used for biological experiments and for alkaloids isolation.
Alkaloids isolation from HSCE
Dried HSCE was dissolved in chloroform, adsorbed on basic alumina (J.T. Baker), dried, and placed on the top of a column (70 × 2 cm) of basic alumina (J.T. Baker). Elution was done with a mixture of methanol/chloroform (1:1) and finally with methanol. The effluent was collected in three fractions. Each fraction was dried
in vacuum and chemical tests were done for alkaloids. Thin-layer preparative chromatography of the fractions was performed on silica gel G plates (Merck), with chloroform/methanol (3
:;2) (v/v); the chromatograms were observed with an ultraviolet lamp (UVGL-25) at 250 nm. The separated fractions were marked, eluted from the plate and examined again for alkaloids with 4-nitroanilindiazothized reagent; thereafter, four principal alkaloids were separated and assayed in biological tests. The alkaloid with higher biological activity was the alkaloid
3 (Guzman et al., 2006), which was isolated and prepared for chemical identification by NMR studies.
Alkaloid identification
1H-NMR spectrum was recorded at 500 MHz 13C-NMR at 75 MHz, on a Varian Inova; chemical shifts (PPM) were relative to (CH3)4 Si as internal reference, and CDClL3 (Aldrich) as solvent. The 1H NMR and 13C NMR data were obtained on a Varian Unity 300 instrument. Comparisons were made with spectra known of these alkaloids using the Organic Chemistry Data Base program.
Effect of HSCE on blood pressure in normotensive rats
The rats were randomly allocated into groups of six each. The Group 1 (control) received vehicle (carboxymethyl cellulose), whereas Groups 2, 3, and 4 received 10, 20, and 40 mg/kg of HSCE for 10 days p.o., respectively. At the beginning and end of treatment, systolic arterial blood pressure (SBP) was measured non-invasively using a tail-cuff computer-aided monitoring device (Automatic Blood Pressure Computer, Model LE 5007; Letica Scientific Instruments, Barcelona, Spain) as follows. The rat was restrained in a size-matched plastic container, and an inflatable latex ring containing a sensor was placed over a tail artery while the rat was kept warm (37°C) in the same device. The rats were trained to be inside the container with the cuff placed on the tail and to get used to the inflation and deflation of the latex ring. The SBP measurements were recorded for each group, and the mean of three measurements obtained in one session was considered.
Effect of HSCE on blood pressure during chronic hypertension caused by inhibition of nitric oxide synthase
Rats were randomly allocated into groups of six each. Group 1 (control) received vehicle; Group 2- Nw-nitro-L-arginine-methyl ester (L-NAME) (70 mg/Kg, Sigma-Aldrich, St. Louis, MO, USA); Group 3- L-NAME + enalapril (5 mg/kg/day, Sigma-Aldrich, St. Louis, MO, USA); Group 4- L-NAME + HSCE (10 mg/kg/day); and Group 5- L-NAME + HSCE (20 mg/kg/day) for 10 days. All drugs were administered in carboxyimethyl cellulose and L-NAME in the drinking water. The actual doses in each group were calculated from the daily water intake. SBP measurement was done using the aforementioned technique.
Rat mesenteric vascular bed assay: functional and mechanistic approaches
The experimental assays were performed in normotensive rat mesenteric vascular bed (MVB) isolated and perfused as a model of vascular resistance (Sousa et al., 2017). Briefly, rats were anaesthetized with sodium pentobarbital (35 mg/kg, i.p). The abdominal cavity was opened and the intestinal loops exposed; the superior mesenteric artery was dissected near to its origin in the abdominal aorta and cannulated with a PE-50 polyethylene (BD Intramedic, Oxford, U.K.) catheter. The vascular bed was covered with moist gauze and perfused with Krebs’ solution, with the following mM composition: 118 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 NaH2PO4, 4.2 MgSO4, 25 NaHCO3, and 11.5 glucose. The solution was bubbled with O2/CO2 (95 %: 5 %) mixture, at 37°C with a 7.4 final pH. The intestinal loops were removed as a block and the MVB was separated cutting near to the intestinal loops. The MVB was placed in a chamber at 37°C and the cannulated superior mesenteric artery connected to a peristaltic pump (Vera, Manostat,
Division of Barnant Company) to provide a constant flow, adjusted to obtain a 48-50 mm Hg basal perfusion pressure. Mean flow rate was 4±0.2 ml.min-1 whereas perfusion pressure was measured using a pressure transducer (Mod. P1000-A, Narco Bio Systems Inc., Houston Texas) placed in the circuit between the outlet of the pump and the preparation, and recorded on a Narco physiograph (Model DMP-4B, Narco Bio Systems Houston Texas). The fFlow was maintained at a constant rate, so vasoconstriction was recorded as an increase in perfusion pressure, and relaxation as a decrease in perfusion pressure. Data are expressed as changes (Δ) of the perfusion pressure in mm Hg. All preparations were allowed to equilibrate for at least 30 min before starting the experiments.
Phenylephrine-induced vasoconstriction
To measure the MVB vasoconstrictor response, concentration-response curves to phenylephrine (Phen) were constructed. Increasing Phen doses (0.5 to 80 µg) were injected in bolus with a 50 µL Hamilton syringe. The injection volume was 0.1 mL. The interval between injections was 5 min or the time needed for the perfusion pressure to return to its initial value, which was never longer than 10 min. In other preparation, the MVB was constricted with Krebs’ solution containing Phen (10-5 M) to induce submaximal vasoconstriction (about 90%) and allowed to reach a plateau. When the MVB contraction was stable (30-45 min), concentration-response curves to acetylcholine (ACh) were performed; ACh was injected in 0.1 mL volumes and 10-90 µg doses, causing a dose-dependent relaxation, recorded as a decrease in perfusion pressure. The injection duration time was 10 s. Concentration- response curves to HSCE (10-90 µg) were drawn using the same method. In some preparations, the endothelium was removed by perfusion with distilled water for 10 min (Criscione et al., 1984).
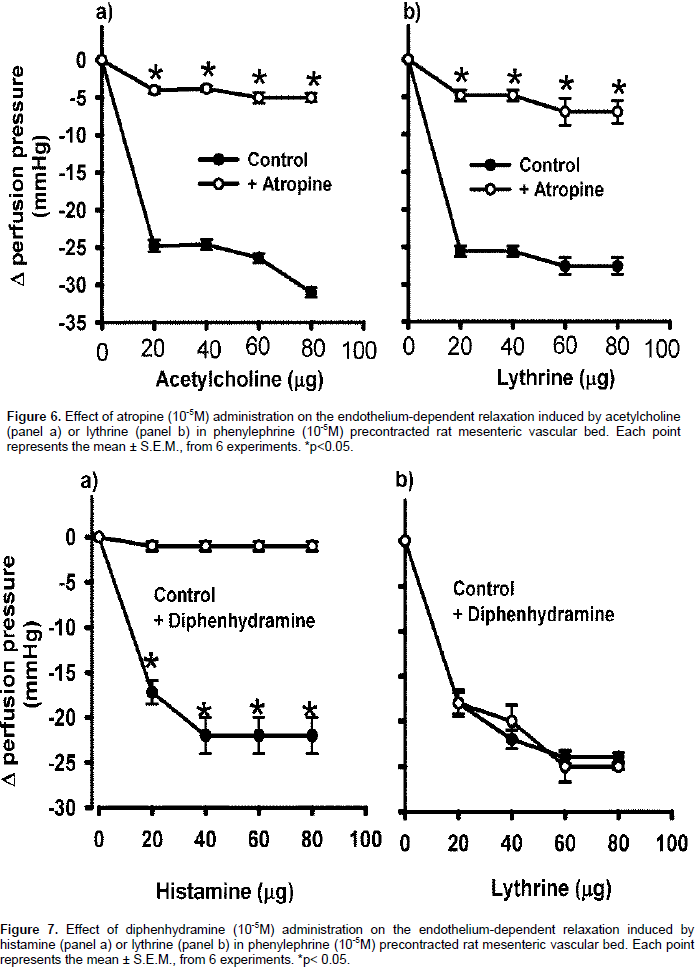
Concentration-response curves were constructed using the previously described parameters with the MVB pre-contracted with Phen. A bolus injection of ACh (80 µg) was applied. Vascular endothelium removal was confirmed by the absence of a relaxation response. To study the possible vasorelaxant mechanism exerted by the alkaloid 3 isolated from HSCE, MVB with intact endothelium was perfused for 10 min with L-NAME (10−4 m), (unspecific nitric oxide synthase inhibitor), methylene blue (10−5 m) (soluble guanylate cyclase inhibitor), wortmannin (10−5 m) (phosphatidylinositol 3-kinase inhibitor). The MVB was perfused for 10 min with atropine (10−5 m) (muscarinic cholinergic receptors antagonist), and/or diphenhydramine (10−5 m) (histamine H1 receptor antagonist) and concentration-response curves to alkaloid 3 (20-80 µg in bolus) were performed. A maximum of 3 curves per preparation wasere constructed.
Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM) of at least five experiments. Means were compared by unpaired Student’s t-test; concentration-response curves by two-ways ANOVA and Bonferroni’s post-hoc test. Differences were considered to be statistically significant with p ≤ 0.05.
HSCE effects on SBP of normotensive and hypertensive rats
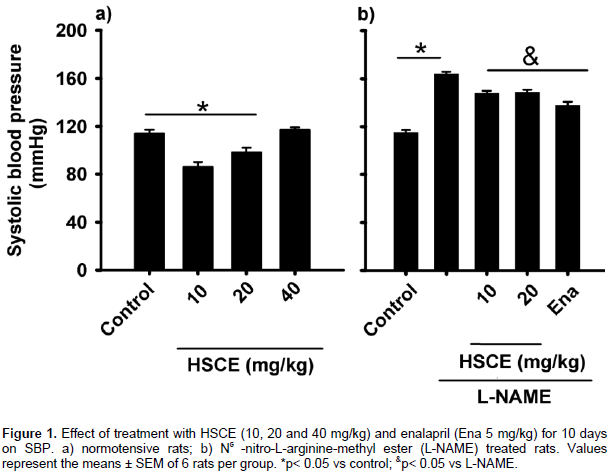
Oral HSCE administration of 10 and 20 mg/kg for 10 days decreased the SBP in normotensive rats (from 115±3 to 85.5±4.5, and 96.8±4.2 mm Hg respectively); the 40 mg/kg dose did not cause SBP reduction (Figure 1a). Chronic NO inhibition by L-NAME was associated with a progressive rise in the SBP, reaching a maximum of 164±2 mm Hg at Day 10, as compared with the control group (115±3 mm Hg). HSCE administration (10 and 20 mg/kg, v. o) produced SBP reduction (148±3 mm Hg). Enalapril also reduced SBP to 131±2.5 mm Hg (Figure 1b).
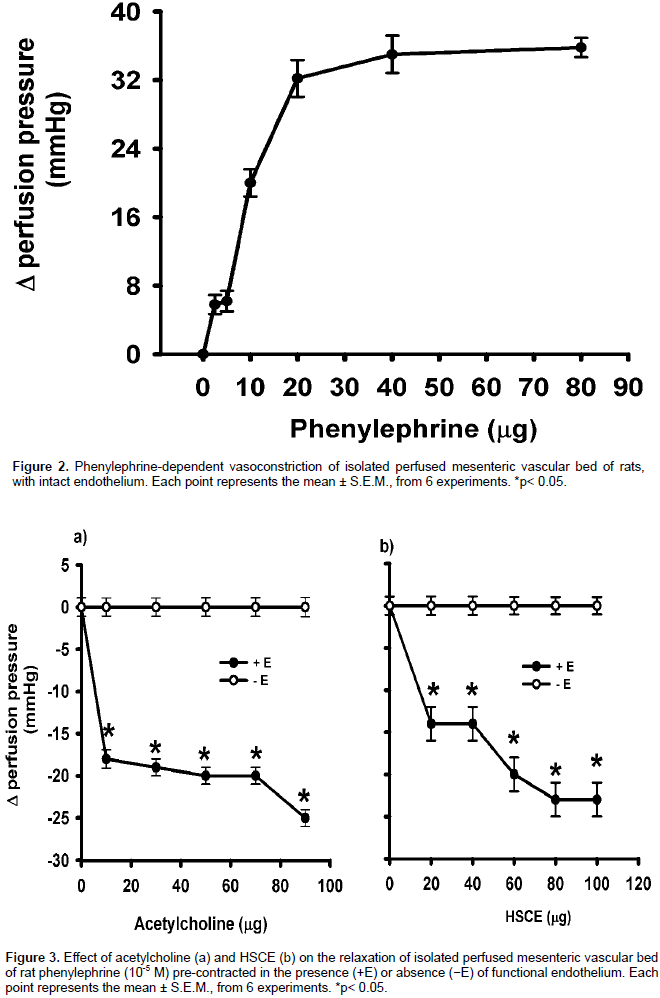
Effect of HSCE on MVB from normotensive rats with and without endothelium
Baseline perfusion pressure of isolated and perfused MVB from normotensive rats was 50±2.2 mm Hg. Phen (0.5-80 µg) bolus administration induced a concentration-dependent vasoconstriction; 40 µg of Phen increased the perfusion pressure to 84±1.2 mm Hg. Constant perfusion with Phen (10-5 M) added to the Krebs’ solution increased the perfusion pressure to 84±1.2 mm Hg. When the perfusion pressure reached a plateau (Figure 2), an ACh bolus injection produced concentration-dependent relaxation, with 90 µg of ACh reducing the perfusion pressure to 25±1 mm Hg (Figure 3a). HSCE administration (10-90 μg) produced concentration-dependent relaxation whereas 70 µg of HSCE decreased the perfusion pressure to 27±3 mm Hg (Figure 3b). When vascular endothelium was removed and pre-contracted with Phen, the relaxant response to ACh and HSCE was significantly diminished (Figures 3a and 3b).
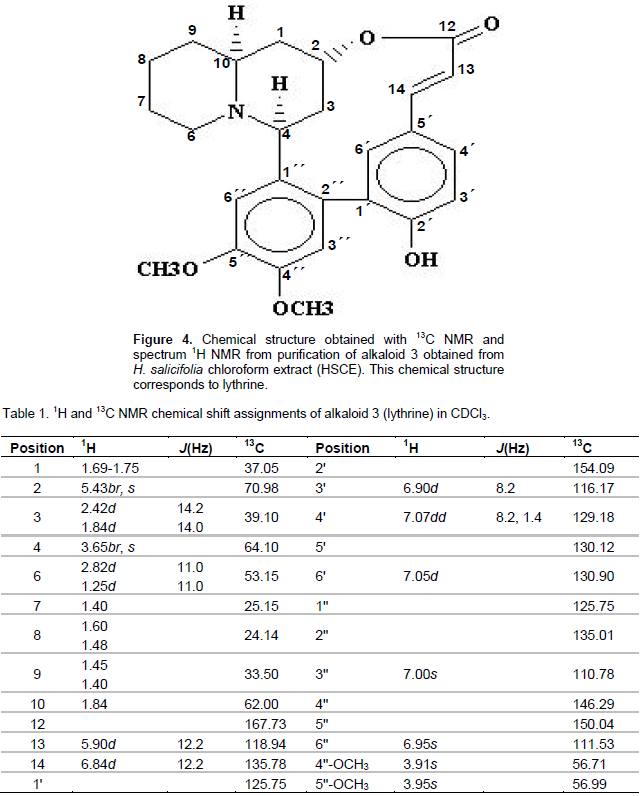
Alkaloids isolation and identification
Four alkaloids were separated by thin layer chromatography; their retention factors (Rf) were as follows: alkaloid 1, Rf = 0.392; alkaloid 2, Rf = 0.87; alkaloid 3, Rf = 0.55; and alkaloid 4, Rf= 0.35. The alkaloids were extracted of silica gel and assayed in normotensive rats and in animals that developed hypertension by angiotensin II administration. Alkaloid 3 showed high anti-hypertensive activity (Guzman et al., 2006), so it was isolated and prepared for structuralchemical identification. Spectroscopic studies identified alkaloid 3 as lythrine, with its chemical structure similar to that of the quinolizidine alkaloids group (Figure 4). The spectroscopic data of the active compound were UV (λmax) 279.9; 1H and 13C NMR (Table 1), corresponding to those reported for lythrine (Rumalla et al., 2008). Thus, in the following experiments, alkaloid 3 is referred as lythrine alkaloid.
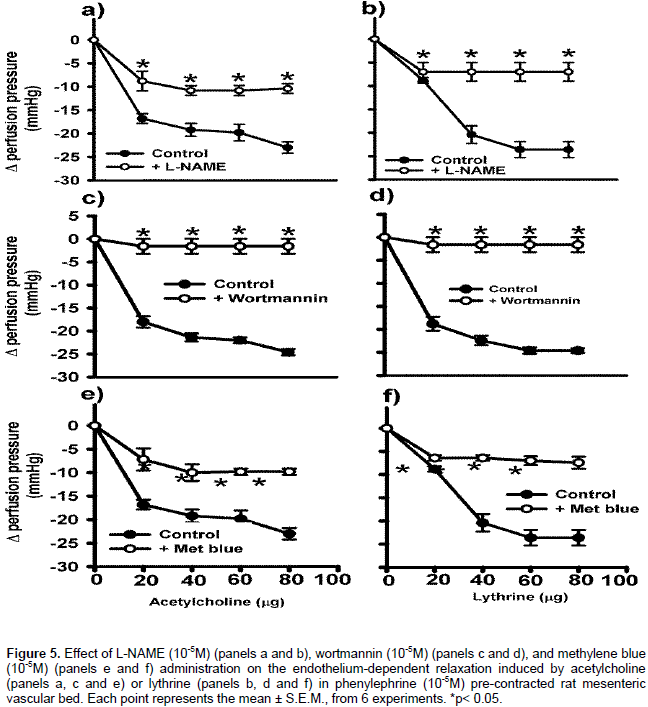
Effect of lythrine on endothelium-derived NO-cyclic GMP pathway in MVB
A concentration-response curve for the lythrine alkaloid on the isolated and perfused MVB from normotensive rats with intact endothelium was constructed. Lythrine did not modify the baseline perfusion pressure (50±2.2 mm Hg). In Phen pre-contracted preparations, constant perfusion with lythrine (20-80 μg) produced concentration-dependent relaxation (8.8±0.6, 20.4±1.9, 23.6±1.7 and 23.6±1.7 mm Hg respectively). In all the concentration-response curves, ACh was the control drug producing similar relaxant effect as lythrine (Figures 5). When the MVB was perfused with 10-4 M l-NAME, the vasodilatation induced by lythrine alkaloid decreased (perfusion pressure 7±2 mm Hg, Figure 5b). A similar effect was observed with ACh (Figure 5a). In the presence of 10−5 M wortmanin, the relaxant effect of lythrine (20-80 μg) was only 1.6±2 mm Hg (Figure 5d), similar to that observed with ACh (Figure 5c). The perfusion with 10−5 M methylene blue decreased the relaxant effect of lythrine. The maximum relaxant effect was 7.4±1.3 mm Hg (Figure 5f), similar to that observed with ACh (Figure 5e). These data suggest that lythrine exerts its relaxant effect onwith functional endothelium through the nitric oxide synthesis pathway.
Lythrine-induced vasorelaxation in MVB treated with atropine and/or diphenhydramine
In an isolated perfused MVB preparation with intact endothelium pre-contracted with Phen in the presence of 10−5 M atropine, the relaxant effect of lythrine (20-80 μg) was decreased (4.8±0.7, 4.8±0.7, 7±1.8, 7±1.5 mm Hg, respectively) (Figure 6b). A similar concentration-response curve was obtained with ACh (Figure 6a), suggesting that the vasodilatory effect of lythrine requires binding to a muscarinic receptor and an intact pathway of the nitric oxide synthesis. In an isolated perfused MVB preparation pre-contracted with Phen, histamine (20-80 μg) produced a relaxant effect (18±1.5, 22±1.03, 24±0.68 and 24±0.51 mm Hg respectively) (Figure 7a). When the preparation was perfused with 10−5 M diphenhydramine, the histamine relaxant effect was blocked. On the other hand, the lythrine relaxant effect was not affected by diphenhydramine (Figure 7b), suggesting that the lythrine vasodilatory effect is independent of the H1 histamine receptors.
H. salicifolia contains alkaloids with antihypertensive activity. Four alkaloids with vasorelaxant effect were obtained from
H. salicifolia chloroform extract (HSCE). The alkaloid with the highest vasorelaxant activity, lythrine, was isolated a decade ago (Rumalla et al., 2008), but pharmacological studies on the compound have not been reported. The results suggest that the HSCE vasorelaxant effect depends on the release of nitric oxide (NO) or a NO-mediated substance. HSCE administration (10 and 20 mg/kg) to normotensive rats or animals with L-NAME-induced hypertension decreased the blood pressure. In MVB preparations with the endothelial cells eliminated by perfusion with distilled water (Criscione et al., 1984), HSCE-induced vasodilatation was significantly reduced, suggesting that it is mediated by the release of endothelium-derived substances. Vascular tone and therefore blood pressure
isare determined by the contractile state of vascular smooth muscle cells within the blood vessel wall. The role of endothelium is of particular importance since it regulates the vascular leiomyocytes tone by releasing potent vasoconstrictor molecules such as endothelin-1 and vasodilator substances such as NO (
Godo and
Shimokawa, 2017).
NO is an important regulator of vascular tone and blood pressure. It has been reported that the pharmacological reduction of NO can lead to hypertension in normotensive rats (Raghavan and Dikshit, 2004; Wang et al., 2017). L-NAME administration decreases NO synthesis and develops hypertension in rats (
Leo et al., 2015). In this study, animals receiving simultaneous administration of L-NAME and HSCE showed lower systolic blood pressure as compared with animals receiving only L-NAME. Similar data were reported for
Nitraria sibirica (Senejouxa et al., 2012),
Bidens pilosa (Bilanda et al., 2017),
Ulmus wallichiana (Syeda et al., 2016),
Antidesma thwaitesianum (Kukongviriyapan et al., 2015), plants with NO-mediated hypotensive effect. The
in vitro studies using the MVB preparation pre-contracted by Phen also support that the vasorelaxant mechanism of lythrine is likely to be mediated by activation of NO release from vascular endothelium, since the removal of endothelial cells led to absence of the response to HSCE.
H. salicifolia exerted an important vasorelaxant effect both
in vivo and
in vitro. The major compound identified in HSCE was the lythrine alkaloid as its vasorelaxant effect has not been previously described.
In this study, its action mechanism was elucidated using in vitro MVB preparations. In the preparation of isolated perfused MVB pre-contracted with phenylephrine and intact endothelium, the effect of the lythrine seemed to be endothelium dependent, as the vasorelaxant action of lythrine was abolished by L-NAME perfusion. The involvement of cGMP in the relaxant and hypotensive effects of lythrine was verified. The soluble guanylate cyclase inhibitor, methylene blue, completely abolished lythrine -induced vasodilatation, an effect similar to that produced by Orthosiphon stamineus (Yam et al., 2016) and Praeruptorin A (Xua et al., 2010). Activation of endothelial nitric oxide synthase (eNOS) depends on the formation of calcium–calmodulin complex (Su et al., 2014). However, eNOS can also be activated by phosphorylation with PI3K, a calcium-independent mechanism (Zhang et al., 2014). The PI3K-dependent activation of eNOS by wine-derived polyphenolic compounds has been reported (Ziberna et al., 2013). In the present study, the MVB was pre-contracted with phenylephrine and wortmannin, a PI3K inhibitor.
Wortmannin strongly reduced lythrine-induced vasodilatation, suggesting that PI3K-dependent activation of eNOS is an important underlying mechanism in the vasodilatory effect of lythrine, similar to that reported for Tapirira guianensis (Rodrigues et al., 2017). Atropine reduced lythrine-induced vasodilatation, suggesting that the vasodilatory effect of lythrine is mediated through the interaction with cholinergic muscarinic receptors. A similar action mechanism has been reported for other herbal remedies, such as O. stamineus (Yam et al., 2016) and Cymbosema roseum (Rocha et al., 2015). To verify if the effect of lythrine is mediated by muscarinic receptors with some degree of specificity, the MVB was perfused with phenylephrine plus diphenhydramine, an H1 receptor antagonist. Lythrine showed vasodilatory effect both in the presence and absence of diphenhydramine, suggesting that it is mediated by muscarinic receptors and not by histamine H1 receptors.