ABSTRACT
The advance of global economy affects developing countries that are facing a major public health problem. The prevalence of obesity triggers a series of non-communicable chronic diseases (NCDs). Several studies have suggested that the chia seed (Salvia hispânica L.) may be an alternative to combat NCDs, as it is rich in alpha-linolenic acid (ALA). This research is an experimental work in which 24 Wistar rats were divided into 4 groups (G1, G2, G3 and G4), out of which G1 was the control group (ad libitum + saline); G2 received a hyperlipidic and hyperglycemic diet (HHD); G3 was given HHD + 0.2 g ground chia and G4 was given HHD + 0.4 g of ground chia, for 4 weeks. The objective was to evaluate biochemical parameters (total cholesterol (TC) and lipoproteins, triglyceride (TG), glucose, homocysteine and high-sensitivity C-reactive protein (hsCRP) and histomorphometric of rats on a diet with chia seed, as well as to ascertain the safety of such treatment proposed evaluating its hepatotoxicity [aspartate transaminase (AST), alanine transaminase (ALT) and gamma-glutamyl transferase (GGT)]. There was no decrease, however, in dyslipidemia parameters (total cholesterol (TC), high-density lipoprotein (HDL), triglycerides) and glucose. The concomitant administration of chia to a hyperglycemic and hyperlipidemic diet was not able to alter these parameters, however with regard to histomorphometric analysis, there was a significant result (p < 0.08) with regard to the thickness of the right ventricle (RV), which leads to a possible cardioprotective effect.
Key words: Chia seed (Salvia hispânica L.), dyslipidemia, biochemical parameters.
The progress of the world economy has been affecting developing countries which are facing a major public health problem, that is the prevalence of obesity, which has been a current issue in developed countries, and at the moment is triggering a series of non communicable chronic diseases (NCDs) (Uauy and Kain, 2002; Popkin and Gordon-Larsen, 2004; Pinheiro et al., 2004). NCDs are currently responsible for the overload of the Unified Health System (SUS) being accountable for innumerous deaths caused by diabetes, dyslipidemia, hypertension, lung problems, cardiovascular disease (CVD) and metabolic syndrome (MS) (Coutinho et al., 2008; Franscischi et al., 2000; Silva et al., 2013). The excessive consumption of products rich in sugars, saturated and trans fats, as well as polyunsaturated n-6 is one of the hypothesis for the current obesity scenario, as these are associated with the increasing development of cardiovascular and inflammatory diseases. On the other hand, fatty acids of the n-3 series and eicosapentaenoic acid (EPA; C20: 5 n-3) to docosahexaenoic acid (DHA C22: 6 n-3) and alpha-linolenic acid (ALA: C18: 3 n-3), the latter coming from the vegetables, have proven to be effective as protectors of these factors (Pinheiro et al., 2004; Silva et al., 2013; Poudyal et al., 2012).
The chia seed (Salvia Hispânica L.) is regarded as a rich source of alpha-linolenic acid (ALA) in approximately 60% of its composition and 20% linoleic acid (LA). It is also rich in fiber, antioxidants and protein. Native from Mexico and Guatemala, chia seeds are currently subject of several studies for its possible protective role in dyslipidemias, as results have shown they were able to reduce total cholesterol (TC) and raise high density lipoprotein (HDL) levels in animal model, in addition to being used in various experiments to a new alternative food as ALA enriched eggs and baked goods (Silva et al., 2013; Poudyal et al., 2012; Vázquez – Ovando et al., 2009; Martínez et al., 2012; Chicco et al., 2009; Ali et al., 2012).
This study aims at evaluating biochemical parameters (TC and lipoproteins, TG, glucose, homocysteine and hsCRP) and histomorphometric of rats on a diet enriched with chia seeds, and ascertain the safety of such treatment evaluating its hepatotoxicity [aspartate transaminase (AST), alanine transaminase (ALT), gamma-glutamyl transferase (GGT)].
Twenty four (24) male albino Wistar animals were used, weighing on average 300 g, from the Vivarium of Faculdade de Medicina do ABC (FMABC). During the experiment, the animals were kept in a photoperiodic cycle of 12 h light and 12 h dark temperature, controlled relative humidity and exhaustion (20 air changes per hour). The 24 animals were divided into 4 groups, with 6 animals in each group: G1 - control group, G2 - HHD group, G3 - HHD + chia seed (0.2 g) and G4 - HHD + chia seed (0 4 g). The Ethics Committee on Animal Experimentation from FMABC approved the study, under registration number 011/2012.
Diet composition
The control group (G1) was given 2.5 ml of saline, in addition to the ad libitum during the entire period of the experiment. The hyperlipidic and hyperglycemic diet (HHD) was composed of 52% (wt/wt) carbohydrate, 24% (wt/wt) fat and 25% (wt/vol) fructose. This mixture was administered by gavage for 2.5 ml. The animals (G2, G3 and G4) were subjected to this diet for four weeks. In the end, total cholesterol levels were checked and fractions of triglycerides and glucose determined. After characterizing the increase in fat diet parameters, G2 continued to receive only HHD, G3 received 0.2 g of grounded chia seed in addition to the HHD, as well as G4 obtained an increase in the ration of 0.4 g of grounded chia seed for another four weeks.
Tissue preparation
After eight weeks, the animals were sacrificed and blood samples were collected from the tail of the animals in dry tubes and kept under refrigeration until the time of analysis. Next, an incision was made in their chests in order to expose the heart of each animal. The hearts were removed and sectioned transversely at the level of papillary muscles. The apical fragments were fixed in formalin 10% buffered (pH 7.2) for 48 h. After the material fixation, a process of dehydration, diaphanization and paraffin inclusion commenced. Five (5) non-consecutive histological transverse sections of 6 microns in thickness of the fragments of the right and left ventricles from each animal were used. The sections were stained with hematoxylin-eosin method and examined under light microscopy.
Morphometric analysis
The Axiovision Zeiss software was used to capture the images used for morphometric studies. 20 micrographs/group with ×30 zoom were captured to estimate the area of the ventricular cavity and the thickness of the ventricles and interventricular septum. To estimate the thickness (E), four measurements per frame obtained at 0, 90, 180 and 270° were used.
Biochemical parameters
Blood samples were collected in dry tubes and kept under refrigeration until the moment of analysis, including the determination of homocysteine (Hcy), alanine transaminase (ALT), aspartate transaminase (AST), gamma- glutamyltransferase (GGT), hsCRP. In addition to the total cholesterol checks and fractions, triglycerides and glucose were also determined. The measurement of plasma homocysteine was taken by enzyme immunoassay using Immulite 2000, automatic chemiluminescence equipment in the Clinical Analysis laboratory at FMABC. Prior to the beginning of the analysis in this system, adjustments were carried out according to the manufacturer's recommendations. Glucose, total cholesterol, HDL-cholesterol, ALT, AST and GGT were performed by enzymatic-colorimetric method following the best practices in clinical analysis. The LDL-cholesterol and VLDL-cholesterol fractions were determined by Friedewald formula. HsCRP dosing was performed by competitive immunoassay method, by boiling in liquid phase, labeled with ligand. The following measurement was performed by chemiluminescence method by binding protein in vitro with immobilized anti-ligand detection system. All analyses were performed in the Clinical Analysis laboratory, at FMABC.
Statistical analysis
Data was expressed as average ± standard error of mean (SEM). After confirming that all continuous variables were normally distributed using the Kolmogorov-Smirnov test, the statistical differences between groups were subjected to analysis of variance 1 (ANOVA). When another significance was detected, comparisons were performed by the post hoc Tukey test. P values less than 0.05 were considered statistically significant.
Twenty four (24) Wistar rats were divided into four groups G1, G2, G3 and G4, which in the first month received 2.5 ml of a HHD except G1 which served as a control group in order to elevate the parameters (total cholesterol, HDL, triglycerides and glucose) in order to analyze attenuation with the use of chia seed the following month. Table 1 shows the results of total cholesterol, HDL, triglycerides, non-HDL cholesterol and glucose levels, held within 4 weeks. It is possible to notice a considerable increase in blood glucose in all groups fed HHD, in contrast with the control group as well as the TG. Although there is variation in the average between the groups for the TC parameters, HDL and HDL non cholesterol, the results were satisfactory for the next phase of the study, in which G2 remained receiving the HHD, G3 received an addition of 0.2 g ground chia seed (plus HHD) and G4 received 0.4 g ground chia seed (plus HHD), for another month.
Table 2 shows the result of chia in the TC parameters, TG, non-HDL cholesterol, HDL cholesterol, homocysteine, hsCRP, glucose. Supplementation with chia seed for four weeks did not cause significant results (p) as shown in Table 2. Although the TC average at G4 has been shown lower than G1, the blood glucose increase was evident in all groups compared to the control group, since the TG average cholesterol non-HDL and HDL were approximate to the control group, demonstrating that the chia seed is ineffective to attenuate the effects of the evaluated parameters. Just as chia seed has not been able to reduce the cardiovascular risk, since no change in hsCRP and Hcy was observed throughout the work. None of the groups had hepatotoxic effect to the seed, the significant values (p) are shown in Table 3.
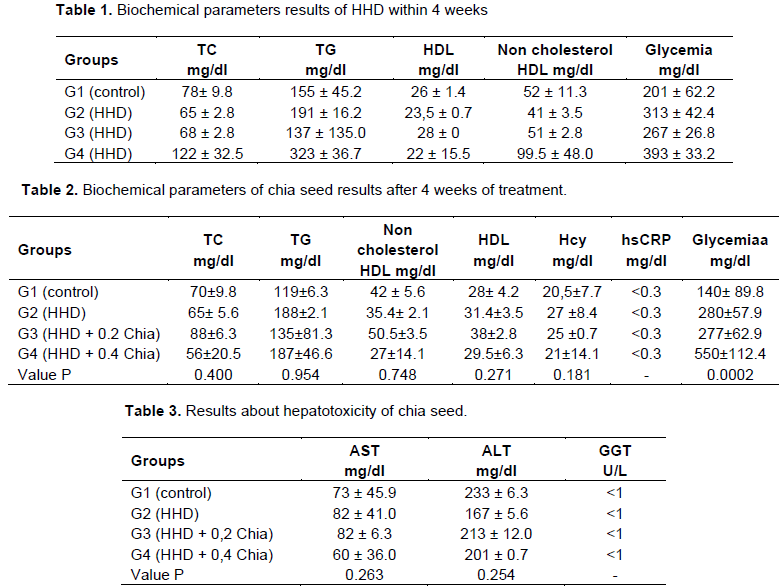
Morphometric analysis
The results (see Figure 1) of morphometric quantification of the thickness of the right ventricle (RV) Is the only one which shows significant trend (p <0.08) when compared to the other groups. This may be related to a possible cardiovascular protective effect. Other parameters such as interventricular septal, left ventricular thickness (VE), RV cavity, and LV cavity did not reach statistical relevance.
In this study the chia seed was incapable of reducing the levels of TC, TG and increase HDL. Although in other studies these results have been satisfactory. Chicco et al. (2009) found that chia seed was able to prevent the onset of dyslipidemia and insulin resistance in rats (Chicco et al., 2009). Another study by Ayerza et al. (2007), also performed in rats, showed that only the seeds, either whole or ground, obtained results compared to the control group, and the whole seeds decreased TG levels, and ground seed increased HDL levels, the chia seed oil, on the other hand, did not show any significant results (Ayerza and Coates, 2007).
The use of chia seed along with a HHD may have influenced in a negative way to mitigate the parameters analyzed, since a balanced diet and physical exercise are widely advocated in order to minimize such factors (Pan and Storlien, 1993; Wang and Peng, 2011). The ALA-rich foods are currently the subject of several studies, as many studies have shown its relevance in the treatment of dyslipidemia and especially in the care of cardio vascular diseases (CVD), for being known as cardio-protective (Pan and Storlien, 1993; Wang and Peng, 2011).
The chia seed, when analyzed in the parameters of non-HDL cholesterol, hsCRP and Hcy, associated factors to quantify the risk of CVD, have not shown significant results (Neves et al., 2004; Silva et al., 2009). As in a study by Nieman et al. (2009), in which the chia seed was not successful in reducing the parameters evaluated for CVD, although there was a rise of plasma ALA, compared to placebo, this result was ineffective, the researchers used overweight individuals and supple-mented their diets with 50 g/day chia seed for 12 weeks (Neiman et al., 2009).
However, in order to perform the histological analysis, we observed a promising outcome (p < 0.08) as the thickness of the RV, related to a possible cardioprotective effect, but the fact that chia seed also contains AL may have influenced for more significant results, since it competes with ALA for the same metabolic enzyme, raising the risk factors associated with CVD (Pan and Storlien, 1993; Vedtofte et al., 2011).
In literature, the relation of high fructose consumption, which can lead to diabetes or insulin resistance is consistently established. The same effect can be generated by the abuse of sweeteners, which are typically derived from fructose, a fact that was recently reported by Suez et al. (2014), to determine the effect of non-caloric sweeteners (NCS) in glucose homeostasis, they administered to a mixture of water with saccharin or aspartame or sucralose to rats, while the control group consumed pure water or water with sucrose or glucose, the control group remained at a similar tolerance curve, unlike the groups that received the NCS developed glucose intolerance, and the same occurred when the rats were given a diet high in fat to verify the effect on obesity, in both situations saccharin was the most efficient for the cause of insulin resistance (Suez et al., 2014; Barreiros et al., 2005).
The same mechanism that causes insulin resistance with the use of NCS, may have occurred in the concomitant administration of chia seed and fructose in this study, since the chia seed was not effective in reducing blood glucose parameters, which increased throughout the study, a fact that again confirms the hypothesis that a balanced diet is necessary and the practice of physical exercises with the use of chia seed, as previously mentioned (Marchon et al., 2015).
Finally, chia seed was ineffective to mitigate the biochemical parameters analyzed in this paper. Although it has shown an improvement in RV thickness, more experimental and clinical studies on the seed should be performed in order to analyze its function in dyslipidemias, since it has low cost and is a great source of ALA, serving as an option to existing products which in many cases are expensive to purchase. Moreover, it is not hepatotoxic, which is an advantage for the consumption of the same, when their effects are more accurate in the literature.
The authors have not declared any conflict of interest.
The present study was carried out with the financial support of the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) no 3407728.
REFERENCES
|
Ali NM, Yeap SK, Ho WY, Beh BK, Tan SW, Tan SG (2012). Review article: The promising future of chia, Salvia hispanica L. J. Biomed. Biotechnol. pp. 1-9.
crossref
|
|
|
|
Ayerza RJ, Coates W (2007). Effect of dietary α-linolenic fatty acid derived from chia when fed as ground seed, whole seed and oil on lipid content and fatty acid composition of rat plasma. Ann. Nutr. Metab. 51:27-34.
crossref
|
|
|
|
Barreiros RC, Bossolan G, Trindade CEP (2005). Frutose em Humanos: efeitos metabólicos, utilização clínica e erros inatos associados. Rev. Nutr. 18(3):377-89.
crossref
|
|
|
|
Chicco AG, D'Alessandro ME, Hein GJ, Olivia ME, Lombardo YB (2009). Dietary chia seed (Salvia hispanica L.) rich in α-linolenic acid improves adiposity and normaliseshypertriacylglycerolaemia and insulin resistance in dyslipaemic rats. Br. J. Nutr. 101:41-50.
crossref
|
|
|
|
Coutinho JG, Gentil PC, Toral N (2008). A desnutrição e obesidade no Brasil: o enfrentamento com base na agenda única da nutrição. Cad. Saúde Pública 24(supl.2):S332-S340.
crossref
|
|
|
|
Franscischi RPP, Pereira LO, Freitas CS, Klopfer M, Santos RC, Vieira P, Lancha Junior AH (2000). Obesidade: Atualização sobre sua etiologia, morbidade e tratamento. Rev. Nutr. 13(1):17-28.
crossref
|
|
|
|
Marchon C, Ornelas EM,Viegas KAS, Lacchini S, Souza RR, Fonseca FLA, Maifrino LBM (2015). Effects of moderate exercise on the biochemical, physiological, morphological and functional parameters of the aorta in the presence of estrogen deprivation and dyslipidemia: An experimental model. Cell Physiol. Biochem. 34:397-405.
crossref
|
|
|
|
Martínez ML, Marín MA, Faller CMS, Revol J, Penci MC, Ribotta PD (2012). Chia (Salvia hispanica L.) oil extraction: Study of processing parameters. Food Sci. Technol. 47:78-82.
crossref
|
|
|
|
Neiman DC, Cayea EJ, Austin MD, Henson DR, Mcanulty SR, Jin F (2009). Chia seed does norpromoteweightlossoralterdiseaserisk factors in overweight adults. Nutr. Search 29:414-418.
|
|
|
|
Neves LB, Macedo DM, Lopes AC (2004). Homocisteína. J. Bras. Patol. Méd Lab. 40(5):311-20.
|
|
|
|
Pan DA, Storlien LH (1993). Dietary lipid profile is a determinant of tissue phospholipid fatty acid composition and rate of weight gain in rats. J. Nutr. 123(3):512-519.
|
|
|
|
Pinheiro ARO, Freitas SFT, Corso ACT (2004). Uma abordagem epidemiológica da obesidade. Rev. Nutr. 17(4):523-533.
crossref
|
|
|
|
Popkin BM, Gordon-Larsen P (2004). The nutrition transition: worldwide obesity dynamics and their determinants. Int. J. Obesity 28:S2-S9.
crossref
|
|
|
|
Poudyal H, Panchal SK, Waanders J, Ward L, Brown L (2012). Lipid redistribution by α-linolenic acid-rich chia seed inhibits stearoyl- CoA desaturase-1 and induces cardiac and hepatic protection in diet – induced obese rats. J. Nutr. Biochem. 23:153-162.
crossref
|
|
|
|
Silva CS, Kanaguchi G, Bruniera FR, Savioli LR M, Radziavicius CR, Feder D, Pires P CR, Perazzo FF, Azzalis LA, Fonseca FLA (2013). A Chia (Salviahispanica L) como nova alternativa alimentar e no tratamento das doenças crônicas não transmissíveis. Rev. Bras. Nutr. Clín. 28(3):234-238.
|
|
|
|
Silva NAO, Morais FFC, Helou T, Bergamin AAC, Teixeira PFS, Vaisman M (2009). Níveis séricos de colesterol não HDL como marcador de risco cardiovascular em pacientes com hipotireoidismo subclínico. Rev. SOCERJ 22(2):80-85.
|
|
|
|
Suez J, Korem T, Zeevi T, Zilberman-Schapira G, Thaiss CA, Maza O, Israeli D, Zmora N, Gilad S, Weinberger A, Kuperman Y (2014). Artificial sweetenersinduce glucose intolerancebyalteringthegut microbiota. Nature 514:181-186.
|
|
|
|
Uauy R, Kain J (2002). The epidemiological transition: need to incorporate obesity prevention into nutrition programmes. Publ. Health Nutr. 5(1):223-229.
crossref
|
|
|
|
Vázquez–Ovando A, Rosado- Rubio G, Chel-Guerrero L, Betancur-Ancona D (2009). Physicochemical properties of a fibrous fraction from chia (Salvia hispânica L.). Food Sci. Technol. 42:168-173.
|
|
|
|
Vedtofte MS, Jakobsen MU, Lauritzen L, Heitmann BL (2011). Dietary α-linolenic acid, linoleic acid, and n-3 long-chain PUFA and risk of ischemic heart disease. Am. J. Clin. Nutr. 94:1097-103.
crossref
|
|
|
|
Wang H, Peng DQ (2011). New insights into the mechanism of low high- density lipoprotein cholesterol in obesity. Lipids Health Dis.10:176.
crossref
|