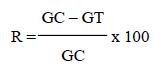ABSTRACT
In this study, phytochemical screening and evaluation were done for the antiplasmodial activity using in vitro test in Plasmodium falciparum and in vivo test in murine model (Plasmodium berghei) of the crude ethanolic extract of roots of Amasonia campestris (Aubl.) Moldenke (CEEAc), popularly known as mendoca and according to surveys ethnobotanically performed in this study, it is used as antimalarial in the State of Amapá, Amazonia, Brazil. The results of the phytochemical screening of CEEAc showed the presence of organic acids, reducing sugars, phenols, tannins, alkaloids and anthocyanins. The CEEAc showed reduction of 96% of the parasitic infection with the dose of 90 μg/mL and moderate antiplasmodial activity, with IC50 value of 42.94 μg/mL on the in vitro assay and partial antimalarial activity just on the highest dose tested (1000 mg/kg), with reduction of parasitic infection of 42.1% on the 5th day and of 37% on the seventh day after inoculation. The other doses were considered inactive. This is the first study reporting the use of A. campestris, to perform more detailed studies on its antiplasmodial and phytochemical activities with the aim to isolate bioactive compounds and elucidate the mechanisms of action.
Key words: Malaria, antiplasmodial activity, phytochemistry, Amasonia campestris (Aubl.) Moldenke.
Amasonia campestris (Aubl.) Moldenke belongs to the Lamiaceae family and Amazon genus. It is widely distributed in the north, northeast, midwest and southeast regions of the Brazil, occurring from the Amazon to the Espirito Santo, and found in Amapá, Pará, Maranhão, Piauí, Ceará, Pernambuco, Bahia and Goiás, This species inhabits the area Amazon in shady locations and edges of forests in latosols, and savanna environments; cerrado and rock field, and transition areas between savanna and cerrado. Mendoca, rabo de arara and bambã de arara, are listed as vernacular names for A. campestris. In folk medicine, the root is the main part of the plant used to treat uterine problems, bleeding and woman inflammation (Coelho-Ferreira, 2009).
Interest in the isolation and identification of chemical substances resulting from the secondary metabolism of plants and the growing search for new compounds that may have biological activity, increase every day. In this sense, the phytochemical covers more space combined with other branches of science such as botany, pharmacology and toxicology, which together contribute to the knowledge of the plant’s chemistry building as well as their pharmacological and toxicological properties, thus for safe use of the species and to confirm or not the indication of the traditional medicinal use (Sasidharan et al., 2011; Carvalho et al., 2015).
According to Bero et al. (2009), the search for new drugs with antimalarial activity is necessary due to the increase in resistant parasites to the main antimalarials used in medicine. The search must be based mainly on the popular medicine of many countries, which use plants for the disease’s treatment and its symptoms. The scientific studies related to the ethnopharmacology and the bioguided fractionations resulted in some of the major antimalarial, such as quinine and artemisinin, and yet have taken to the isolation of promising new antimalarial with a variety of structures.
The intensification of the studies related to natural substances to combat malaria resulted in many herbal extracts which have been tested to verify the antiplasmodial activity, but only a limited number of active compounds, belonging to different molecular classes, were isolated and identified (Burrows et al., 2013).
Although, many types of researches, reported on antiplasmodial activity in vivo and in vitro of several extracts and/or compounds isolated from plants, it is important to note that these activities are often considered moderate, suggesting that the species in question may have a limited effect in man and that the cure is improbable. However, this does not mean that the use of these species have no value, since many of them act as symptomatic in malaria treatment, for example, by reduction of anemia, relief of pain, fever and increasing immunity (Nogueira and Rosário, 2010; Veiga and Scudeller, 2015). It is noteworthy that, from the results obtained in these studies, the ethnopharmacology is a rich source for the discovery of new antimalarial compounds.
In general, evaluation of the antimalarial activity of the compounds is fulfilled by in vivo and in vitro tests, which may be complementary, as data obtained by in vitro tests require confirmation with live models and are fundamental to the development of new therapeutic agents. However, the analysis procedure and validation of antimalarial drugs are relatively time-consuming and complicated. The first phase of evaluation of a drug commonly consists of two subphases. The first one involves the use of assays for determining the effect of the compound on the growth of the human parasite covers analysis of the in vivo efficiency of drugs selected from small malaria models of animals, mainly the rodent’s parasites, Plasmodium berghei, Plasmodium yoelii and Plasmodium chabaudi in laboratory mice (Nogueira and Rosário, 2010).
In the State of Amapá, Amazon, Brazil, the traditional knowledge of medicinal plants is one of the greatest richness of the population. However, with the spread of the use of allopathic medicines, the natural medicine transmitted from generation to generation may end up forgotten. It is noteworthy that the majority of the cities in the state are considered a malaria-risk area and the resident population in these places has the habit of using medicinal plants to solve their health problems. Despite the constant and indiscriminate use, these species need to be widely researched and scientifically confirmed.
It is noteworthy that there is lack of studies on Amasonia genus, being described so far and there are only systematic and reproductive biology studies, which makes it a promising group for conducting chemical, pharmacological and toxicological studies. Therefore, the species, Amasonia campestris (Aubl.) Moldenke was investigated with regards to the chemical and pharmacological aspects of its crude ethanol extract through antimalarial tests, in vivo and in vitro and qualitative analysis through phytochemical screening of the main metabolites present in the extract.
Collection and preparation of the CEEAc
The A. campestris (Aubl.) Moldenke samples (Figure 1) were collected in the city of Porto Grande (0.335043N, -51.641241W), in the state of TableAmapá, Amazonia, Brazil in August 2012, and identified in the Botany Laboratory of the Federal University of Amapá, and the voucher specimen was deposited in the Amapaense Herbarium (HAMAB) under number 018 681.
The ethanolic extract was obtained by cold maceration for 48 h. The process was repeated twice and the extraction solution was subjected to rotary evaporation to remove the solvent and obtain the CEEAc.
Phytochemical screening
A sample of CEEAc was submitted for the prospecting tests, according to the techniques adapted from Carrera et al. (2014). These analyses aimed at the qualitative analysis of the secondary metabolites present in the extract.
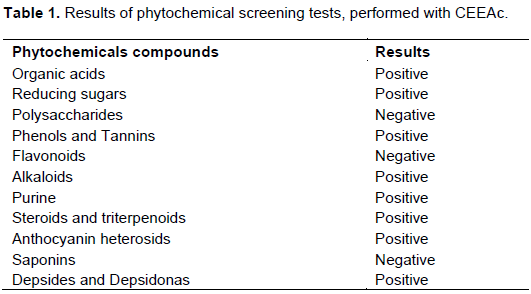
In this study, tests were done to analyze organic acids, reducing sugars, anthraquinones, polysaccharides, alkaloids, flavonoids, phenols and tannins, steroids and triterpenes, anthocyanin glycosides, saponins, depsides and depsidones.
Chromatographic profile
The CEEAc was subjected to column chromatography through the dry packaging technique. The adsorbent used was silica 60 gel, using the gradient eluents with increasing polarity gradient (hexane; hexane/ethyl acetate; ethyl acetate; ethyl acetate/methanol)
In thin layer chromatography, analytical (0.3 mm) stationary phasem used was silica gel GF-254 from Merck. The chromato-graphic revelations were made through direct observation of the chromatoplates in ultraviolet light, with a wavelength of 254 and 399 nm and sputter of chromatoplates with methanol/sulfuric acid.
In vitro antimalarial tests
The P. falciparum K1 strains used in the experiment were provided by Prof. Dr. Valter Ferreira de Andrade Neto (LABMAT/UFRN). The CEEAc was initially prepared in a stock solution of 5 mg/mL using the RPMI 1640 medium. From the stock solution, different dilutions of the CEEAc were prepared to be tested (90, 30, 10, 3.3, 1.1, 0.37 and 0.12 μg/mL).
To assess the response to P. falciparum samples to be tested, parasitized erythrocytes with approximately 1-2% parasitemia and predominance of young trophozoites (ring-shaped) were resuspended in a complete culture environment to a 3% hematocrit (Trager and Jensen 1976; 1977). This suspension was distributed in microplate (96 wells) of polyethylene, putting up 175 μl per well. Hereafter, approximately 2 h of incubation in a humidified CO2 incubator at 37°C (time required for sedimentation of red blood cells), the medium was removed from all wells and blood smears were prepared and randomly chosen from 6 to 8 wells. The procedure was performed to ensure the uniformity of the distribution of parasites in microplates. After that, only the culture mediums were added to the control well containing different concentrations of the extract.
The test was performed according to Andrade-Neto et al. (2004). 25 μl of each dilution and the control with chloroquine were transferred to the microplate containing the parasitized erythrocytes. The concentrations were tested in duplicate. The plate was incubated for 48 h at 37°C. After 48 h incubation period, smears were prepared from all samples and examined under an optical microscope to determine the percentage of parasitized erythrocytes. The growth inhibition of the parasites was determined by comparison of the growth without the samples according to the formula below:
In vitro activity criterion was established as: active sample (A) = parasites’ inhibition of growth in 80 to 100%. Partially active sample (PA) = parasites’ inhibition of growth in 50 to 79%. Inactive sample (I) = when the inhibition was less than 50%.
To evaluate the CEEAc antiplasmodial activity, the proposed scheme by Basco et al. (1994), modified by Dolabela (2008), was adopted, in which the extract was classified in accordance with the range of IC50 values: active (IC50 < 10.0 μg/mL); moderately active (IC50 between 10.0 and 100.0 μg/mL); inactive (IC50 > 100.0 μg/mL).
In vivo antimalarial tests
This study was approved by the Ethics Committee of the Federal University of Amapá under the Protocol 002A/2012 August 6, 2012. The P. berguei from NNK-65 lineage was provided by Biology Laboratory of Malaria and Toxoplasmosis (LABMAT) of Federal University of Rio Grande do Norte (UFRN).
This study used 25 male black mice (age, 6 weeks; weight 18 ± 2g; lineage C57BL/6); in the experimental animal, the mice were maintained in room at a temperature of 22 ± 2°C, relative humidity and 12-h light/12-h dark cycle. They were acclimatized for two weeks to the experimental tests with commercial food and water. In the experiment, 0.2 mL with 1x105 erythrocytes infected with P. berghei were inoculated intraperitoneally in each animal and then, they were randomly divided into five groups with five animals per cage. The experiment used the following scheme: a) group I: treated with CEEAc, dose of 250 mg/kg; b) group II: treated with CEEAc, dose of 500 mg/kg; c) group III: treated with CEEAc, dose of 1000 mg/kg; d) group IV: control group treated with chloroquine, dose of 10 mg/kg; e) group V: negative control group, treated only with water.
Chloroquine was used as reference drug, and the CEEAc were diluted in distilled water. All samples in the volume of 200 μL per animal (gavage) were administered orally. The mice were treated for four consecutive days. This test is called Peters Suppression Test (Peters, 1965), modified by Carvalho et al. (1991).
The CEEAc activity evaluation was accompanied from the 5th day after inoculation. The determination of the percentage of parasitemia was made through blood smears of the mice, made at 5th, 7th and 9th days. The smears were fixed and stained with dye Panoptic and examined by an optical microscope (1000x).
The parasitemia was determined by the reading of the smears by counting the number of parasitized erythrocytes among the 3000 total erythrocytes (Carvalho et al., 1991). The calculation of the parasitemia reduction, in percentage terms, was made in relation to the untreated control group and based on the following formula:
In which, GC = average of parasitized erythrocytes of the negative control group and GT = average parasitized erythrocytes of the test group. It was established as activity criteria of CEEAc in vivo for the inhibition of 30% or more.
Statistical analysis
The assays in vivo and in vitro were realized in duplicate and express in percentage. Then, for the parasitemia and antimalarial trials analysis ANOVA following the Tukey's test were used. Results with p < 0.05 were considered significant. For the IC50 calculation, the Microcal Origin 5.0 software was used, with the probit analysis.
The phytochemical test results are shown in Table 1. The CEEAc column chromatography generated 25 fractions. The fractions collected with hexane/AcOEt 25% (B, C and D) were analyzed by thin layer chromatography (TLC- Hexane/AcOEt 9:1), and the chromatograms revealed with sulfuric acid/methanol showed purple chromatographic zones, typically of triterpenes class substances (Martelanc et al., 2009).
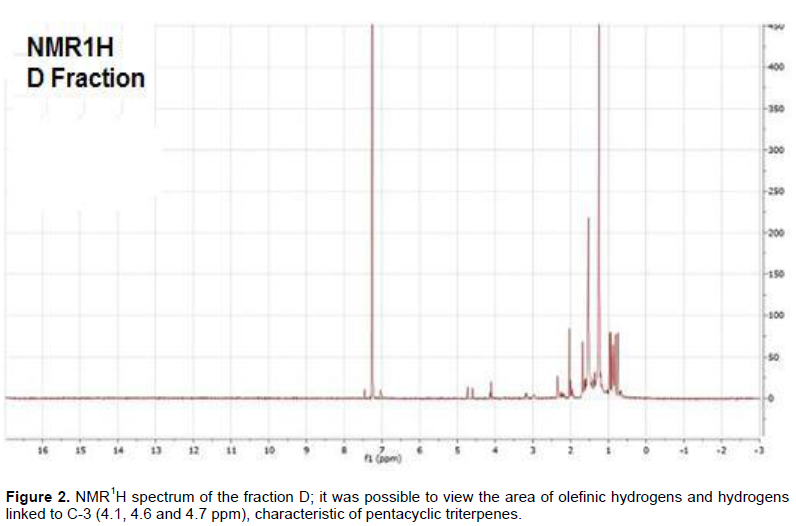
The D-fraction, apparently purest, appeared as a white solid, amorphous aspect and through the results of the TLC and of the NMR1H spectrum of the fraction (Figure 2); it was possible to view the area of olefinic hydrogens and hydrogens linked to C-3 (4.1, 4.6 and 4.7 ppm) of pentacyclic triterpenes, and it is assumed to be a mixture of substances belonging to the class of terpenes.
The CEEAc showed in vivo antimalarial activity only at the highest dose tested (1000 mg), with 42.1% reduction in parasitemia on the 5th day and 37% on 7th day after inoculation (Table 2). The remaining doses were found inactive by the criteria established in this study, where the activity is considered from inhibition of 30% or more (Carvalho et al., 1991).
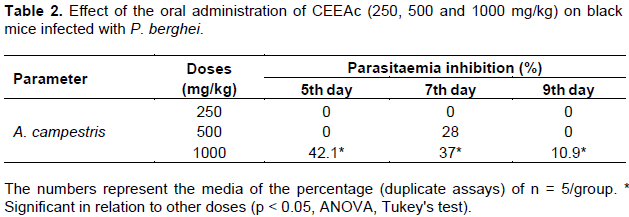
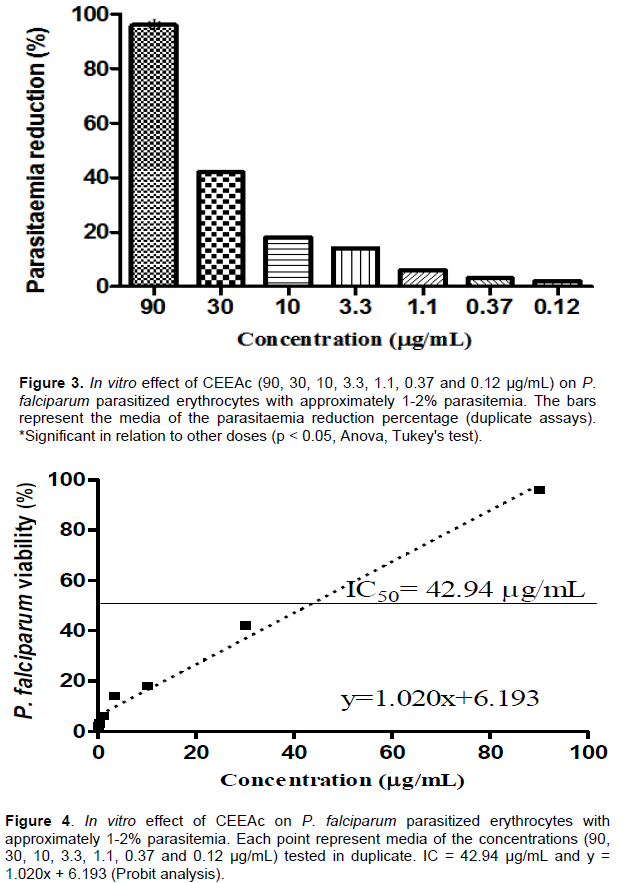
Regarding the treated animals mortality, it was observed that it was very close to the control untreated group, with survival average of 30 days for the control group and between 31 and 33 days for the treated groups. The small difference in mortality time between treated and untreated animals may be related by the half-life of the extract and metabolization in the organism, or due to immunological variations between the animals used in the study (Table 2). A criterion was established for in vitro activity that the doses that inhibit the growth of parasites from 80 to 100% are considered active; 50 to 79% partially active and less than 50% inactive. After the experiment and the results analyses, it was observed that the CEEAc was considered active in the highest tested dose (90 μg/mL), with reduction of parasite growth by 96% (Figure 3). The results indicate that the CEEAc has a moderate schizonticidal activity, with IC50 value = 42.94 μg/mL (Figure 4).
According to Sasidharan et al. (2011), the preliminary phytochemical analysis is used to identify the groups of secondary compounds, when chemical studies are not available on species of interest or may be relevant to direct research on isolating and structural elucidation of these substances. In this study, this preliminary information on the chemical composition of the species was extremely important, because despite the extensive literature search, chemical studies on the species of the Amasonia genus were not found.
Regarding the use of species as antimalarial treatment, the positive result for the presence of alkaloids in the extract suggests that the species might have anti-plasmodial activity (Basco et al., 1994). Saxena et al. (2003) reported that the antiplasmodial activity of plant-derived alkaloids has been widely reported in literature, and in 1990 and 2000, over a hundred of substances of this class are described, some of them are even more potent than chloroquine but in this study, a substance with characteristic of pentacyclic triterpenes was detected (Figure 2).
The mixture of substances with the class of terpenes, although preliminary results are fundamental, due to its huge structural diversity, have large numbers of biological properties as anti-cancer and anti-malarial activity (Maimore and Baran, 2007). They may be, therefore, associated with antimalarial activity related to the species.
The malaria treatment aims at blood schizogony interruption, which is the cause of pathogenesis and clinical symptoms of the disease. According to Oliveira et al. (2009), the oral treatment prevents disease progression to severe conditions; in other words, if drugs are administered properly, there is a decrease in morbidity and mortality from disease. In this study, CEEAc samples were administered orally (gavage), which is considered the appropriate choice in malaria treatment.
About the results, in which the CEEAc had partial antimalarial activity only at the highest tested dose (1000 mg), several factors must be considered (Table 2). The crude extracts obtained from plants contain many substances that can interfere with antagonistic form with the active substance, for example tannins, that was detected in the CEEAc (Table 1), often resulting in inactivity of them, facing the Plasmodium (Carvalho, 2016).
This is the first study that analyzed the antimalarial activity of A. campestris, and there is no data at this moment on the pharmacological studies or chemical compositions of the species and the Amasonia genus, and there is no data to compare this species with other species of the genus. However, A. campestris belongs to the family Lamiaceae, which in Brazil is represented by approximately 496 species (Harley, 2004), wherein some were already mentioned in ethnopharmacological survey as antimalarial, such as for invigorating antimalarial drugs used in Guiana French performed by Vigneron et al. (2005), which were cited as species Leonotis nepetifolia (L.) R.Br., Ocimum campechianum P. Mill. and Plectranthus barbatus Andrews. However, there are no guarantees that these species present compounds resulting from secondary metabolism that are similar and that may provide antimalarial activity, although they belong to the same family, but not to the same genus.
Despite the fact that value of IC50 in this study ranks CEEAc as moderately active, it is observed that it reduced the parasitemia in 96% at a dose of 90 μg/mL (Figures 3 and 4), as preliminary results, as this is an initial screening, supporting at this moment the traditional use of A. campestris (Aubl.) Moldenke (Mendoca) as antimalarial, according to ethnodirected survey of plants used as antimalarials in the State of Amapá.
This study evaluated, for the first time, the antimalarial activity of the roots of A. campestris, and the results showed antimalarial activity both in in vitro tests with infected erythrocytes with strains of P. falciparum and in vivo tests with mice infected with strains of P. berghei; however, these tests are preliminary, requiring more detailed studies of their antiplasmodial activity and chemical composition. The results obtained for antimalarial tests, although preliminary, corroborate with the popular statement obtained by ethnodirected studies on antimalarial medicinal plants in the state of Amapá.
The authors declare that there is no conflict of interest.
REFERENCES
|
Andrade-Neto VF, Goulart MO, Silva Filho JF, Pinto MC, Pinto AV, Zalis MG, Carvalho LH, Krettli AU (2004). Antimalarial activity of phenazines from lapachol, β-lapachone and its derivatives against Plasmodium falciparum in vitro and Plasmodium berghei in vivo. Bioorg. Med. Chem. Lett. 14(5):1145-1149.
Crossref
|
|
|
|
Basco LK, Mitaku S, Skaltsounis AL, Ravelomanantsoa N, Tillequin F, Kock M, Le Bras J (1994). In vitro activities of furoquinoline and acridone alkaloids against Plasmodium falciparum. Antimicrob. Agents Chemother. 38(5):1169-1171.
Crossref
|
|
|
|
|
Bero J, Frederich M, Quetin-Leclercq J (2009). Antimalarial compounds isolated from plants used in traditional medicine. J. Pharm. Pharmacol. 61(11):1401-1433.
Crossref
|
|
|
|
|
Burrows JN, Burlot E, Campo B, Cherbuin S, Jeanneret S, Leroy D, Spangenberg T, Waterson D, Wells TN, Willis P (2013). Antimalarial drug discovery-the path towards eradication. Parasitology 141(1):128-39.
Crossref
|
|
|
|
|
Carrera GC, Benedito EF, Souza-Leal T, Pedroso-de-Moraes C, Gaspi FOG (2014). Testes fitoquímicos em extratos foliares de Oeceoclades maculata Lindl. (Orchidaceae). Rev. Bras. Pl. Med. 16(4):938-944.
Crossref
|
|
|
|
|
Carvalho HO, Lima CS, Sanches AA, Silva JO, Fernandes CP, Carvalho JCT (2015). Study of the in vitro release profile of sesquiterpenes from a vaginal cream containing Copaifera duckei Dwyer (Fabaceae) oleoresin. J. Appl. Pharm. Sci. 5:1-6.
Crossref
|
|
|
|
|
Carvalho LH, Brandão MGL, Santos-Filho D, Lopes JLC, Kretlli AU (1991). Antimalarial activity of crude extracts from brazilian plants studied in vivo in Plasmodium berghei-infected mice and in vitro against Plasmodium falciparum in culture. Braz. J. Med. Biol. Res. 24(11):1113-1123.
|
|
|
|
|
Carvalho JCT (2016). Interacoes de principios ativos naturais. Formulário Médico-Farmaceutico de Fitoterapia. Editora Pharmabooks. Sao Paulo, Brasil.
|
|
|
|
|
Coelho-Ferreira M (2009). Medicinal knowledge and plant utilization in an Amazonian coastal community of Marudá, Pará State (Brazil). J. Ethnopharmacol. 126(1):159-175.
Crossref
|
|
|
|
|
Dolabela MF, Oliveira SG, Nascimento JM, Peres JM, Wagner H, Póvoa MM, de Oliveira AB (2008). In vitro antiplasmodial activity of extract and constituents from Esenbeckia febrifuga, a plant traditionally used to treat malaria in the Brazilian Amazon. Phytomedicine 15(5):367-372.
Crossref
|
|
|
|
|
Harley RM, Atkins S, Budantsev AL, Cantino PD, Conn BJ, Grayer R, Harley MM, De Kok RD, Krestovskaja T, Morales R, Paton AJ (2004). Labiatae. In Flowering Plants• Dicotyledons. Springer Berlin Heidelberg pp. 167-275.
Crossref
|
|
|
|
|
Maimone TJ, Baran PS (2007). Modern synthetic efforts toward biologically active terpenes. Nat. Chem. Biol. 3(7):396-407.
Crossref
|
|
|
|
|
Martelanc M, Vovk I, Simonovska B (2009). Separation and identification of some common isomeric plant triterpenoids by thin- layer chromatography and high-performance liquid chromatography. J. Chromatogr. A. 1216(38):6662-6670.
Crossref
|
|
|
|
|
Nogueira F, Rosário VE (2010). Métodos para avaliação da atividade antimalárica nas diferentes fases do ciclo de vida do Plasmodium. Rev Pan-Amaz Saude. 1(3):109-124.
|
|
|
|
|
de Oliveira FC, de Albuquerque UP, da Fonseca-Kruel VS, Hanazaki N (2009). Avanços nas pesquisas etnobotânicas no Brasil. Acta Bot. Bras. 23:590-605.
Crossref
|
|
|
|
|
Peters W (1965). Drug resistance in Plasmodium berghei. Vinke and Lips 1948. I. Chloroquine resistance. Exp. Parasitol. 17(1):80-89.
Crossref
|
|
|
|
|
Sasidharan S, Chen Y, Saravanan D, Sundram KM, Yoga Latha L (2011). Extraction, Isolation and Characterization of Bioactive Compounds from Plants' Extracts. Afr. J. Tradit. Complement Altern. Med. 8:1-10.
|
|
|
|
|
Saxena S, Pant N, Jain DC, Hakuni BRS (2003). Antimalarial agents from plant sources. Curr. Sci. 85:1314-1329.
|
|
|
|
|
Trager W, Jensen JB (1977). Plasmodium falciparum in culture: use of outdated erythrocytes and description of the candle jar method. J. Parasitol. 63:883-886.
Crossref
|
|
|
|
|
Trager W, Jensen JB (1976). Human malaria parasites in continuous culture. Science. 193:673-675.
Crossref
|
|
|
|
|
Veiga JB, Scudeller VV (2015). Etnobotânica e medicina popular no tratamento de malária e males associados na comunidade ribeirinha Julião – baixo Rio Negro (Amazônia Central). Rev. Bras. Pl. Med. 17:737-747.
Crossref
|
|
|
|
|
Vigneron M, Deparis X, Deharo E, Bourdy G (2005). Antimalarial remedies in French Guiana: A knowledge attitudes and practices study. J. Ethnopharmacol. 98(3):351-360.
Crossref
|
|