ABSTRACT
The quality of botanicals has been reported to be influenced by their places of cultivation. Although, ginger remains a common health remedy both in Ghana and China, no comparative study exists to the best of the authors’ knowledge, on the anti-inflammatory properties of ginger from these two countries. The aim of this study was to comparatively assess the anti-inflammatory effect of the methanolic extracts of fresh ginger samples from Ghana and China and identify biomarker(s) that is/are diagnostic of acute inflammation via untargeted metabolomics. A phytochemical assessment of the ginger extracts was initially performed with high performance liquid chromatography- quadrupole time-of-flight mass spectrometry (HPLC-Q/TOF-MS). Low (200 mg/kg) and maximum (400 mg/kg) doses the ginger extracts from each country were assessed using Sprague-Dawley (SD) rats against groups for positive (indomethacin, 10 mg/kg) and negative controls (no drug). Blood samples were taken from the retro orbital vein at four time points: 1, 4, 8 and 24 h after induction of inflammation. The levels of tumor necrosis factor-α (TNF-α) and prostaglandin E2 (PGE2) were determined in the plasma samples. The metabolomics study was also performed with HPLC-QTOF/MS and the data analyzed with MetaboAnalyst 3.0 and SIMCA 14.1. Phytochemical evaluation revealed marked differences in terms of the compounds deemed to have anti-inflammatory activities in the ginger samples from the two countries. Ginger extract from Ghana showed superior anti-inflammatory effects over that from China. In the metabolomics study, L-valine was identified as the diagnostic biomarker of acute inflammation from 8 differential metabolites identified.
Key words: Anti-inflammatory effects, biomarker, ginger, China, Ghana, L-valine, metabolomics.
Abbreviation:
SD, Sprague-Dawley; TNF-α, tumor necrosis factor-α; PGE2, prostaglandin E2; HPLC/QTOF-MS, high performance liquid chromatography-quadrupole time-of-flight mass spectrometry; ELISA, enzyme-linked immunosorbent assay; OPLS-DA, orthogonal partial least-squares discrimination analysis; VIP, variable importance in projection; ANOVA, analysis of variance; ACN, acetonitrile; NR, normal rats; NC, negative control; SIMCA, soft independent modelling by class analolgy.
Inflammation is often characterized by redness, oedema, fever, pain and loss of function. These usually constitute the biological responses of the body to local injury and infection. It involves a cascade of events initiated by numerous stimuli which include ischaemia, thermal and physical injury, infectious agents and antigen-antibody
interaction (Alolga et al., 2015; Wang et al., 2014). Non-steroidal anti-inflammatory drugs (NSAIDs) remain the mainstay in the treatment of acute and chronic inflammation. However, the use of these drugs comes with its attendant side effects such as peptic ulcer, nausea, salt and water retention, severe gastritis, vomiting, worsening of renal function in renal or cardiac and cirrhotic patients, hypersensitivity, etc (Kumari et al., 2014). Based on these adverse effects, attention has been drawn to alternative sources of drugs that offer similar therapeutic effects and devoid of all the aforementioned issues. The use of natural medicine is currently receiving a lot of scientific attention in this regard, particularly the herbs that have been recognized and used in traditional medicinal practice.
Metabolomics has emerged as an invariably reliable analytical technology in systems biology, complementary to genomics and proteomics. It gives a quantitative measurement of metabolic perturbations of living systems exposed to pathophysiological stimuli or genetic modifications using biological samples such as urine, plasma, serum, tissues and single cells. Using metabolomics, biomarkers which are diagnostic of the prognosis of diseases can be discovered. The mechanisms underlying the perturbed biochemical states can then be further investigated with the ultimate aim of finding the appropriate therapeutic interventions. The rhizome of Zingiber officinale Roscoe (ginger) aside from its popular use as a spice, has a long history of use as a medicinal herb. It is commonly used to treat cold, fever, headache, nausea, etc. Generally the reported pharmacological effects of ginger include anti-neuroinflammatory (Ho et al., 2013), anti-viral (Chang et al., 2013), anti-ulcerative colitis (Hsiang et al., 2013; El-Abhar et al., 2008), anti-oxidant (Kota et al., 2012), anti-dyslipidemic (Bhandari et al., 2005), anti-emetic (Sharma et al., 1997) and immunomodulatory effects (Zhou et al., 2006) among a host of others.
The major bioactive compounds responsible for these activities are the gingerols and their dehedydrated derivatives, shogaols and gingerone (a dehydrated derivative of shogaols) (Ho et al., 2013). Even though there is scientific data justifying the anti-inflammatory potential of ginger, there is no report to the best of the authors’ knowledge on a comparative investigation in this regard on ginger from China and Ghana. The aim of this study therefore was to comparatively assess the anti-inflammatory potentials of the methanolic extracts of fresh ginger from these two countries. The authors’ decision was informed by the fact that from a previous study (Mais et al., 2018), they observed phenotypic differences between forty batches of fresh ginger samples from these two geographical locations. This study therefore aimed to investigate whether or not these differences translate to significant differences in terms of the bioactivities of the ginger samples. It also sought to determine the effects of these ginger extracts at the metabolic level via metabolomics. By that, the authors identified the biomarkers of inflammation, from which they would find those that are diagnostic. Thus, using a modified form of the Carrageenan-induced paw oedema model, the anti-inflammatory potential of extracts from the two countries was investigated.
Chemicals and plant material
HPLC grade acetonitrile and formic acid were purchased from Merck (Darmstadt, Germany) and analytical grade methanol was obtained from Nanjing Chemical Factory (Nanjing, China). Distilled water was purified by Milli-Q system (Millipore, USA). Carrageenan was bought from Aladdin Chemistry Co.Ltd and Kits for enzyme-linked immunosorbent assay (ELISA) were purchased from Calvin Co., Ltd (Suzhou, China). Twenty (20) batches of fresh rhizomes of ginger were purchased from three different places in Ghana: Makola market in Accra, Cape Coast central (Cape coast) and Community 3 market (Tema). In a similar way, 20 batches of fresh ginger rhizomes were bought from four Provinces in China: Sichuan, Yunnan, Jiangsu and Shandong. The samples were identified and authenticated as true samples of Z. officinale Roscoe based on their macroscopic, organoleptic, microscopic and phytochemical characteristics. Reference standards of (6)-Shogaol, (6)-gingerol and (10)-gingerol were purchased from Sigma-Aldrich Co. (St. Louis, MO).
Preparation of ginger extracts
For phytochemical characterization
The fresh rhizomes were peeled manually, washed and milled. All batches of the ginger samples were milled separately. From the milled samples, 5 g of each batch was weighed and to each quantity, 20 mL of methanol was added and left under room temperature for forty-eight hours for cold extraction to take effect. After extraction, the samples were ultrasonicated for 1 h 30 min at 100 Hz and filtered. The filtrate was centrifuged at 16627 xg for 5 min at 4°C in an Eppendorf 5430R centrifuge and 100 µL aliquots of the supernatant from each batch was transferred into and mixed in separate Eppendorf tubes, thus, producing two mixed samples of the extracts, one for each country. The mixed samples were vortexed for 30 s and finally filtered through a sintered glass filter of pore size 0.22 µm and a 100 mL aliquot transferred to sample vials for HPLC/QTOF-MS analysis.
For animal studies
A quantity (43 g) of each pulverized sample from Ghana was mixed together and macerated with 1 L of methanol for 48 h. The same was done for the samples from China. The macerated samples were ultrasonicated at 100 Hz for 90 min at 27°C and filtered. The filtrate was then concentrated with the rotary evaporator at 65°C. The resultant concentrates were air-dried at room temperature and reconstituted with water to be given to the rats.
Animals and treatment
Male Sprague-Dawley (42) rats 4 weeks old (60 to 90 g) were obtained from the Qinglong Mountain Animal Center. They were housed in acryl fibre cages at 24 ± 2°C, humidity 50± 1.0% and kept on a 12 h light/dark cycle and fed with standard pellet diet and water ad libitum. They were kept for 7 days before the study so as to enable them acclimatize with their new environment. They were fasted 12 h prior to the study. The animal care and use complied with the Provisions and General Recommendations of the Chinese Experimental Animals Administration Legislation. Ethical clearance was given by the Animal Ethics Committee of China Pharmaceutical University. Inflammation was induced using the carrageenan-induced paw oedema method with modifications (Winter et al., 1962). The 42 rats were divided into 7 groups, 6 in each group.
The drug administration and animal treatment was done as follows: Group 1 received only distilled water and no induced inflammation (Normal rats); Group 2 received distilled water but had induced inflammation (Negative Control); Group 3 received 10 mg/kg of indomethacin and had induced inflammation (Positive Control); Group 4 was given 200 mg/kg of ginger extract from Ghana and induced inflammation (Low Dose, Ghana); Group 5 was given 400 mg/kg of ginger extract from Ghana and induced inflammation (Maximum Dose, Ghana); Group 6 was administered 200 mg/kg of ginger extract from China and induced inflammation (Low Dose, China) and Group 7 was given 400 mg/kg of ginger extract from China and induced inflammation (Maximum Dose, China). After 1 h of administering the various doses of the drugs, 0.1 ml of carrageenan (0.1% in normal saline) was injected into the subcutaneous tissue of the right hind paw of each animal.
Blood collection and preparation
Blood samples were taken from the retro orbital plexus of the rats in each group at the following time points: 1, 4, 8 and 24 h after the induction of inflammation into heparinized tubes. At each time point, the blood samples were centrifuged at 9838 xg for 10 min at 4°C and the supernatant collected and stored at -80 °C until analysis. For analysis, the plasma samples were thawed at room temperature. To a 50µL aliquot of plasma was added 150 µL of methanol, vortexed for 1 min and centrifuged at 9838 xg and 4°C for 10 min. The supernatant was collected, dried under a gentle stream of nitrogen gas and reconstituted with 100 µL of standard solution (ketoprofen 1 µg/mL and 2-Chloro-L-phenylalanine 100 ng/mL in 20% acetonitrile aqueous solution). The samples were centrifuged again at 9838 xg and 4°C for 10 min and the supernatant of each sample drawn into the sample vials for HPLC/QTOF-MS analysis.
LC-MS analyses
Phytochemical characterization of methanolic extracts of ginger
Chromatographic evaluations were performed with an Agilent 1290 series (Agilent Corp., Santa Clara, CA, USA) HPLC system equipped with a binary pump, micro degasser, an auto sampler and a temperature-controlled column compartment. Chromatographic separations were done on a Zorbax RRHD Eclipse Plus C18 column (2.1 mm x 50 mm, 1.8 µm). The mobile phase consisted of 0.1% aqueous formic acid (A) and acetonitrile, ACN (B). This mobile phase system was run in a gradient elution as follows: 45% B at 0-10 min; 45-48% of B at 10-15 min; 48-60% of B at 15-17 min; 60% of B at 17-43 min; 60-67% of B at 43-45 min; 67-69% of B at 45-48 min; 69-71% at 48-58 min; 71-45% of B at 58-66 min at a flow rate of 0.5 mL/min and sample injection volume of 1 µL. Before each injection, the column was equilibrated for 10 min with 45% of phase B. Separated components were detected with Agilent 6530 Q/TOF mass spectrometer (Agilent Corp., Santa Clara, CA, USA) equipped with an ESI interface. The operating parameters were as follows: drying N2 gas flow rate, 10.0 L/min; temperature, 330°C; nebulizer, 35 psig; capillary, 3000 V; skimmer, 60 V; OCT RFV, 250 V. The samples were analyzed in the positive ion mode and mass spectra data recorded across the m/z range of 100-2000.
Analysis of plasma samples
Chromatographic separations were performed with an Agilent 1290 series (Agilent Corp., Santa Clara, CA, USA) HPLC system equipped with a binary pump, micro degasser, an autosampler and a temperature-controlled column compartment. Chromatographic separations were done on an ACQUITY UPLC HSST3 column (1.8 µm, 2.1 mm ×100 mm; Waters, Ireland). The mobile phase consisted of two solvents, A and B. Mobile phase A was 0.1% aqueous formic acid and B was acetonitrile and 0.1% formic acid. This mobile phase system was run in a gradient elution as follows: 1-10 % B at 1 min; 70% of B at 1-3 min; 70-85% of B at 3-8 min; 100% of B at 8-11 min; 1% of B at 11-13 min. The oven temperature was set to 25°C, the injection volume was 0.5 µL and the flow rate set at 0.4 mL/min. Before each injection, the column was equilibrated for 3 min with 1% of phase B. Separations were detected with Agilent 6545A Q/TOF mass spectrometer (Agilent Corp., Santa Clara, CA, USA) equipped with an ESI interface. The operating parameters were as follows: drying N2 gas flow rate, 8 L/min; temperature, 320 °C; nebulizer, 35 psig; capillary, 3000 V; skimmer, 65 V; OCT RFV, 750 V, fragmentor 100 V. The samples were analyzed in both the positive ion mode and mass spectra data recorded across the m/z range of 50-1000. The reference masses, 121.0509 (Purine) and 922.0098 (HP-0921) were used for internal mass calibration during the runs.
Test for TNF-α and PGE2
The TNF-α and PGE2 levels in the supernatants were measured using enzyme-linked immunosorbent assay (ELISA) kits (Calvin Co. Ltd, Suzhou, China) according to the manufacturer’s instructions.
Data processing, statistical analysis and metabolite identification
LC-MS raw data files generated from the analyses were converted to mzData format using DA reprocessor (Agilent) with the threshold set at 5000 counts. Using the open-source software XCMS, the following were carried out, peak finding, filtering and alignment. MetaboAnalyst 3.0 was then used to achieve the following: (1) Missing value processing; (2) Data filtering (IQR); (3) Sample Normalization (using median); (4) Data transformation (Log); (5) Data scaling using Auto scaling option. These normalized data were then fed into SIMCA 14.1 for multivariate analysis. Using the normalized data for the group of normal rats (NR) against the negative control (NC) at the various time points, differential metabolites responsible for the discrimination and indicative of inflammation were obtained using SIMCA 14.1 supervised orthogonal partial least-squares discrimination analysis (OPLS-DA).
The criteria for the differential metabolites included those with VIP values greater than 1 (VIP >1), fold change greater than 2 (FC > 2) and p-value < 0.05. Differential metabolites were deemed diagnostic if they were found in all time points. The metabolites were identified based on their mass spectral data using the Metlin database with a similarity of more than 70%. The amounts of L-valine and the pro-inflammatory cytokine (TNF-α) and PGE2 in the various groups were expressed as mean ± standard error of mean (S.E.M). Differences between control and treatment groups were analyzed using one-way analysis of variance (ANOVA) with GraphPad Prism 5.0 software as described in Dunnett’s test. p-values < 0.05 were considered significant, p-values < 0.01 were considered as very significant while p< 0.001 were considered as highly significant.
Phytochemical evaluation
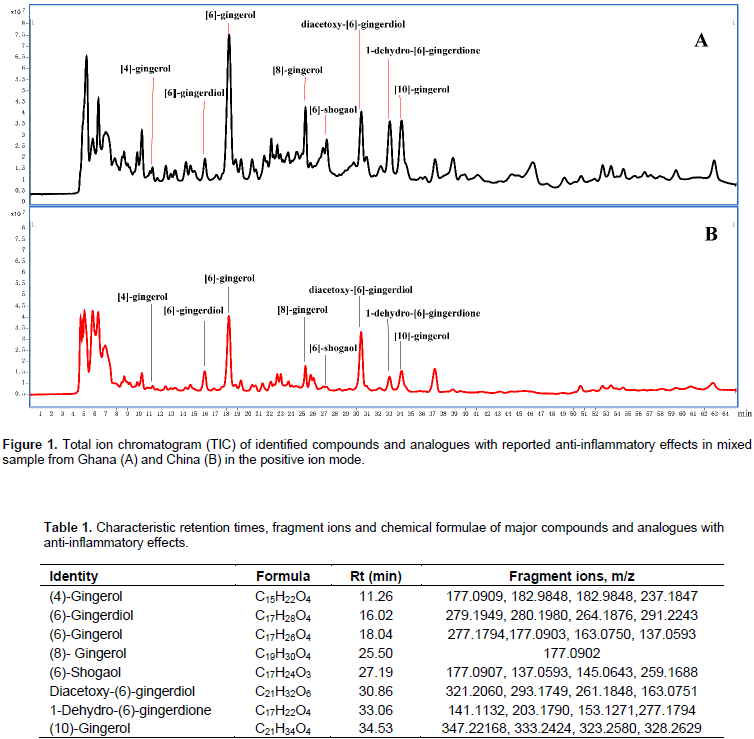
Attention was paid to the major peaks accounting for the difference between the samples from Ghana and China. The compounds and their analogues that have been reported to possess anti-inflammatory activities were also specifically focused on. Hence, eight compounds were assigned their corresponding peaks as shown in Figure 1. The identification was based on fragmentation patterns in comparison with literature and reference compounds (Table 1).
Metabolic changes in response to inflammation
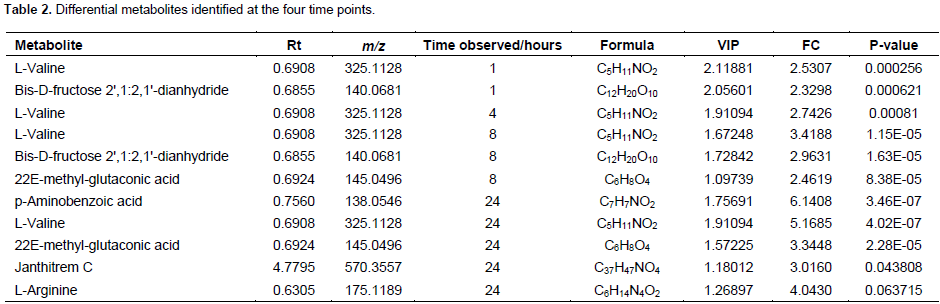
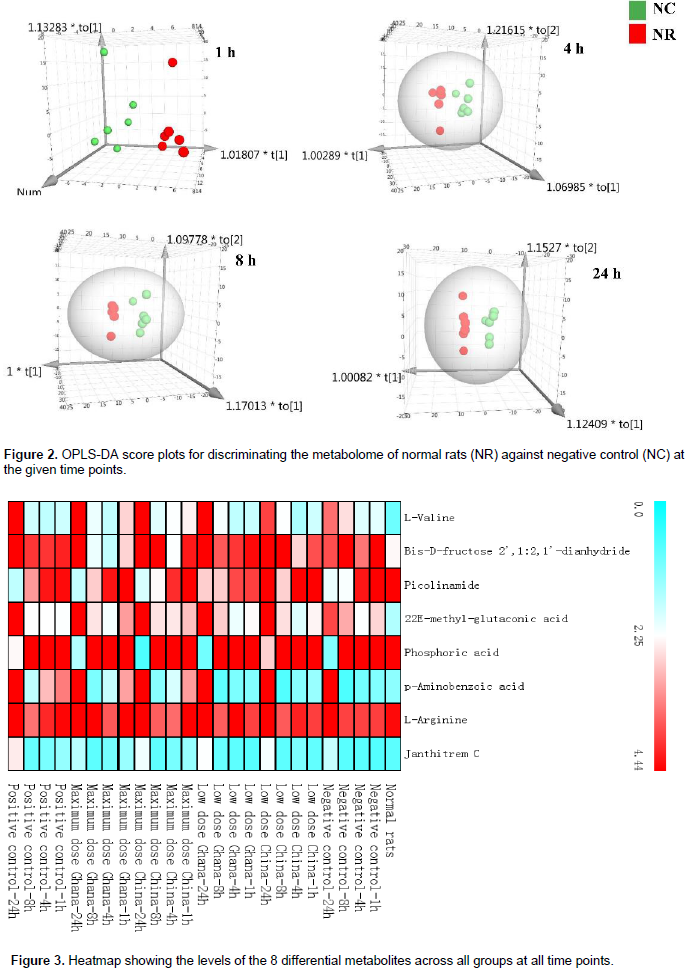
A total of 156 metabolite peaks were extracted from all time points using the NR and NC groups. Based on the selection and inclusion criteria earlier highlighted (VIP >1, FC >2, p-value <0.05), eight metabolites were obtained. From these metabolites, two were detected after 1 h, one metabolite was detected after 4 h, three metabolites after 8 h and five metabolites after 24 h of inflammation as shown in Table 2. Figure 2 gives the OPLS-DA plots discriminating the metabolome of NR from NC groups at the selected time points. There is discrimination between the two groups at the four time points but this becomes more obvious with increase in time of inflammation. Thus, at 24 h post inflammation induction, the metabolomes of the NR and NC groups were much more distinct than at 1, 4 and 8 h. Figure 3 is the heatmap presentation of all eight metabolites in the various groups. From the results, it was observed that L-valine which was detected from the first time point, 1 h, was consistent at all-time points in increasing amount. This was thus, selected as the diagnostic biomarker of acute inflammation from the 8 eight differential metabolites identified.
Effect of ginger extracts on L-valine
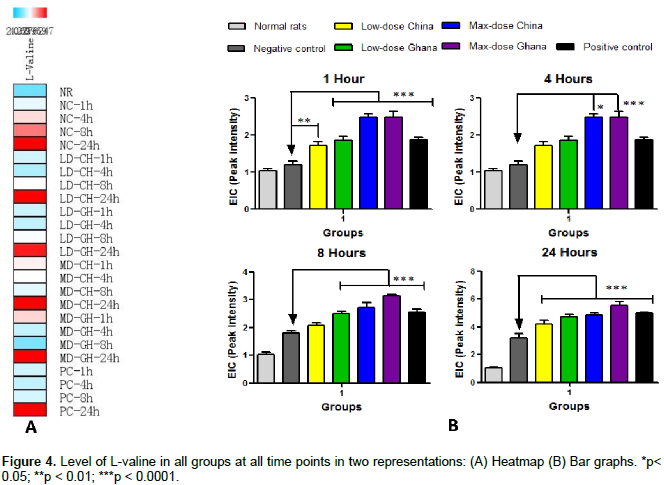
The effect of the ginger extracts on the level of L-valine at the four time points is summarized in Figure 4.The amount of L-valine increased with time upon the administration of both the low and high doses of the ginger extracts from the two countries. Significant difference in the level of L-valine as a result of the administration of the extracts from the two sources was observed at 8 and 24 h. However, the differences between the various treatment groups and the NC group was significant at all the time points.
Effect of ginger extracts on TNF- α and PGE2 production
The levels of pro-inflammatory cytokines as a result of the administration of the extracts of ginger from the two countries decreased in a dose-dependent manner. The anti-inflammatory effect of the extract from Ghana was more pronounced than that from China. As shown in Figure 5, significant differences were observed for the levels TNF-α and PGE2 at 4 and 8 h.
In a recent comparative metabolomics study, six marker compounds responsible for the discrimination of forty batches of fresh ginger samples from Ghana and China were reported (Mais et al., 2018). The use of metabolomics revealed little difference between the ginger samples. The study therefore proved that geographical location and hence differential climatic conditions have an influence on the chemical composition of the ginger rhizomes. This study aimed to investigate the effect of these differences earlier reported on the bioactivities of the ginger samples, that is, against carrageenan-induced paw oedema. The comparative anti-inflammatory effect of the methanolic extracts of the ginger samples in SD rats was also investigated from the lenses of metabolomics. The carrageenan-induced paw oedema is a good acute inflammatory experimental animal model. The biological response to inflammation due to carrageenan injection is biphasic. In the early phase (0-2.5 h post-carrageenan injection), there is release of histamine and serotonin and the involvement of platelet activating factor and arachidonic acid metabolites (Wang et al., 2014; Boughton-Smith et al., 1993).
Consequently, there is infiltration of neutrophils and accumulation of plasma fluid into the interstitial spaces as a result of increased vascular permeability (Kumari et al., 2014; Vinegar et al., 1987). All these processes lead to the formation of oedema. The second phase of oedema formation is due to increased synthesis of prostaglandins, production of free oxygen radical and massive infiltration of neutrophils usually occurring 3-6 h post-carrageenan injection. In the present study, even though the extracts of ginger from the two countries inhibited the inflammation at both phases, the anti-inflammatory effects were more pronounced at 4 and 8 h post-carrageenan injection. The ginger extract from Ghana exhibited better anti-inflammatory effects at both the low and maximum doses than those from China. The levels of the pro-inflammatory cytokine (TNF-α) and PGE2 were significantly lower in the groups treated with the extracts of ginger from Ghana when compared with those treated with the ginger extracts from China. This can be accounted for by the differential amounts of (6)-shogaol, (6)-gingerol, (8)-gingerol and (10)-gingerol in the extracts of ginger from the two countries.
As shown in Figure 1, the corresponding peaks of these compounds are higher in the mixed sample from Ghana than that from China. These compounds and their analogues have been reported to be responsible for the anti-inflammatory potential of ginger (Ho et al., 2013; Young et al., 2005; Dugasani et al., 2010; Li et al., 2012; Lantz et al., 2007). The outcome of the phytochemical evaluation also revealed (6)-gingerol as the most abundant chemical constituent of the fresh ginger. This finding is in agreement with the study of Ho et al. (2013). From the perspective of metabolomics, eight differential metabolites were identified at the four time points. From these eight differential metabolites, L-valine was taken as the diagnostic biomarker of acute inflammation. The effect of the extracts and the positive control on the general state of inflammation with particular focus on L-valine was analyzed. It was realized that the level of L-valine increased more upon drug treatment than in the negative control group. The levels of increase corresponded with the doses, thus, higher amount of L-valine was present in the groups given maximum doses of the ginger extract than the low dose groups.
However, the groups treated with the ginger extracts from Ghana gave higher levels of L-valine at both low and maximum doses (Figure 4). L-valine is part of a group of amino acids known as the branched chain amino acids, BCAA, thus, L-leucine, L-isoleucine and L-valine. These proteins together promote normal growth, regulate blood sugar levels, energize the body and are crucial in tissues repair (Cruzat et al., 2014). The BCAA were found in clinical studies to be instrumental in the treatment and management of hepatic encephalopathy (Rossi et al., 1986). L-valine has been reported to have marked anti-inflammatory and moderate analgesic activities (Meyers et al., 1979; Mine and Zhang, 2015; Khanna et al., 1982). It has also been found to play a supportive role in the central nervous system and cognitive function (Jellinger et al., 1978). As an essential amino acid, it cannot be biosynthesized in the body of the rats but rather gotten from diet. Therefore, its increased amount with time could be due to the contributions of the extracts of ginger (since it is an endogenous metabolite) and as a defense mechanism by the rats to combat the inflammation. The findings confirm the hypothesis that differences at the metabolite level of the ginger samples do translate into marked differences in bioactivity, that is, as anti-inflammatory agents. This study lends credence to the fact that differences in geographical location and climate does have effect on the quality of medicinal plants cultivated.
The methanolic extract of ginger from Ghana exhibited better anti-inflammatory effect than that from China. From this metabolomics study, L-valine proved to be a reliable biomarker of acute inflammation. It is recommended that more samples from other countries in Africa be compared with those from other continents so as to determine the region that produces the best ginger in terms of safety and pharmacological activity. The complementary negative ion mode of LC-MS should be employed in the metabolomics study so as to aid in the identification of more diagnostic biomarkers of acute inflammation.
The authors have not declared any conflict of interests.
REFERENCES
|
Alolga RN, Amadi SW, Onoja V, Assanhou AG, Muyaba M, Kassim SA (2015). Anti-inflammatory and antipyretic properties of Kang 601 heji, a traditional Chinese oral liquid dosage form. Asian Pac. J. Trop. Biomed. 5(11):921-927.
Crossref
|
|
|
|
Bhandari U, Kanojia R, Pillai KK (2005). Effect of ethanolic extract of Zingiber officinale on dyslipidaemia in diabetic rats. J. Ethnopharmacol. 97(2):227-230.
Crossref
|
|
|
|
|
Boughton-Smith NK, Deakin AM, Follenfant RL, Whittle BJ, Garland LG (1993). Role of oxygen radicals and arachidonic acid metabolites in the reverse passive Arthus reaction and carrageenin paw oedema in the rat. Br. J. Pharmacol. 110(2):896-902.
Crossref
|
|
|
|
|
Chang JS, Wang KC, Yeh CF, Shieh DE, Chiang LC (2013). Fresh ginger (Zingiber officinale) has anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J. Ethnopharmacol. 145(1):146-151.
Crossref
|
|
|
|
|
Cruzat VF, Krause M, Newsholme P (2014). Amino acid supplementation and impact on immune function in the context of exercise. J. Int. Soc.Sport Nutr.11(1):61.
Crossref
|
|
|
|
|
Dugasani S, Pichika MR, Nadarajah VD, Balijepalli MK, Tandra S, Korlakunta JN (2010). Comparative antioxidant and anti-inflammatory effects of (6)-gingerol, (8)-gingerol, (10)-gingerol and (6)-shogaol. J. Ethnopharmacol. 127(2):515-520.
Crossref
|
|
|
|
|
El-Abhar HS, Hammad LN, Gawad HS (2008). Modulating effect of ginger extract on rats with ulcerative colitis. J. Ethnopharmacol. 118(3):367-372.
Crossref
|
|
|
|
|
Ho SC, Chang KS, Lin CC (2013). Anti-neuroinflammatory capacity of fresh ginger is attributed mainly to 10-gingerol. Food chem. 141(3):3183-3191.
Crossref
|
|
|
|
|
Hsiang CY, Lo HY, Huang HC, Li CC, Wu SL, Ho TY (2013).Ginger extract and zingerone ameliorated trinitrobenzene sulphonic acid-induced colitis in mice via modulation of nuclear factor-kappaB activity and interleukin-1beta signalling pathway. Food chem. 136(1):170-177.
Crossref
|
|
|
|
|
Jellinger K, Riederer P, Rausch WD, Kothbauer P (1978). Brain monoamines in hepatic encephalopathy and other types of metabolic coma. J. Neural. Transm. 14:103-120.
|
|
|
|
|
Khanna N, Jain P, Pendse V (1982). L-valine as an anti-inflammatory agent. Indian J. Pharmacol. 14(2):149.
|
|
|
|
|
Kota N, Panpatil VV, Kaleb R, Varanasi B, Polasa K (2012). Dose-dependent effect in the inhibition of oxidative stress and anticlastogenic potential of ginger in STZ induced diabetic rats. Food Chem. 135(4):2954-2959.
Crossref
|
|
|
|
|
Kumari KD, Weerakoon TC, Handunnetti SM, Samarasinghe K, Suresh TS (2014). Anti-inflammatory activity of dried flower extracts of Aegle marmelos in Wistar rats. J. Ethnopharmacol. 151(3):1202-1208.
Crossref
|
|
|
|
|
Lantz RC, Chen GJ, Sarihan M, Solyom AM, Jolad SD, Timmermann BN (2007).The effect of extracts from ginger rhizome on inflammatory mediator production. Phytomedicine 14(2):123-128.
Crossref
|
|
|
|
|
Li F, Nitteranon V, Tang X, Liang J, Zhang G, Parkin KL, Hu Q (2012). In vitro antioxidant and anti-inflammatory activities of 1-dehydro-(6)-gingerdione, 6-shogaol, 6-dehydroshogaol and hexahydrocurcumin. Food chem. 135:332-337.
Crossref
|
|
|
|
|
Mais E, Alolga RN, Wang SL, Linus LO, Yin X, Qi LW (2018). A Comparative UPLC-Q/TOF-MS-based Metabolomics approach for distinguishing Zingiber officinale Roscoe of two geographical origins. Food chem. 240:239-244.
Crossref
|
|
|
|
|
Meyers BE, Moonka DK, Davis RH (1979). The effect of selected amino acids on gelatin-induced inflammation in adult male mice. Inflammation 3(3):225-233.
Crossref
|
|
|
|
|
Mine Y, Zhang H. (2015).Calcium-sensing receptor (CaSR)-mediated anti-inflammatory effects of L-amino acids in intestinal epithelial cells. J. Agric. Food Chem. 63(45):9987-9995.
Crossref
|
|
|
|
|
Rossi Fanelli F, Cangiano C, Capocaccia L, Cascino A, Ceci F, Muscaritoli M, Giunchi G (1986). Use of branched chain amino acids for treating hepatic encephalopathy: clinical experiences. Gut. 27 (Suppl 1):111-115.
Crossref
|
|
|
|
|
Sharma SS, Kochupillai V, Gupta SK, Seth SD, Gupta YK (1997). Antiemetic efficacy of ginger (Zingiber officinale) against cisplatin-induced emesis in dogs. J. Ethnopharmacol. 57(2):93-96.
Crossref
|
|
|
|
|
Vinegar R, Truax JF, Selph JL, Johnston PR, Venable AL, McKenzie KK (1987). Pathway to carrageenan-induced inflammation in the hind limb of the rat. Fed. Proc. 46:118-126.
|
|
|
|
|
Wang Y, Chen P, Tang C, Wang Y, Li Y, Zhang H (2014). Antinociceptive and anti-inflammatory activities of extract and two isolated flavonoids of Carthamus tinctorius L. J. Ethnopharmacol.151(2):944-950.
Crossref
|
|
|
|
|
Winter CA, Risley EA, Nuss GW (1962). Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc. Soc. Exp. Biol. Med. 111(3):544-547.
Crossref
|
|
|
|
|
Young HY, Luo YL, Cheng HY, Hsieh WC, Liao JC, Peng WH (2005). Analgesic and anti-inflammatory activities of (6)-gingerol. J. Ethnopharmacol. 96(1):207-210.
Crossref
|
|
|
|
|
Zhou HL, Deng YM, Xie QM (2006).The modulatory effects of the volatile oil of ginger on the cellular immune response in vitro and in vivo in mice. J. Ethnopharmacol. 105(1):301-305.
Crossref
|
|