Guiera senegalensis is a medicinal plant that is widely used in West Africa against many illnesses. In this work, the antiasthmatic properties of ethanol leaf extract of the G. senegalensis were evaluated by using various in vitro animal models. In vitro models like isolated guinea pig tracheal chain preparation, isolated guinea pig ileum and rabbit jejunum preparations were studied to evaluate possible mechanism by which extract shows relaxant activity. The preliminary phytochemical screening of the extract revealed the presence of alkaloids, anthraquinones, flavonoids, saponins and tannins. The study showed that the extract contained phytochemicals such as flavonoids, alkaloids, steroids, tannins and saponins. Isolated tracheal smooth muscles contractions induced by histamine was significantly (p<0.01) reduced by the extract, so also that induced by acetylcholine at concentrations of 4 and 8 mg/ml. The findings were similar to that of 0.4 µg/ml isoprenaline. Guinea pig and rabbit jejunum contractions induced by histamine and acetylcholine were significantly (p<0.01) reduced by the extract. These observed effects can be attributed to the presence of phytochemicals such as flavonoids, alkaloids and steroids in the extract. The results of these studies indicated ethanol extract of G. senegalensis possess antiasthmatic activity.
Asthma is a respiratory disease characterized by recurrent episodes of wheezing, chest tightness, cough and difficulty breathing brought about by bronchial constriction, inflammation, and excessive mucus secretion due to bronchial hyperesponsiveness (GINA, 2014). Although advancement in the treatment of asthma is on the increase, a great number of individuals have been affected by asthma. It has been estimated that about 300 million people in both developed and developing countries suffer asthma attacks worldwide (GINA, 2015). Global prevalence of asthma has been approximated to be 10% among children and 5% among adults population (Rathore et al., 2011). In Nigeria, asthmatic children has been reported to be between 5.1 and 14.3% (Musa and Aliyu, 2014) while estimate for adult Nigerians having asthma has been put to 10% (Desalu et al., 2013). Prevalence of asthma has been estimated to increase by 59% worldwide in 2025 (Masoli et al., 2004). This can be attributed to increased industrialization and urbanization.
Although, pharmaceutical drugs are the major treatments given, a large number of patients worldwide (especially in Africa and Asia) still patronize traditional medicines as an alternative (WHO, 2013). It has been reported that 75 to 90% of the rural populations worldwide still depend on herbal preparations as their main source of medicines (Shettima et al., 2013). Research in traditional medicine is also increasing worldwide and has been encouraged by the World Health Organization because of the large number of patronizers of such kind of treatment for their healthcare (WHO 2013). Guiera senegalensis is used as a medicinal plant by many cultures and researches carried out on the medicinal uses of the plant turn out to give good results (Jigam et al., 2011). The plant has been used traditionally to treat various diseases including asthma and its use in treatment of pulmonary problems has been documented by Dénou et al., (2016) and Saraswathy et al. (2014). The plant could therefore be considered important in medical interventions and its potential as anti-asthmatic be exploited. This may generate data that can be used to complement pharmaceutical drugs for the benefit of humanity. This study therefore, aimed at evaluating the potential of ethanol extract of G.senegalensis (ELEGS) as anti-asthmatic.
Drugs and chemical
The following drugs and chemicals were used in the experiment: Acetylcholine chloride (Sigma Chemicals, USA), histamine diphosphate (Sigma Chemicals, USA), atropine sulphate (Shandong Shenglu Pharmaceutical Co., Ltd.), isoprenaline hydrochloride (Sigma-Aldrid), mepyramine, sodium hydrogen carbonate (BDH Chemicals Ltd Poole, England), sodium chloride (Johnson and Solomon Ltd. London, England), D-glucose (BDH Chemicals Ltd. Poole, England), calcium chloride (BDH Chemicals Ltd. Poole, England), sulfuric acid, ferric chloride, acetic anhydride and ethanol.
Experimental animals
Adult guinea pigs (weighing 400 to 450 g) and rabbits (weighing 3.0 to 3.5 kg) were obtained from the animal house of the Department of Pharmacology and Therapeutics, Faculty of Pharmaceutical Sciences, Ahmadu Bello University, Zaria. The animals were kept in propylene cages under favourable conditions prior to the study. Water and feed were provided ad libitum according to the ethical guidelines of Ahmadu Bello University for the care and use of animals in research.
Plant
Fresh leaves of G. senegalensis were collected from Tofa Local Government Area in Kano State of Nigeria. Taxonomic identification was done and voucher number (BUKHAN0032) was deposited at the herbarium of Biological Sciences Department, Bayero University, Kano.
Extraction
Leaves of the plant material were thoroughly washed with distilled water to remove dirt and soil and dried under the shade. The plant material was then pulverized into fine powder and the powder macerated in 70% ethanol for 48 h. The resultant extract was filtered and then through Whatman® Filter Paper No.1, 185 mm. The filtrate was concentrated in an oven at 40°C.
Preliminary phytochemicals screening
Phytochemicals screening of the ethanol leaf extract of G. senegalensis (ELEGS) were carried out to determine the presence of secondary metabolites such as alkaloids, steroids and flavonoids as described by Tiwari et al. (2011).
Isolated guinea pig trachea preparation
Guinea pig of either sex weighing 400 to 450 g were starved overnight but allowed free access to water. The animals were then sacrificed and the entire tracheae was dissected out and removed and cut into individual rings. Twelve rings were tied together and mounted in 25 ml organ bath containing Krebs physiological solution. The tissue was maintained at 37°C under tension of 1 g and constantly aerated with carbogen (Vogel and Vogel, 2002). The tissue was allowed to equilibrate during which the bath solution was replaced every 10 min. After the equilibrium period, the action of the extract alone was evaluated; three graded concentrations were selected and used for the study. Contraction was then induced by adding acetylcholine or histamine separately. When the contraction reached maximum (initial spasm), the concentration was recorded and after 5 min the standard drug (isoprenaline 0.4 µg/ml) was added (Kulkarni, 2005). Thereafter, the test extract (2, 4 and 8 mg/ml) was added serially in the presences of histamine and then acetylcholine. The tissues were observed for bronchodilation.
Isolated guinea pig ilea preparations
Guinea pig of either sex weighing 400 to 450 g were starved overnight but allowed free access to water. The animals were then sacrificed and then dissected. The intestine was removed and the distal portion was cut into pieces 2 to 3 cm long. Mesentery was removed and tyrode solution passed through the pieces of the tissue until the effluent was clear. The tissue was then mounted in 25 ml organ bath containing Tyrode physiological solution. The tissue was maintained at 37°C under tension of 1 g and constantly aerated (Vogel and Vogel, 2002). At the end of the equilibration period, increasing concentrations of histamine and the extract were added separately. The concentration of histamine that produced the submaximal response was then added. Five minutes after, the standard drug (mepramine 0.04 µg/ml) was added. The tissue responses were recorded. The tissue was then rinsed and allowed to equilibrate after which the test extracts (2, 4 and 8 mg/ml) was added serially in the presence of histamine. The tissues were observed for bronchodilation using microdynanometer. In another set up of isolated guinea pig ileum preparation, effects of the acetylcholine and acetylcholine in presence of atropine, and graded concentrations of the extract were studied as described by Kulkarni (2005). Responses of the tissues were recorded.
Isolated rabbit jejunum preparations
According to the method of Amos et al. (2000), overnight fasted rabbits (2.0 to 2.5 kg) were sacrificed and dissected. Segments of the rabbit jejunum of about 2 to 3 cm long were removed, washed with Tyrode solution and mounted in 25 ml organ bath and maintained at 37°C. The tissue was allowed to equilibrate and then, the effect of the various concentrations of the extract alone on the tissue was determined. After a washout period, various concentrations of the acetylcholine alone were added and the concentration that produced submaximal response determined. Using the concentration of acetylcholine that produced submaximal response, the procedure was repeated in the presences of atropine and then increasing concentrations of the extract. In another set up, the procedure was repeated using propranolol and isoprenaline in place of acetylcholine and atropine respectively.
Membrane stabilization assay
Bovine red blood cells (RBC) were obtained from slaughter house and made into 5% suspension. To 1 ml of the RBC suspension, 0.1 ml of 50 mM H2O2 was added and incubated at 37°C to initiate hemolysis. After 30 min, 4 ml distilled water was added to the mixture and centrifuged at 1000 rpm for 10 min and absorbance of the supernatant was then taken at 540 nm. This procedure was repeated in the presence of the various concentrations of the extract and percentage inhibition of hemolysis was calculated (Su et al., 2009).
Data analysis
The data obtained were expressed as Mean ± standard error of mean (SEM) or percentages protection. Data were analyzed using one way analysis of variance (ANOVA), followed by Dunnett’s post-hoc tests. Values of p<0.05 were considered statistically significant. Results were presented in tables and charts.
Extraction
Maceration of the G. senegalensis leaf in 70% ethanol yields 19.2% dark coloured extract.
Phytochemical screening
Preliminary qualitative phytochemical screening of ELEGS indicated the presence of alkaloids, flavonoids, saponins, steroids and tannins (Table 1). Similar study on the leaf extract conducted by Akuodor et al. (2013) also found these phytochemicals with the exception of anthraquinones.
Effects of ethanol leaf extract of G. senegalensis on isolated guinea pig trachea
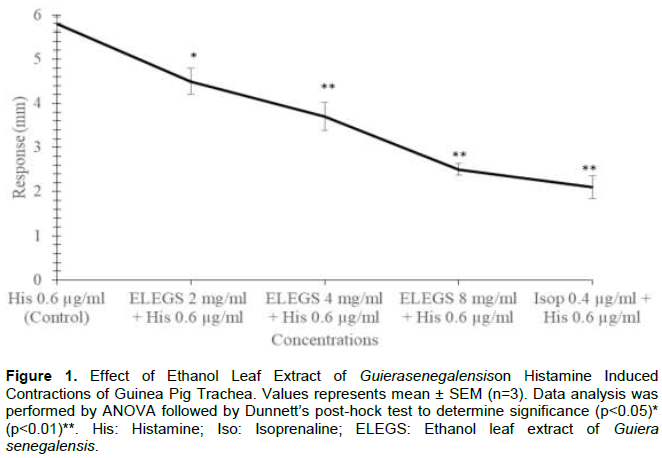
The extract alone produced neither contraction nor relaxation. However, histamine (0.6 µg/ml) induced-contraction was dose-dependently inhibited by the extract. The effect of the extract at 4 and 8 mg/ml was statistically significant (p<0.01) and was comparable to that of 0.4 µg/ml isoprenaline (Figure 1). The extract also significantly (p<0.05) inhibited acetylcholine (0.4 µg/ml) induced-contraction as compared to control, but lower than isoprenaline 0.4 µg/ml (Figure 2).

Effect of ethanol leaf extract of G. senegalensis on isolated guinea pig ileum
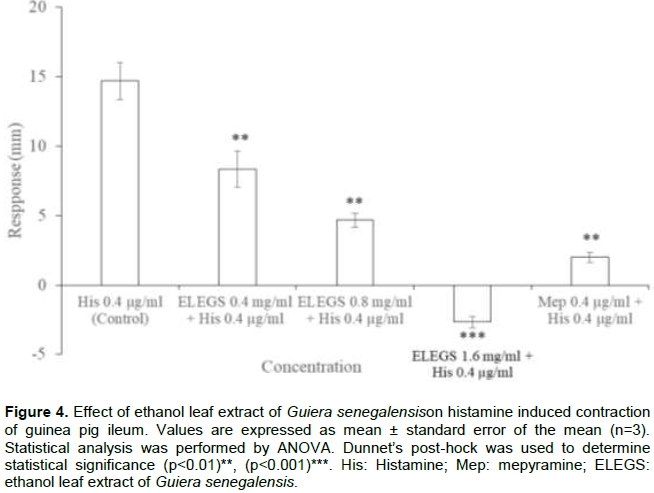
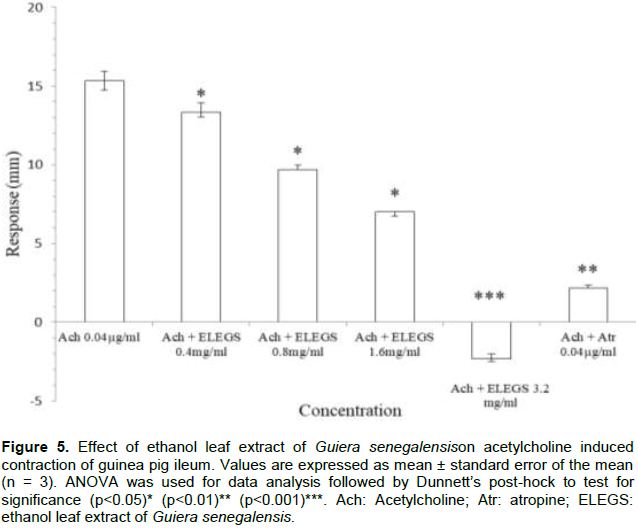
Graded doses of the extract produced dose-dependent relaxation of isolated guinea pig ileum. Responses were all significant (p<0.05) compared to the baseline. Maximum relaxation was obtained at 3.2 mg/ml (Figure 3). On challenging the extract to histamine-induced contraction, significant and dose-dependent inhibition was observed. The extract at concentration of 1.6 mg/ml resulted to higher inhibition (p<0.001) than 0.2 µg/ml mepyramine (Figure 4). Similar result was obtained on acetylcholine-induced contraction (Figure 5).

Effect of ethanol leaf extract of G. senegalensis on rabbit jejunum
Dose-dependent inhibition of the spontaneous basal contractions of isolated rabbit jejunum was observed when ELEGS was used alone. The contraction was almost abolished at 6.4 mg/ml (Figure 6). The contractile responses induced by acetylcholine (0.04 µg/ml) were significantly (p<0.01) inhibited by graded doses of ELEGS in a dose-dependent manner. Atropine (0. 04 µg/ml) also significantly (p<0.001) inhibited the contractile response induced by acetylcholine (Table 2).
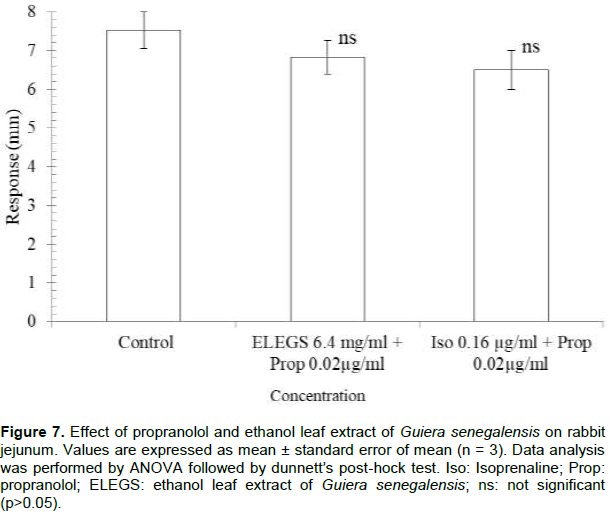
Effect of ethanol leaf extract of G. senegalensis and propranolol on rabbit jejunum
Responses obtained from rabbit jejunum after addition of ELEGS (6.4 mg/ml) to baths pretreated with propranolol were not significantly different from the control. Similar effect was observed with 0.16 µg isoprenaline (Figure 7).
Membrane stabilization effect of ethanol leaf extract of G. senegalensis
The membrane stabilizing effect of ELEGS was assessed using erythrocytes hemolysis inhibition assay. Maximum protection was observed at ELEGS concentration 1 mg/ml compared to the control (Table 3).
Bronchial constriction in asthma is primarily mediated by histamine released from degranulated mast cells and by acetylcholine which cause contractions of tracheal smooth muscles and increased mucus secretion. Inflammation of the bronchi also sets due to lipid mediators from arachidonic acid pathway generated from membrane phospholipids (Gosh, 1984). Clinical interventions so far aim at maintaining normal airway muscles tone by the use of bronchodilators and anti-inflammatory agents to reverse bronchoconstriction. Therefore, substance that can reverse bronchoconstrictions will possibly relieve symptoms of asthma. For this, the antihistaminic as well as the antimuscarinic activities of the plant extract were investigated using animals’ isolated tissues. Smooth muscles are rich in many kinds of receptors with different localization specifications. Airway smooth muscles are rich in histamine, acetylcholine, and β2 adrenergic receptors. Distribution of these receptors and their responses to agonists and antagonists is similar in both humans and guinea pigs. Therefore, guinea pigs are used in contrast to rats and mice.
In the present study, in vitro anti-asthmatic effect of the ethanol leaf extract of Guiera senegalensis (ELEGS) was evaluated. In isolated trachea guinea pig tracheal chain preparations, ELEGS was also found to possess antihistaminic and antimuscarinic effects. Although it did not exhibit any effect when used alone, the extract at concentrations of 8 mg/ml was able to significantly (p<0.01) inhibit contractions induced by both spasmogens (histamine and acetylcholine) on the tracheal chains. The action of the extract was found to be similar to that of the standard drug (isoprenaline). Histamine and muscarinic receptors are the dominant receptors in guinea pig ileum and this makes it a good tissue for studies involving these spasmogens. ELEGS exhibited smooth muscle relaxing effect on guinea pigs ileal strips and was able to significantly inhibit contractions induced by histamine (p<0.01 to 0.001) and acetylcholine (p<0.05 to 0.01) in a dose-dependent manner. Atropine (antimuscarinic) was used against acetylcholine while mepyramine (antihistamine) was used against histamine for comparison with the extract. These pharmacological antagonists significantly (p<0.01) inhibited the ileal induced contractions but to a lesser effect than 3.2 mg/ml of the extract. At the 3.2 mg/ml, ELEGS completely abolished the contractions-induced by both histamine and acetylcholine and resulted to relaxation of the tissues.
On isolated rabbit jejunum, the extract when given alone was able to significantly (p<0.05 to 0.01) reduced the basal spontaneous contractions as well as contractions induced by acetylcholine (p<0.01 to 0.001) in dose-dependent pattern. Similar result was observed with atropine. These indicate that ELEGS has a nonspecific spasmolytic activity on smooth muscles that may probably be due to β-adrenergic stimulatory (Martin et al., 1994), histamine H1 (Popa et al., 1984) and/or muscarinic inhibition (Linden et al., 1993). However, result obtained from rabbit jejunum after addition of ELEGS up to 6.4 mg/ml to baths pretreated with propranolol was not significantly different from the control. Similar effect was observed with 0.16 µg isoprenaline. Propranolol is a nonselective β adrenergic receptor blocker that binds to all β receptors and inhibits their activity. This suggests that, ELEGS might be acting on the same receptors resulting in smooth muscle relaxation by activation of β2 adrenoceptors. Allergic asthma is a chronic inflammatory process occurring due to exposure to allergen resulting in the activation of T-lymphocyte with subsequent release of inflammatory mediators. Immuno-modulating agents are useful in the treatment of asthma by inhibiting the antigen-antibody (AG-AB) reaction and thereby inhibiting the release of inflammatory mediators.
Membrane stabilization assay was used to study the possible inhibition of lipid mediators of inflammation in asthma. Percent inhibition of erythrocytes hemolysis was calculated and the ELEGS at all concentrations used was able to protect the membranes of erythrocytes from possible damage. Maximum effective concentration was observed at 1 mg/ml. Drugs effective in asthma are mostly steroidal in nature. ELEGS contained steroid, alkaloids, flavonoids, saponins, anthraquinones and tannins. These secondary metabolites have been known to possess various biological activities including antibacterial, antioxidant, anticancer, spasmolytic and anti-inflammatory effects (Parmar et al., 1999). Flavonoids have been known to inhibit the release of several mediators of inflammations such as prostaglandins, histamine, serotonin and bradykinins by inhibiting the biosynthetic pathways of inflammatory mediators (Macauder, 1986). Steroids are known for their maintenance of membrane integrity by inhibiting lipoxygenase and cyclo-oxygenase pathways and are used for their anti-inflammatory properties (Speroni et al., 2005). So antiasthmatic activity showed by G. senegalensis might be because of these chemical moieties.
The authors have not declared any conflict of interest.