ABSTRACT
This paper describes the anticholinesterase and antioxidant activities of Eugenia dysenterica DC. (O. Berg. (Myrtaceae) essential oils from leaves (EOED). EOED were obtained by hydrodistillation using a Clevenger-type apparatus and the products were analyzed by gas chromatography-mass spectrometry (GC-MS) and gas chromatography-flame ionization detector (GC-FID). The main constituents of EOED were caryophyllene oxide (66.3%), isoledene (3.9%), 1,3,8-p-menthatriene (3.5%), mustakone (3.46%), β-phellandrene (1.7%), and selin-11-en-4-α-ol (1.7%). The antioxidant assay was performed based on the formation of thiobarbituric acid reactive substances (TBARS), hydroxyl radical, and nitric oxide production. By performing the Ellman assay, it was observed that EOED was able to inhibit the enzyme acetylcholinesterase (AChE) with an IC50 = 0.92 ïg.ml-1 promising better value when compared with the drug rivastigmine (IC50 = 1.87 ïg.ml-1), used in the treatment of Alzheimer's disease. The caryophyllene oxide (the main compound) was tested after purification on the AChE with an IC50 = 0.31 ïg.ml-1. Caryophyllene oxide (the majority compound) was tested on the AChE and presented the IC50 = 0.31 ïg.ml-1. At concentrations of 0.9, 1.8, 3.6, 5.4, and 7.2 ïg.ml-1, it was found out that EOED prevented lipid peroxidation inhibiting amount of TBARS formed in a similar manner to ascorbic acid. In addition, there was a reduction in the production of hydroxyl radical as well as the production of nitric oxide. To the best of our knowledge, this is the first report on compounds from this species that have activity for potentially preventing neurodegenerative disorders.
Key words: Eugenia dysenterica, essential oil, antioxidant, anticholinesterase.
A variety of essential oil of plants has shown acetylcholineterase (AChE) inhibitory activity and may be relevant to the treatment of neurodegenerative disorders, such as Alzheimer’s disease (AD). The essential oils of
Cistus species have functional properties in prevention of neurodegenerative disorders (Loizzo et al., 2013).
Centella asiatica essential oil and various other essential oils from plant species, for example
Cistus salvifolius and
Ocimum canum have shown pharmacological activities relevant to the treatment of cognitive disorders, indicating potential for therapeutic use in disorders, such as AD (Houghton and Howes, 2003). Though recent intensive efforts have been made to understand the mechanism of neurodegeneration involved in AD and to discover new drugs combating the symptoms; at present, there is a deficit in the number of efficient and safe therapeutic agents to treat the disease. No new drugs have been approved by the US Food and Drug Administration (FDA) since 2003, likely because the abnormal brain deposits of Aβ and τ-proteins still cannot be considered causes or by-products of the disease (
Buckholtz,
. 2011). Since the approval of galantamine for the treatment of AD patients, the search for new anticholinesterase alkaloids has escalated, leading to promising candidates, such as huperzine A (Konrath et al., 2013).
Many monoterpenes and sesquiterpenes for example 1,8-cineole, α-pinene, and linalool have been cited in promising research due to their potent anticholinesterase activity (Kiendrebeogo et al., 2013). However, few reports exist that deal with the inhibition of AChE by plant essential oils (Chaiyana and
Okonogi, 2012).
Acetylcholinesterase inhibitors (IAChE) have therapeutic applications in AD and in addition, the central cholinergic system is considered one of the more important neurotransmitter systems involved in the regulation of cognitive functions. Cholinergic neuronal loss in the hippocampal area is the major feature of AD and enhancement of central cholinergic activity by using IAChE is presently the mainstay of pharmacotherapy of senile dementia of Alzheimer type (Enz et al., 1993; Siddiqui and Levey, 1999).
Pharmacological activities from plants and their constituents may be relevant for the treatment of cognitive disorders, including enhancement of cholinergic function in the central nervous system, anti-inflammatory, and antioxidant activities (Houghton and Howes, 2003). A variety of plants has been reported to show AChE inhibitory activity and so may be relevant to the treatment of neurodegenerative disorders, such as AD (Mukherjee
et al., 2007). Research and interest in essential oils is on the increase. Recently, our research group published studies with essential oils with antioxidant and antinociceptive effects from Citrus limon Osbeck as studied on mice (Campelo et al., 2012).
Eugenia dysenterica (DC).O. Berg and several species of Eugenia are used in folk medicine with anti-inflammatory, anti-diarrheic, diuretic, and other properties. In Brazil, this species is popularly known as ‘cagaiteira’, with opposite leaves, simple, ovate or elliptical limbo; has edible white flowers (Rizzini, 1970), holders of laxative properties. Its fruits are consumed raw or in the form of juices, or processed to ice cream and liqueurs and when fermented can produce alcohol and vinegar. Sensory evaluation of fruit wine from cagaita showed over 70% acceptability for colour, flavor, and taste for all cagaita beverages (Oliveira et al., 2011; Oga and Fonseca, 1994). Tea from the leaves is used to combat diarrhea and the bark is used as anti-inflammatory agent. Daily consumption of ‘cagaita’ (100 g) contributed significantly to the supply of daily requirements of vitamin C (on average 71.0%), vitamin A (on average 7.5%), and folates (on average 7.9%). The ‘cagaiteira’ has a high pulp yield, reduced total energy value and is considered a source of vitamin C, which is an important role in human health (Cardoso et al., 2011).
This paper describes the anticholinesterase and antioxidant activities of E. dysenterica DC. (cagaiteira) essential oil from leaves (EOED). This is the first report on the activities of EOED.
Plant
E. dysenterica fresh leaves were collected in october 2013 in Uruçuí, Piauí State, Brazil, coordinates [07°14′02″S and 44°33′14″W]. Plant identification was confirmed by Dr. Roseli Farias Melo de Barros, Department of Biology, Piauí Federal University (UFPI), Brazil and a voucher specimen (number 28824) have been deposited at the Graziela Barroso herbarium of the UFPI.
Pure compound, solvents and enzymes
AChE enzyme, (−)-Caryophyllene oxide (95% of purity), ascorbic acid, and thiobarbituric acid (TBA) were purchased from Sigma-Aldrich.
Hydrodistilation of the essential oils
The EOED (3 samples of 300 g each were used) were extracted
using hydrodistillation for 3 h with a Clevenger-type apparatus. The essential oil was dried over anhydrous sodium sulphate and the percentage content was calculated on the basis of the dry weight of the plant material. The essential oils were stored in a freezer (-20°C) until analyzed.
Gas chromatography-flame ionization detector (GC-FID) and gas chromatography-mass spectrometry (GC-MS) analysis of the essential oils
GC analyses were carried out using a Shimadzu GC-17A fitted with a FID and an electronic integrator. Separation of the compounds was achieved employing a ZB-5MS fused capillary column (30 m × 0.25 mm × 0.25 µm film thickness) coated with 5% phenyl-arylene and 95% methylpolysiloxane. Helium was the carrier gas at 1.0 ml.min-1 flow rate. The column temperature program was: 40°C/3 min, followed by a rate of 4°C.min-1 to 240°C, then a rate of 10°C.min-1 to 300°C and then 300°C/3 min. The injector and detector temperatures were 250 and 280°C, respectively. Samples (0.5 µl in CH2Cl2) were injected with a 1:50 split ratio. Retention indices were generated with a standard solution of n-alkanes (C8-C20). Peak areas and retention times were measured by an electronic integrator. The relative amounts of individual compounds were computed from GC peak areas without FID response factor correction.
GC/MS analyses were performed on a Shimadzu QP5050A GC/MS system equipped with an AOC-20i auto-injector. A J&W Scientific DB-5MS (coated with 5% phenyl and 95% dimethylpolysiloxane) fused capillary column (30 m × 0.25 mm × 0.25 µm film thickness) was used as the stationary phase. MS were taken at 70 eV with scan interval of 0.5 s and fragments from 40 to 500 Da. The other conditions were similar to the GC analysis.
Identification of constituents
The essential oil components were identified by comparison of (i) their retention times (tR) with those of the same standard compounds (caryophyllene oxide) analyzed under identical conditions, (ii) their retention indices (RIs), determined on a DB-5MS column relative to the tR of a series of n-alkanes (C8-C20), according to Van Den Dool and Kratz (1963) with those published in the literature (Van Den Dool andKratz, 1963) and their mass spectra with those listed in the NIST (05, 05s, 21 and 107) and Wiley 8 mass spectral libraries and those published in the literature (Adams, 2007).
AChE inhibition assay
The inhibitory effect of EOED on AChE activity is evaluated by an adaptation of the spectrophotometric method of Ellman (Ellman et al., 1961).The EOED and caryophyllene oxide were dissolved in methanol to prepare solutions of 10 mg.ml-1. Then, 1.5 μl of the methanol EOED was spotted onto silica gel thin layer chromatography (TLC) plated and developed with chloroform: methanol 9:1 after which the enzyme inhibitory activity was detected using Ellman’s method “in situ” on the plate (Ahmad et al., 2015; Ellman et al., 1961; Rhee et al., 2001). The developed plates were sprayed with 1 mM 5,5-dithiobis (2-nitrobenzoic acid) (DTNB) and 1 mM ATCI in buffer A. The plate was dried for 3 to 5 min and then an enzyme solution of AChE from an electric eel (type VI-s lyophilized, 261 U.mg-1 solid, and 386 U.mg-1 protein) dissolved in buffer A (500 U.ml-1 stock solution) was diluted with buffer A to obtain 5 U.ml-1 enzyme and was then sprayed on the plate. A yellow background with white spot for inhibiting EOED was visible after about 5 min. The observation must be recorded within 15 min, because they fade after 20 to 30 min. To observe whether the positive results of the extract in TLC or the microplate assay are due to enzyme inhibition or to the inhibition of the chemical reaction between DTNB and thiocholine (the product of the enzyme reaction), 5 units.ml-1 of AChE was premixed with 1 mM acetylthiocholine (ATCI) in buffer A and incubated for 15 min at 37°C. The enzyme-substrate mixture was used as thiocholine spray. The extract was spotted on the silica gel TLC plate developed as described earlier and sprayed with 1 mM solution DTNB followed by the thiocholine spray. White spot on a yellow background was observed for false positive extract.
The inhibitory effect quantitative of EOED on AChE activity was evaluated using and adaptation of the spectrophometric method of Ellman modified by Rhee (Ellman et al., 1961; Rhee et al., 2001). Six different concentrations were prepared in triplicate, starting from the EOED and caryophyllene oxide (0.9, 1.8, 2.7, 3.6, 5.4, and 7.2 μg.ml-1). The reaction is monitored for 5 min at 412 nm inspectrophotometer. Test tube placed in 100 μl of the sample (concentration 0.1% solution in 50 mM Tris-HCl, pH 8, and methanol 10%) was mixed with 100 μl of AChE 0.22 U.ml-1 (22U of enzyme diluted in 100 ml of 50 mM Tris-HCl, pH 8, and 0.1% bovine serum albumin (BSA)) and 200 μl of buffer (50 mM Tris-HCl, pH 8, and BSA 0.1%). The mixture was incubating for 5 min at 30°C. Subsequently, was added 500 μl of DTNB acid (concentration of the 3 mM in Tris-HCl, pH 8, 0.1 M NaCl, and 0.02 M MgCl2) and 100 μl of ATCI (4mM in water).
The quantitative inhibitory effect ofv EOED on AChE activity was evaluated using an adaptation of the spectrophometric method of Ellman modified by Rhee (Ellman et al., 1961; Rhee et al., 2001). Six different concentrations were prepared in triplicate, starting with the EOED and caryophyllene oxide (0.9, 1.8, 2.7, 3.6, 5.4, and 7.2 μg.ml-1). The reaction was monitored at 412 nm for 5 min inspectrophotometer. 100 μl of the sample (concentration of 0.1% solution in 50 mM Tris-HCl, pH 8, and methanol 10%) was mixed with 100 μl of AChE 0.22 U.ml-1 (22U of enzyme diluted in 100 ml of 50 mM Tris-HCl, pH 8, and 0.1% BSA) and 200 μl of buffer (50 mM Tris-HCl, pH 8, and BSA 0.1%). The mixture is incubated for 5 min at 30°C. Subsequently, was add 500 μl of DTNB (concentration of the 3 mM in Tris-HCl, pH 8, 0.1 M NaCl, and 0.02 M MgCl2) and 100 μl of ATCI (4 mM in water). A blank is prepared by substituting AChE with 100 μl of buffer (50 mM Tris-HCl buffer pH 8, and 0.1% BSA). The reaction is monitored for 5 min at 412 nm and the initial velocity (V0) recorded. Anticholinesterase activity (%) was calculated using Equation 1 (Kiendrebeogo et al., 2011): Sample V0 and blank V0 represents the initial velocities of samples and blank. Inhibition concentration of 50% (IC50) values are obtained using Log-Probit. Rivastigmine (commercial lAChE) is used as positive control at the same concentration of the essential oil.
I (%) = (1 - V0 Sample/V0 Blank) × 100 (1)
Equation 1- Anticholinesteraseactivity(%)
Evaluation of in vitro potential against production of thiobarbituric acid reactive substances (TBARS) in essential oil from E. dysenterica
Determination of TBARS was performed to quantify the lipid peroxidation level (Esterbauer and Cheeseman, 1990). This method was used to determine the EOED, using homogenized egg yolk as a lipid rich substrate (Guimarães et al., 2010). Briefly, egg yolk was homogenized (1% w/v) in 20 mM phosphate buffer (pH 7.4). A volume of 1 ml of this homogenate was homogenized with 0.1 ml of EOED, at concentrations of 0.9, 1.8, 3.6, 5.4, and 7.2 μg.ml-1 of EOED. Lipidperoxidation was induced by adding 0.1 ml of 2,2-azobis-2-midinopropane (AAPH, 0.12 mol.L-1). Control was carried out only with the solution (0.05% Tween 80 dissolved in 0.9% saline solution) used to emulsify the substance that was evaluated. Reactions were performed for 30 min at 37°C. After cooling, samples (0.5 ml) were centrifuged with 0.5 ml of trichloroacetic acid (15%) at 1200 g for 10 min. An aliquot of 0.5 ml of the supernatant was mixed with 0.5 ml of thiobarbituric acid (0.67%) and heated at 95°C for 30 min. After cooling, absorbance of the samples was measured on a spectrophotometer at 532 nm. The results were expressed as the percentage of TBARS was formed by AAPH alone (inducedcontrol). Ascorbic acid is used as control in this assay (Ahmad et al., 2015).
Evaluation of EOED in vitro potential against production of hydroxyl radical (OH•)
Production of hydroxyl radical (OH•) was quantified by the Fenton reaction. During this reaction, the in vitro effect of EOED against the production of OH•, produced by the oxidative degradation of 2-deoxyribose, was determined (Lopes et al., 1999). The principle of the test is to quantify the degradation product of 2-deoxyribose, malonaldehyde (MDA), by its condensation with TBA. Briefly, the reactions were initiated by the addition of Fe2+(FeSO4) with 6 mmol.L-1 final concentration for solutions containing 2-deoxyribose 5 mmol.L-1, H2O2 100 mmol.L-1 and phosphate buffer 20 mmol.L-1 (pH 7.2). Concentrations of 0.9, 1.8, 3.6, 5.4, and 7.2 μg.ml-1 of EOED were added to the system before the addition of Fe2+ in order to determine EOED in vitro antioxidant activity against hydroxyl radical formation. The reactions were performed for 15 min at room temperature and they were stopped by the addition of phosphoric acid at 4% (v/v), followed by addition of TBA (1% v/v in NaOH 50 mmol.L-1). The solutions were heated in a water bath for 15 min at 95°C. The absorbance was measured at 532 nm and results were expressed as equivalents of MDA formed by Fe2+and H2O2.
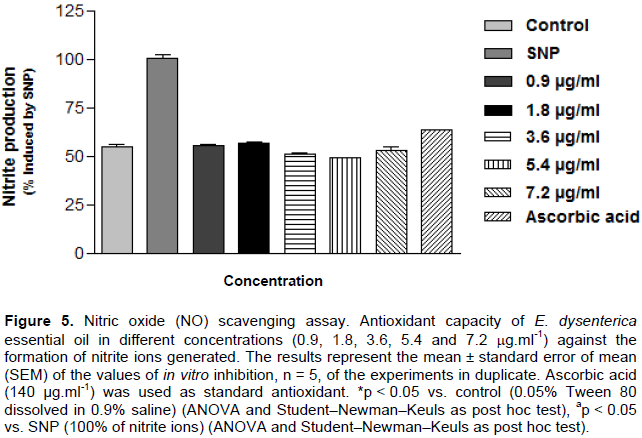
Evaluation of EOED in vitro potential against production of nitrite ion (NO2)
Nitric oxide was generated from the spontaneous decomposition of sodium nitroprusside (SNP) in 20 mM phosphate buffer (pH 7.4). Once generated, NO interacts with oxygen to produce nitrite ions, which were measured using the Griess reaction (Tsikas, 2007). The reaction mixture (1 ml) containing 10 mM SNP in phosphate buffer and EOED evaluated at concentrations of 0.9, 1.8, 3.6, 5.4, and 7.2 μg.ml-1 concentrations was incubated at 37°C for 1 h. A 0.5 ml aliquot was taken and homogenized with 0.5 ml Griess reagent. The absorbance of the chromophore formed was measured at 540 nm. The extent to which the nitric oxide generated was inhibited was measured by comparing the absorbance values of negative controls (only 10 mM SNP and blank) and assay preparations. Results were expressed as percentages of nitrite formed by SNP alone.
Chemical composition
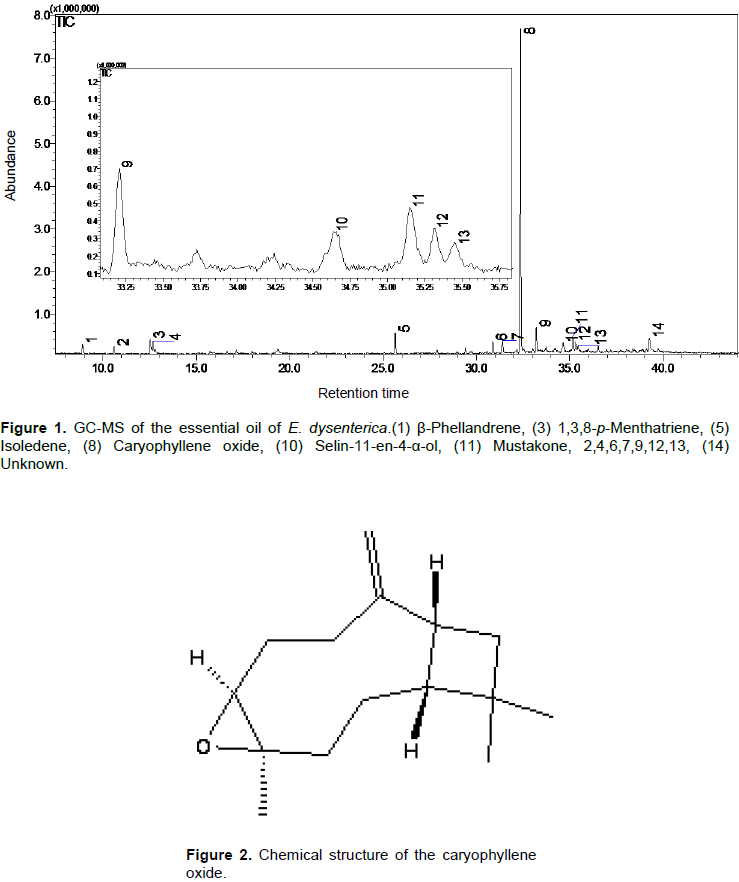
Hydrodistillation of the leaves of E. dysenterica gave a light-yellowish crude essential oil (EOED), with a yield of 1.45±1.48% (w/w), in relation to the dry weight of the plant material. As shown in Table 1, it was possible to identify 6 compounds (80.67% of the total composition) and the sesquiterpenes (75.4%) were the majority (Figure 1 and Table 1). The major compounds identified were caryophyllene oxide (66.4%) (Figure 2), isoledene (3.9%), 1,3,8-p-menthatriene (3.5%), mustakone (3.5%), β-phellandrene (1.7%), and selin-11-en-4-α-ol (1.7%)
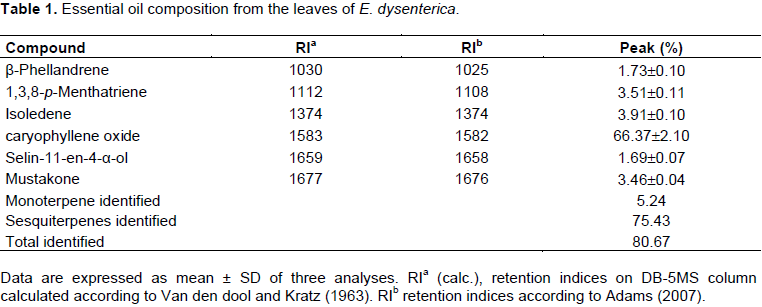
These results show a high content of caryophylleneoxide (66.37%) and there are also new constituents different from what have been found in other species of E. dysenterica collected in another region of Brazil as reported by Costa et al. (2000). β-caryophyllene (14.8%), α-humulene (10.9%), α-terpineol (6.1%), limonene (5.5%), α-thujene (5.6%), caryophyllene oxide (5.4%), and sabinene (3.9%).
In addition to the major constituent, caryophyllene oxide (66.37%) and β-caryophyllene have been reported in the essential oils of several other species of Eugenia (higher than 20%), indicating that this species is a typical The member of the Myrtaceae family (Costa et al., 2000). substances isoledene (3.91%), 1,3,8-p-menthatriene (3.51%), mustakone (3.46%), β-phellandrene (1.73%), and selin-11-en-4-α-ol (1.69%) were identified for the first time as chemical constituents of EOED.
Inhibition of AChE activity
The qualitative results of inhibition of enzyme AChE in TLC showed that the EOED and caryophyllene oxide inhibited the enzyme by the appearance yellow
backgrounds with white spots for inhibiting compounds were visible after about 5 min. This are the results of the first tests, yellow backgrounds with white spots for inhibiting compounds were visible after about 5 min.
The best anti-AChE activity was found for EOED (IC50 = 0.92 µg.ml-1) when compared with Eucalyptus camaldulensis (IC50 = 18.98 µg.ml-1), Ocimum canum (IC50=36.16 µg.ml-1) and Cistus salvifolius (IC50 = 58.10 μg.ml-1) (Kiendrebeogo et al., 2011). When starting the Ellman assay, it was observed that EOC was able to inhibit the enzyme AChE with an IC50 = 0.92 μg.ml-1 (Means from independent experiments were then expressed as means ± standard deviation (SD).
For all statistical analyses, p<0.001 was considered as statistically significant with a promising value when compared with drug rivastigmine (IC50 = 1.87 μg.ml-1), the conventional AChE inhibitor used in treatment of AD.
In another study, Eugenia sulcata essential oil contained monoterpenes known for their anticholinesterase activity (for example α-cubebene and β-copaene), with an inhibitory capacity of the enzyme AChE and value of IC50 = 4.66 μg.ml-1 (Lima et al., 2012).
In research, the caryophyllene oxide pure was tested on the enzyme AChE and showed a value for CI50 = 0.31 μg.ml-1. In studies, there are reports of analgesic and anti-inflammatory activities for caryophyllene oxide, major constituent of the EOED (Chavan et al., 2010).
Testing of antioxidants TBARS
To evaluate the antioxidant activity of EOED, two other methods were adapted which are based on the ability of a substance to scavenge free radicals through direct interaction with a substance reactive molecules, converting the less reactive free radical species and therefore more stable (Hoelzl et al., 2005).
Homogenized egg yolk as a lipid rich substrate was used to determine the antioxidant activity of EOED (Guimarães et al., 2010). TBARS is a complex formed by APPH (soluble water-azo compound is used as free radical generator) and TBARS.
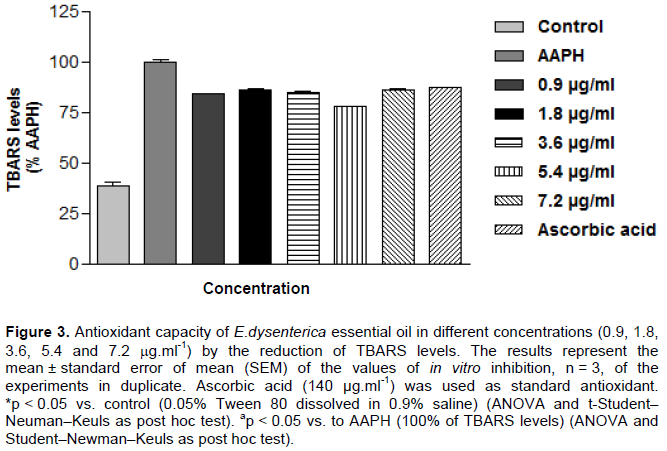
From the methods used in vitro, it was demonstrated that EOED was able to reduce the production of free radicals at all concentrations tested, with a better performance in antioxidant TBARS test, which is a method used to quantify the peroxidation which corresponds to a lipid in the cell membrane damage caused by oxidative stress. The EOED, at all concentrations tested, 0.9, 1.8, 3.6, 5.4 to 7.2 μg.ml-1, were capable of preventing lipid peroxidation inhibiting the amount of TBARS 16.07, 14.11, 15.02, 22.07, and 13.95%, respectively, as shown in Figure 3.
Similar results were obtained with ascorbic acid as antioxidant used provided standard 12.67% inhibition of TBARS formed. It was also found that 50% inhibitory concentration (IC50) of the oil is 1.2 μg.ml-1 against the formation of TBARS with variation margin on the effective concentration 0.3 to 5.8 mg.ml-1 (with 95% confidence interval).
There is evidence to indicate that free radicals cause oxidative damage to lipids, proteins, and nucleic acids (Uttara et al., 2009). AD is the most common form of and dementia, characterized by progressive neurodegeneration. Pathogenetic mechanisms, triggered by β-amyloid (Aβ) accumulation, include oxidative stress, deriving from energy homeostasis deregulation involving mitochondria and peroxisomes. At severe pathological stages, when senile plaques disrupt cortical cytoarchitecture, antioxidant capacity is gradually lost. Porcellotti et al. (2015) reported that oxidative stress occurs during the progression of β-amyloid pathology in the neocortex of the Tg2576 mouse model of AD, suggesting early therapeutic intervention in AD, also targeting peroxisomes.
In small quantities, antioxidants have great therapeutic potential for some conditions caused by free radicals such as arthritis, AD, heart disease, aging, cancer, among other (Halliwell et al., 1995). Antioxidants may thus be used as neuroprotectors agents, for example: aryl amines and indoles-carotene, lycopene polyenes-carotene, lycopene, and retinol selenium containing compounds ebselen, polyphenols-favonoids, stilbenes, and hydroquinone monophenols: tocopherols (vitamin E), 17-estradiol (estrogen), 5-hydroxytryptamine (serotonin), since oxidative damage may be observed before the formation of β-amyloid specific pathological signs (Behl et al., 1992).
The use of antioxidants has been explored in an attempt to slow AD progression and neural degeneration. Given the complex pathology of AD, current strategies for the development of new agents focus on compounds with various powers and plants are a major source of these
compounds (Konrath et al., 2012).
Evaluation of EOED in vitro potential against production of hydroxyl radical (OH•)
The radical OH• is a more toxic moiety known, since they can oxidise non-specifically all classes of biological macromolecules, including lipids, proteins, and nucleic acids with virtually limited diffusion rates (Imlay and Linn, 1988). Therefore, OH• may result in oxidative damage that gives rise to various diseases, including arthritis, atherosclerosis, cirrhosis, diabetes, cancer, AD, emphysema, and aging (Ozyurek et al., 2008).
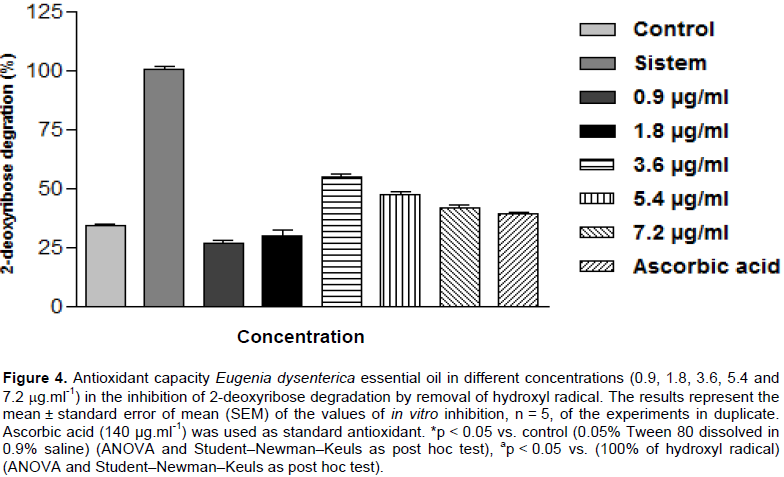
In this procedure OH• generated from a Fenton reaction with deoxyribose produce malondialdehyde (MDA) and similar substances (Halliwell and Gutteridge, 2007). After antioxidants testing in vitro was observed at all concentrations tested, 0.9, 1.8, 3.6, 5.4, and 7.2 µg.ml-1 to hydroxyl radical removal capacity in 73.46, 69.90, 45.09, 52.65, and 58.1%, respectively, as shown in Figure 4, results similar to that of ascorbic acid removed60.96% of the produced hydroxyl radical. IC50 was established at 5.814 µg.ml-1 against the formation of the hydroxyl radical with a variation range of 253 to 10.39 (with a 95% confidence interval).
Antioxidant potential evaluation in nitric oxide removal (NO)
In our studies, in vitro, was also possible to determine the 50% inhibitory concentration (IC50) of the sample 0.1684 μg.ml-1 against the formation of nitrite radical, with variation margin on the effective concentration from 0.06630 to 0.4276 μg.ml-1 with 95% confidence interval, was observed at all concentrations tested, 0.9, 1.8, 3.6, 5.4, and 7.2 µg.ml-1 to remove nitrite metabolic capacity in 44.62, 43.45, 48.97, 51.09, and 47.05% as shown in Figure 5 results similar to that of ascorbic acid removed 36.73%.
Antioxidants comprise a broad and heterogeneous family of compounds that share the common task of interfering with stopping, retarding, or preventing the oxidation or autoxidation of an oxidizable substrate (Savelev et al., 2003). Numerous physiological and biochemical processes in the human body may produce oxygen containing free radicals and other reactive oxygen or nitrogen species as by-products (Chavan et al., 2010). Overproduction of such radicals can cause oxidative damage to biomolecules, eventually leading to many diseases, such as atherosclerosis, cancer, diabetes, or inflammatory conditions and pain (Jeon et al., 2011).
Oxidative stress is implicated in the pathophysiology of a wide variety of neurodegenerative disorders, including Parkinson’sdisease, AD, Friedreich’s ataxia, amyotrophic lateral sclerosis, and stroke. This is the first report of biological properties of E. dysenterica, a candidate to be a source of antioxidant and anticholinesterase compounds for use as drugs.
The main constituents of E. dysenterica DC. (cagaiteira) essential oil from a water extract of leaves were caryophyllene oxide (66.37%), isoledene (3.91%), 1,3,8-p-menthatriene (3.51%), mustakone (3.46%), β-phellandrene (1,73%), and selin-11-en-4-α-ol (1,69%). The pure caryophyllene oxide (the main compound) was tested on the AChE and presented the IC50 = 0.31 mg.ml-1. This is the first report, that the essential oil from leaves E. dysenterica exhibits antioxidant effects preventing lipoperoxidation and AChE activity. The results are encouraging and it is suggested that they should be followed up with in vivo testing.
The authors have not declared any conflict of interests.
REFERENCES
|
Adam RP (2007). Identification of essential oil components by Gas Chromatography Mass Spectrometry. 4th ed. Carol Stream, Illnois - USA: Allured Publishing Corporation.
|
|
|
|
Ahmad S, Ullah F, Ayaz M, Sadiq A, Imran M (2015). Antioxidant and anticholinesterase investigations of Rumexhastatus D. Don: potential effectiveness in oxidative stress and neurological disorders. Biol. Res. 48:20.
Crossref
|
|
|
|
Behl C, Davie J, Cole GM, Schubert D (1992). Vitamin E protects nerve cells from amyloid protein toxicity. Biochem. Biophys. Res. Commun. 186:944-950.
Crossref
|
|
|
|
Buckholtz NS (2011). Perspective: In search of biomarkers. Nature. 475:(7355)S8.
Crossref
|
|
|
|
Campelo LML, De Almeida AA, Freitas RM, Cerqueira GS, de Sousa GF, Saldanha GB, Feitosa CM, de Freitas RM (2012). Antioxidant and antinociceptive effects of Citrus limon essential oil in mice". J. Biomed. Biotechnol. 2011(2011):1-8.
|
|
|
|
Cardoso LM, Martino HSD, Moreira AVB, Ribeiro SMR, Pinheiro-Sant'Ana HM (2011). Cagaita (Eugeniadysenterica DC.) of the Cerrado of Minas Gerais, Brazil: Physical and chemical characterization, carotenoids and vitamins. Food Res. Int. 44(7):2151-2154.
Crossref
|
|
|
|
Chaiyana W, Okonogi S (2012). Inhibition of cholinesterase by essential oil from food plant. Phytomedicine 19(8-9):836-839.
Crossref
|
|
|
|
Chavan MJ, Wakte PS, Shinde DB (2010). Analgesic and anti-inflammatory activity of Caryophyllene oxide from Annonasquamosa L. bark. Phytomedicine. 17(2):149-151.
Crossref
|
|
|
|
Costa TR, Fernandes OFL, Santos SC, Oliveira CM, Lião LM, Ferri PH, Paula JR, Ferreira HD, Sales BH, Silva MR (2000). Antifungal activity of volatile constituents of Eugenia dysenterica leaf oil. J. Ethnopharmacol. 72:111-117.
Crossref
|
|
|
|
Ellman GL, Courtney DK, Andres VJR, Featherstone RM (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 7(2):88-90.
Crossref
|
|
|
|
Enz A, Amstutz R, Boddeke H, Gmelin G, Malanowski J (1993). Brain selective inhibition of acetylcholinesterase: a novel approach to therapy for Alzheimer's disease. Progress Brain Res. 98:431-438.
Crossref
|
|
|
|
Esterbauer H, Cheeseman KH (1990). Determination of aldehydic lipid peroxidation products:Malonaldehyde and 4-hydroxyno- nenal. Methods Enzymol. 186:407-421.
Crossref
|
|
|
|
Guimarães AG, Oliveira GF, Melo MS, Cavalcanti SC, Antoniolli AR, Bonjardim LR, Silva FA, Santos JP, Rocha RF, Moreira JC, Araújo AA, Gelain DP, Quintans-Júnior LJ (2010). Bioassay-guided evaluation of antioxidant and antinociceptive activities of carvacrol. Basic Clin. Pharmacol. Toxicol. 107(6):949-957.
Crossref
|
|
|
|
Halliwell B, Gutteridge JMC (2007). Free Radicals in Biology and Medicine (4nd edition). Oxford University Press, New York, 704p.
|
|
|
|
Halliwell B, Aeschbach R, Lölinger J, Aruoma OI (1995). The characterization on antioxidants.Food Chem. Toxicol. Oxford 33(7):601-617.
Crossref
|
|
|
|
Hoelzl C, Bichler J, Ferk F, Simic T, Nersesyan A, Elbling L, Ehrlich V, Chakraborty A, Knasmüller S (2005). Methods for the detection of antioxidants which prevent age related diseases: a critical review with particular emphasis on human intervention studies. J. Physiol. Pharmacol. 56(2):49-64.
|
|
|
|
Houghton PJ, Howes MJR (2003). Plants used in Chinese and Indian traditional medicine for improvement of memory and cognitive function. Pharmacol. Biochem. Behav. 75:513-527.
Crossref
|
|
|
|
Imlay J, Linn S (1988). DNA damage and oxygen radical toxicity. Science. 240(4857):1302-1309.
Crossref
|
|
|
|
Jeon S, Bose S, Hur J, Jun K, Kim YK, Cho KS, Koo BS (2011). A modified formulation of Chinese traditional medicine improves memory impairment and reduces Abeta level in the Tg-APPswe/PS1dE9 mouse model of Alzheimer's disease. J. Ethopharmacol. 137 91):783-789.
|
|
|
|
Van Den Dool H, Kratz PD (1963). A Generalization of the Retention Index System including Linear Temperature Programmed Gas—Liquid Partition Chromatography. J. Chromatogr. 11:463-471.memory impairment and reduces Abeta level in the Tg-APPswe/PS1dE9 mouse model of Alzheimer's disease. J. Ethopharmacol. 137(91):783-789.
|
|
|
|
Kiendrebeogo M, Coulibaly AY, Nebie RCH, Zeba B, Lamien CE, Lamien-Meda A, Nacoulma OG (2011). Antiacetylcholinesterase and antioxidant activity of essential oils from six medicinal plants from Burkina Faso. Braz. J. Pharmacogn. 21(1).
Crossref
|
|
|
|
Konrath EL, Neves BM, Lunardi PS, Passos CS, Simões F, Ortega MG, Gonçalves CA, Cabrera JL, Moreira JCF, Henriques AT (2012). Investigation of the in vitro and ex vivoacetylcholinesterase and antioxidant activities of traditionally used Lycopodium species from South America on alkaloid extracts. J. Ethnopharmacol.139(1):58-67.
Crossref
|
|
|
|
Konrath EL, Passos CS, Klein-Júnior LC, Henriques AT (2013). Alkaloids as a source of potential anticholinesterase inhibitors for the treatment of Alzheimer's disease. J. Pharm. Pharmacol. 65:1701-1725.
Crossref
|
|
|
|
Lima BG, Tietbohl LAC, Fernandes CP, Cruz RAS, Botas GS, Santos MG, Silva-Filho MV, Rocha L (2012). Chemical Composition of Essential oils and Anticholinesterasic Acticity of Eugenia sulcata Spring ex Mart. Lat. Am. J. Pharm. (formerly Acta Farmacéutica Bonaerense), 31(1):152-155.
|
|
|
|
Loizzo MR, Jemia MB, Senatore F, Bruno M, Menichini F, Tundis R (2013). Chemistry and functional properties in prevention of neurodegenerative disorders of five Cistus species essential oils. Food Chem. Toxicol. 59:586-594.
Crossref
|
|
|
|
Lopes GKB, Schulman HM, Lima MH (1999). Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ions. Biochim. Biophys. Acta - General Subjects. 1472(1-2):142-152.
|
|
|
|
Mukherjee PK, Kumar V, Mal M, Houghton PJ (2007). Acetylcholinesterase inhibitors from plants. Phytomedicine 14:289-300.
Crossref
|
|
|
|
Oga M, Fonseca CEL (1994). A fast methodto estimate leafarea incagaiteiraseedlings (Eugenia dysenterica D.C.). BrazilianAgricultural Research.Brasília. 29(4):571-577.
|
|
|
|
Oliveira MES, Pantoja L, Duarte WF, Collela CF, Valarelli LT, Schwan R, Dias DR (2011). Fruit wine produced from cagaita (Eugenia dysenterica DC) by both free and immobilised yeast cell fermentation. Food Res. Int. 44(7):2391-2400.
Crossref
|
|
|
|
Ozyurek M, Bektasoglu B, Guclu K, Apak R (2008). Hydroxyl radical scavenging assay of phenolics and flavonoids with a modiï¬ed cupric reducing antioxidant capacity (CUPRAC) method using catalase for hydrogen peroxide degradation. Anal. Chim. Acta, Istanbul, Turkey. 616(2):196-206.
|
|
|
|
Porcellotti S, Fanelli F, Fracassi A, Sepe S, Cecconi F, Bernardi C, Cimini AM, Cerù MP, Moreno S (2015). Oxidative stress during the progression of β-amyloid pathology in the neocortex of the Tg2576 mouse model of Alzheimer's disease. Oxidative Med. cellular longevity. 1-18.
Crossref
|
|
|
|
Rhee IK, Van de Meent M, Ingkaninan K, Verpoorte R (2001). Screening for acetylcholinesterase inhibitors from Amaryllidaceae using silica gel thin-layer chromatography in combination with bioactivity staining. J. Chromatogr. A915(1-2):217-223.
Crossref
|
|
|
|
Rizzini C (1970). Efeito tegumentar na germinação de Eugenia dysentericaDC (Myrtaceae). Braz. J. Biol. 30(3):381-402.
|
|
|
|
Savelev S, Okello E, Perry NSL, Wilkin RM, Perry EK (2003). Synergistic and antagonistic interactions of anticholinesterase terpenoids in Salvia lavandulaefolia essential oil. Pharmacol. Biochem. Behav. 75 (3):661-668.
Crossref
|
|
|
|
Siddiqui MF, Levey AI (1999). Cholinergic Therapies in Alzheimer's Disease.Drugs Future. 24(4):417-444.
Crossref
|
|
|
|
Tsikas D (2007). Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: Appraisal of the Griess reaction in the L-arginine/nitric oxide area of research. J. Chromatogr. B851(1-2):51-70.
Crossref
|
|
|
|
Uttara B. Singh AV, Zamboni P,Mahajan RT(2009). Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr Neuropharmacol. 7:65-74.
Crossref
|