ABSTRACT
Alchornea cordifolia has been shown to be hepatoprotective against hepatotoxicity induced by high dose paracetamol in a model animal. However, its hepatoprotective effects against the hepatotoxicity induced by anti-tubercular drugs have not yet been studied, whereas anti-tubercular drugs are known to be hepatotoxic at therapeutic dose. The aim of this work was to evaluate the hepatoprotective effect of a methanol extract of A. cordifolia leaves in order to overcome hepatotoxicity induced by anti-tubercular drugs. Isoniazid, Rifampicin and Pyrazinamid have been used to induce hepatotoxicity in rats. The animals were administered hepatotoxic agent. Two hours later they were given methanol extract of A. cordifolia (MEAC) leaves or silymarin. One group of animals received only the anti-tubercular drugs, one group received MEAC only and another group received physiological saline. The animals were thus treated for 10 consecutive days. Blood sample was taken on the 11th day for evaluation of the biochemical parameters, as well as markers of hepatotoxicity. Isoniazid increased transaminases (ALT and AST), MEAC and silymarin reduced these biochemical parameters, Isoniazid + Rifampicin increased ALT and AST levels, MEAC reduced alanine transaminase (ALT) and aspartate transaminase (AST) levels, Isoniazid + Rifampicin + Pyrazinamid combination resulted in significant ALT elevation and MEAC reduced the ALT levels. MEAC alone did not significantly alter ALT and AST values. Phytochemical screening revealed the presence of flavonoids, polyphenols, saponosides and alkaloids. A. cordifolia leaves would thus have a protective effect against anti-tubercular drugs induced hepatotoxicity in rats.
Key words: Hepatoprotective, Alchornea cordifolia, antitubercular drugs.
The liver is indeed the main organ involved in metabolism and detoxification for the excretion of various endogenous and exogenous substances from the body. However, liver cells are prone to attack and necrosis by free radicals (Pramod et al., 2008). The liver therefore needs protection, whereas there are very few medicines which possess a protective effect of this liver. Herbal treatments are becoming increasingly important in the population. Some of these plants have demonstrated some hepatoprotective activity such as Trichilia roka (Germano et al., 2004), Hemidesmus indicus (Prabakan et al., 2004), Cassia fistula leaf extract (Bhakta et al., 2004), legumes (Wu et al., 2004), Acanthus ilicifolius (Babu et al., 2004) and Alchornea cordifolia (Olaleye et al., 2006; Osadebe et al., 2012). Several works regarding this have dealt with A. cordifolia (Effo et al., 2013; Kouakou-Siransy et al., 2010).
The hepatoprotective effect of A. cordifolia has been evaluated and reported against hepatotoxicity induced by paracetamol at high doses (Olaleye et al., 2006; Olaleye et al., 2007) in an animal. However, the protective effect of A. cordifolia on the hepatotoxicity induced by anti-tubercular drugs has not been reported, whereas anti-tubercular drugs are known to be hepatotoxic at therapeutic dose.
Anti-tubercular drugs (Isoniazid (INH), Rifampicin (RIF), Pyrazinamide (PZA) and Ethambutol (EMB) are the first line anti-tubercular drugs for the treatment of pulmonary tuberculosis. These drugs are responsible for many adverse effects (Blumberg et al., 2003; Yee et al., 2003) such as cytolytic, and result in an increase in serum transaminases level (Nolan et al., 1999; Shakya et al., 2004). Several authors reported that INH taken alone, in normal doses, was responsible for liver biochemical markers disorders. Aouam et al. (2007) reported hepatic disorders in 10 to 20% of users in Tunisia, and Blumberg et al. (2003) reported 0.5 to 2% of patients in the USA. When RIF was associated with INH, this liver disorder affected a greater number of patients (Aouam et al., 2007) and 2.5 to 6% of patients (Blumberg et al., 2003). The work of Aouam et al. (2007) also showed that INH + RIF + PZA was responsible for cytolytic hepatitis in 0.5 to 10% of treated patients.
In continued investigation on this plant, this present work seeks to evaluate the hepatoprotective effect of a methanol extract of A. cordifolia leaves in vivo in rats in order to overcome hepatotoxicity induced by anti-tubercular drugs.
Plant materials
The plant material consisted of leaves of A. cordifolia (Schum. And Thonn.) collected at Yakasse-Mé (In the city of Adzopé about 75 km from Abidjan, Ivory Coast). Voucher samples (AC 2016) are kept in the Pharmacology Laboratory. The leaves were authenticated at the National Floristic Center of Abidjan, affiliated to Université Félix Houphouët Boigny (Abidjan) and air-dried in the laboratory at 18°C.
Extraction method
The fine powder of dried leaves (100 g) was macerated for 24 h at room temperature in 1 L of 70% methanol. The resulting filtrate was evaporated using a rotary evaporator (Büchi R180). The obtained dry extract (methanolic extract of A. cordifolia: MEAC) was conserved at 4°C and aliquots of dry powder were used for pharmacological studies after being suspended in physiological saline.
Animal material
The animal material consisted of rats, Rattus Norvegicus, Wistar strain weighing between 150 and 220 g which were obtained from the laboratory animals of the Pharmacology Laboratory of the Faculty of Pharmacy and Biological Sciences of Université Félix Houphouët Boigny Abidjan (Côte d'Ivoire). All animals were kept under controlled environmental conditions of 24 ± 1°C with a cycle of 12 h of light and 12 h of darkness. The animals had free access to water and food. Before the beginning of the experiment, they were subjected to fasting for 12 h with free access to water.
Chemical materials used
In this study, we used isotonic saline solution 0.9%, ether (Gifrer), distilled water, anti-tubercular drugs (INH (Lupine LTD), RIF (Remedica LTD), PZA (Cadila Pharmaceuticals Limited)), silymarin (Sigma Aldrich), methanol (VWE chemicals). Silymarin was used as a reference liver protector substance in this study. It is a mixture of three flavonoids (silychristin, silydianine and silybin) used as a hepatoprotective agent extracted from the seeds and fruits of Silybum marianum (Parthasarathy et al., 2007).
Study of the hepatoprotective activity of A. cordifolia in rats
Principle
The study involved inducing hepatotoxicity in laboratory rats by using anti-tubercular drugs in different combination (Santhosh et al., 2007; Saraswathy et al., 1998), and then evaluating the effect of different preparations on hepatic markers.
Procedure
Effect of MEAC alone on hepatotoxicity markers: Rats of both sexes were divided into 4 batches of 6 rats each and were treated for 10 days as follows:
- The rats in lot 1 (negative control) received saline solution by gavage;
- The rats in lot 2 received MEAC at 200 mg/kg/day by gavage;
- The rats in lot 3 received MEAC at 400 mg/kg/day by gavage;
- The rats in lot 4received MEAC at 800 mg/kg/day by gavage.
Hepatoprotective effect against INH-induced hepatotoxicity: Rats of both sexes were divided into 6 batches of 6 rats each and were treated for 10 days as follows:
- The rats in lot 1 (negative control) received saline solution by gavage;
- The rats in lot 2 received INH (100 mg/kg/day) by gavage;
- The rats in lots 3, 4 and 5 received MEAC (200, 400 and 800 mg/kg) orally 2 h after administration of INH (100 mg/kg/day).
- The rats in lot 6 (positive control) received sylimarin (100 mg/kg/day) orally, 2 h after administration of INH (100 mg/kg/day).
Hepatoprotective effect against INH+RIF-induced hepatotoxicity: Rats of both sexes were divided into 6 batches of 6 rats each and were treated for 10 days as follows:
- The rats in lot 1 (negative control) received saline solution by gavage;
- The rats in lot 2 received INH (100 mg/kg/day) +RIF (100 mg/kg/day) by gavage;
- The rats in lots 3, 4 and 5 received MEAC (200, 400 and 800 mg/kg) orally 2 h after administration of INH (100 mg/kg/day) +RIF (100 mg/kg/day).
- The rats in lot 6 (positive control) received sylimarin (100 mg/kg/day) orally, 2 h after administration of INH (100 mg/kg/day) +RIF (100 mg/kg/day).
Hepatoprotective effect against INH+RIF+PZA-induced hepatotoxicity: Rats of both sexes were divided into 6 batches of 6 rats each and were treated for 10 days as follows:
- The rats in lot 1 (negative control) received saline solution by gavage;
- The rats in lot 2 received INH (100 mg/kg/day) +RIF (100 mg/kg/day) + PZA (100 mg/kg/day) by gavage;
- The rats in lots 3, 4 and 5 received MEAC (200, 400 and 800 mg / kg) orally 2 h after administration of INH (100 mg/kg/day) +RIF (100 mg/kg/day) + PZA (100 mg/kg/day).
- The rats in lot 6 (positive control) received sylimarin (100 mg/kg/day) orally, 2 h after administration of INH (100 mg/kg/day) +RIF (100 mg/kg/day) + PZA (100 mg/kg/day).
Liver biochemical indices measured
At the end of 10 days of treatment, a blood sample was taken on the 11th day by cardiac puncture for the determination of markers of hepatotoxicity. They were alanin aminotransferase (ALT) and aspartat aminotransferase (AST) (Reitman et al., 1957).
Phytochemical screening
Screening for different chemical groups was done using the method as described in the works of Békro et al. (2007), Ronchetti and Russo (1971) and Wagner et al. (1983).
Statistical analysis
The results were expressed as mean ± SD. Statistical analysis used the Wilcoxon test. The difference between the mean values was considered significant at p <0.05.
Extraction yield
Extraction with 70% methanol (MEAC) gave 15.96 g of dry residue, that is, a yield of 15.96%.
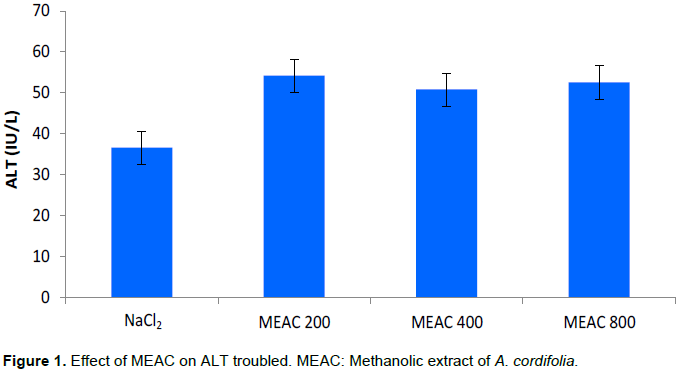
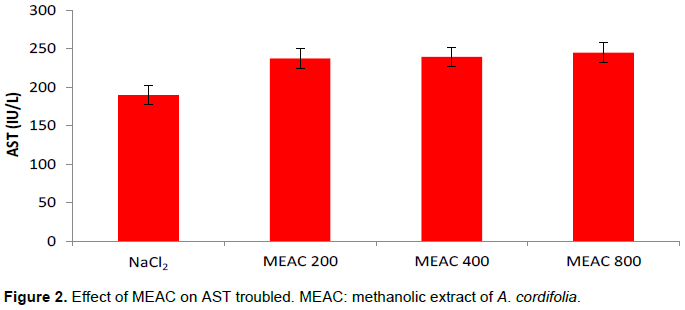
Effect of EMAC on transaminases
The effect of MEAC only was evaluated on serum transaminases and the various mean values are recorded in Figures 1 and 2. Different doses of MEAC only resulted in a non-significant increase in transaminase (AST and ALT) values compared to the NaCl2 batch (p > 0.05).
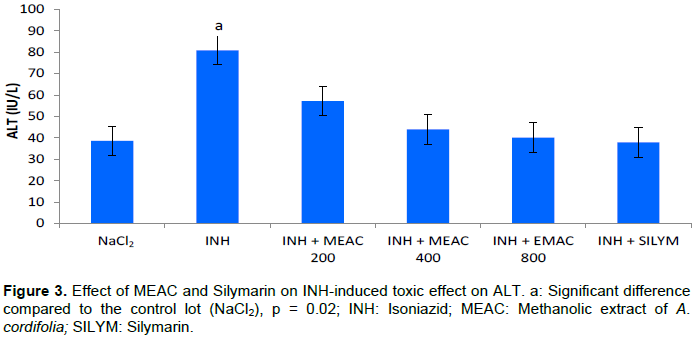
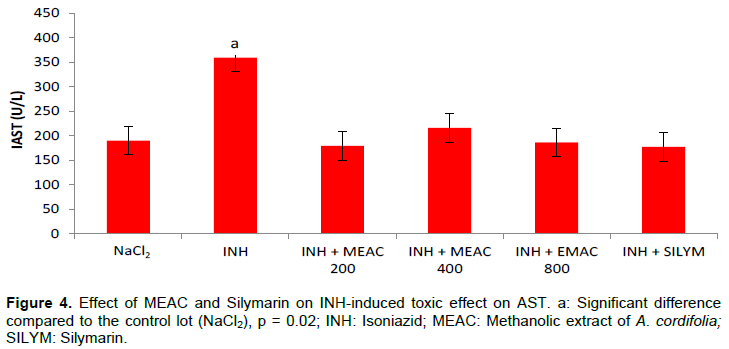
Hepatoprotective effect against INH induced hepatotoxicity
The mean values of serum transaminases are shown in Figures 3 and 4. INH caused increased transaminases (ALT and AST) (p = 0.028) in rats receiving it compared to rats given only NaCl2. The doses of MEAC and silymarin reduced significantly the different elevated values (p < 0.05). The activity of the various extracts was compared with the standard reference drug used, silymarin, and showed no significant difference whatever the dosage used. The comparison of the different doses of MEAC between them showed no significant difference (p > 0.05).
Hepatoprotective effect against INH + RIF induced hepatotoxicity
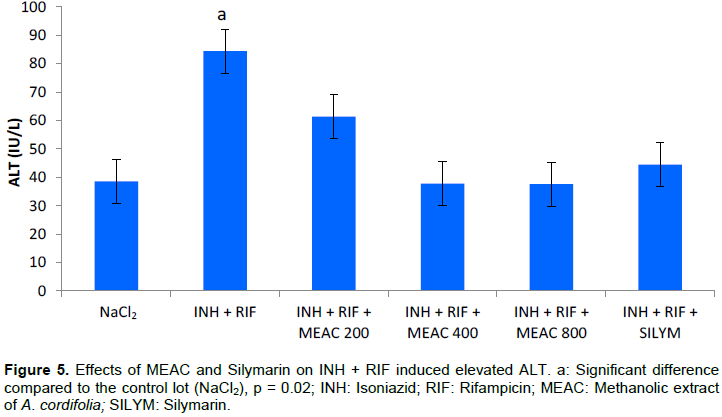
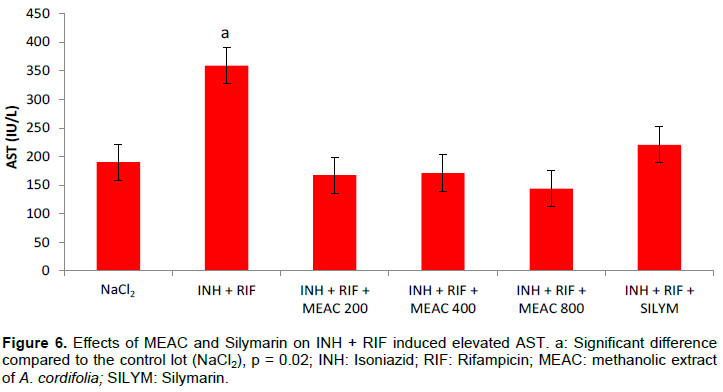
The mean values of the serum transaminases are recorded in Figures 5 and 6. The combination of INH + RIF resulted in an elevation of ALT and AST (p = 0.02) in rats compared to rats given only NaCl2. The administration of MEAC and silymarin in rats resulted in a significant reduction in abnormally high ALT and AST levels (p < 0.05). Comparison of the different doses of MEAC with silymarin did not show any significant difference (p > 0.05).
Hepatoprotective effect against INH + RIF + PZA induced hepatotoxicity
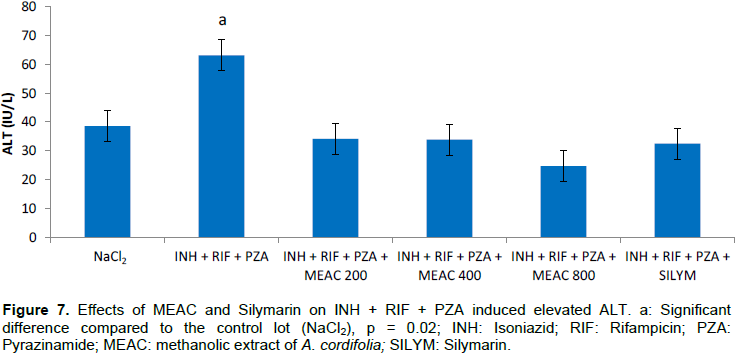
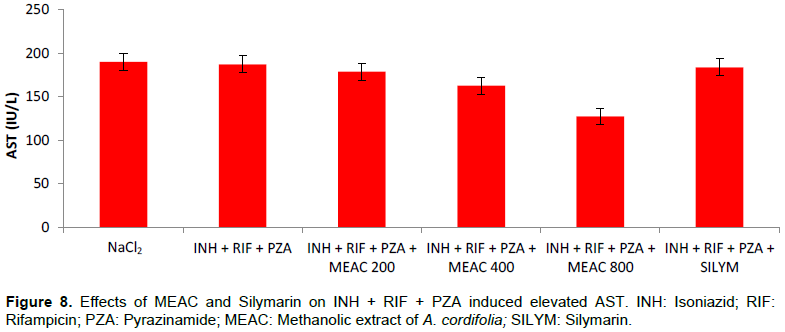
The mean serum transaminase values are shown in Figures 7 and 8. The combination of INH + RIF + PZA resulted in a significant elevated ALT (p = 0.02) in rats compared to rats that were given only NaCl2. The AST was not disturbed (p > 0.05). The administration of MEAC and silymarin in rats significantly reduced abnormally elevated ALT levels (p < 0.05).
Phytochemical screening
The phytochemical screening carried out on MEAC revealed the presence of large phytochemical groups flavonoids, polyphenols, saponosides and alkaloids, but an absence of tannins.
The purpose of this study was to evaluate the hepatoprotective effect of a methanol extract of A. cordifolia leaves in vivo in rats in order to overcome hepatotoxicity induced by anti-tubercular drugs. Isoniazid only and Isoniazid + Rifampicin combination resulted in a significant increase in ALT and AST levels. The combination of Isoniazid + Rifampicin + Pyrazinamid, on the other hand, caused an increase in the ALT values only. ALT and AST are two well-known diagnostic indicators of liver damage, with a higher specificity for ALT. In liver injury involving hepatocellular lesions and parenchymal cell necrosis, these enzymes are released from the damaged tissues and thus increase their level in the blood flow (Nkosi et al., 2005). They are two hepatic enzymes linked to the subcellular functions of mitochondria (Dwivedi et al., 1993).
In this present study, anti-tubercular drugs were used in high doses to induce hepatotoxicity in laboratory animals (Santhosh et al., 2007; Saraswathy et al., 1998). These produced lesions in the liver, including centrilobular necrosis and deterioration of liver cells (Graham et al., 2004) resulting in an increase in serum transaminases (Nolan et al., 1999; Shakya et al., 2004) as observed in rats given the hepatotoxic agents.
The administration of the 200 mg/kg methanolic extract, 400 and 800 mg/kg, as well as sylimarin at 100 mg/kg, resulted in a significant decrease in the values ​​of transaminases caused by anti-tubercular drugs. The effect of the extract on ALT and AST is comparable to that of silymarin, the standard reference hepatoprotective agent used in this study (Parthasarathy et al., 2007).
This ability of the extract to reduce these values ​​would suggest a hepatoprotective effect of A. cordifolia leaves. This effect would be manifested by stabilization of the hepatic membrane and regeneration of the hepatocytes. The extract also prevents the release of hepatic enzymes to the blood stream by reducing tissue lesions (Madhu et al., 2012; Singh et al., 2012). The hepatoprotective activity of A. cordifolia has already been demonstrated in rats against hepatotoxicity induced by high doses of paracetamol (Arhoghro et al., 2015; Olaleye et al., 2006, 2007) and tetrachloride (Osadebe et al., 2012). The results of our work on the methanol extract of A. cordifolia is therefore in agreement with the results of previous work and could confirm the hepatoprotective effect of the leaves of A. cordifolia, a plant very popular in African traditional medicine.
The extracts itself administered at 200, 400 and 80 mg/kg over 10 days did not cause hepatotoxicity, although it caused a non-significant increase in transaminases. However, a significant increase in transaminases was found in male rats as reported by other authors by administering a methanol extract of A. cordifolia leaves at 800 and 1600 mg/kg over 8 days (Ajibade and Olayemi, 2015). A. cordifolia is however considered to be a plant with a high margin of safety in a single administration (Organisation de coopération et de développement économiques - OCDE, 1998). In this study for the extraction, 70% methanol was used, contrary to Ajibade and Olayemi (2015) who used pure methanol. Lowering the degree of methanol could explain the absence of toxicity of the extract this study. Moreover,
the place of collection of plants could explain this absence of toxicity of our extract at 800 mg/kg. The plant was collected from the forest in Côte d'Ivoire while Ajibade and Olayemi (2015) collected theirs in a botanical garden in Nigeria.
The phytochemical screening has revealed the presence of flavonoids, polyphenols, saponosides and alkaloids in the methanol extract which could be responsible for its hepatoprotective effect. Indeed, Manga et al. (2004) found that flavonoids possess antioxidant and anti-inflammatory activities. Other studies have shown that the antioxidant activity of plants used in the traditional medicine was mainly due to the presence of flavonoids (Czinner and Yunes, 2001). Huong et al. (1998) also showed that saponosides were endowed with antioxidant properties. The protective effects of the extract could be due to the presence of one or more of the active ingredients in the plant (Banzouzi et al., 2002).
This study demonstrated that the methanol extract of A. cordifolia leaves could provide significant protection against the hepatotoxic effects of anti-tubercular drugs in rats. These finding results are promising, considering the hepatotoxic adverse effects of anti-tubercular drugs observed in patients during the treatment of pulmonary tuberculosis.
REFERENCES
|
Ajibade TO, Olayemi FO (2015). Reproductive and toxic effects of methanol extract of Alchornea cordifolia leaf in male rats. Andrologia 47(9):1034-1040.
Crossref
|
|
|
|
Aouam K, Chaabane A, Loussaïef C, Romdhane FB, Boughattas NA, Chakroun M (2007). Les effets indésirables des antituberculeux : épidémiologie, mécanismes et conduite à tenir. Med. Mal. Infect. 37(5):253-261.
Crossref
|
|
|
|
|
Arhoghro EM, Ikeh CH, Eboh AS, Angalabiri-Owei B (2015). Liver function of wistar rats fed the combined ethanolic leaf extract of alchornea cordifolia and costus afer in paracetamol-induced toxicity. World J. Pharmaceut. Res. 4(5):01-12.
|
|
|
|
|
Babu BH, Shylesh BS, Padikkala J (2004). Antioxidant and hepatoprotective effect of Acanthus ilicifolius. Fitoterapia 72(3):272-277.
Crossref
|
|
|
|
|
Banzouzi JT, Prado R, Menan H, Valentin A, Roumestan C, Mallie M, Pelissier Y, Blache Y (2002). In vitro antiplasmodial activity of extracts of Alchornea cordifolia and identification of an active constituent: ellagic acid. J. Ethnopharmacol. 81(3):399-401.
Crossref
|
|
|
|
|
Bekro YA, Mamyrbekova JA, Boua BB, Bi FT, Ehile EE (2007). Etude ethnobotanique et screening phytochimique de Caesalpinia benthamiana (Baill.) Herend. et Zarucchi (Caesalpiniaceae). Rev. Sci. Nat. 4 (2):217-225.
|
|
|
|
|
Bhakta T, Banerjee S, Mandal SC, Maity TK, Saha BP, Pal M (2001). Hepatoprotective activity of Cassia fistula leaf extract. Phytomedicine 8(3):220-224.
Crossref
|
|
|
|
|
Blumberg H, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, Fujiwara P, Grzemska M, Hopewell PC, Iseman MD, Jasmer RM (2003). American Thoracic Society/Center for Disease Control and Prevention/Infectious Discases Society of America treatment of tuberculosis. Am. J Respir Crit. Care Med.167:603-62
Crossref
|
|
|
|
|
Czinner E, Hagymasi K, Blazovics A, Kery A, Szoke E, Lemberkovics E (2001). The in vitro effect of Helichysi flos on microsomal lipid peroxidation. J. Ethnopharmacol. 77(1):31-35.
Crossref
|
|
|
|
|
Dwivedi Y, Rastogi R. Chander R. Sharma SK, Kapoor NK, Garg NK, Dhawan BN (1993). Hepatoprotective activity of picroliv against carbon-tetrachloride induced damage in rats. Ind. J. Med. Res. 92:1995-2000.
|
|
|
|
|
Effo KE, Kouakou-Siransy G, Irie-Nguessan G, Sawadogo RW, Dally IL, Kamenan AB, Kouakou LS, Kablan-Brou J (2013). Acute toxicity and antipyretic activities of a methanolic extract of Alchornea cordifolia leaves. Pharmacol. Pharm. 4(07):1-6.
Crossref
|
|
|
|
|
Germano MP, D'Angelo V, Sanogo R, Morabito A, Pergolizzi S, De Pasquale R (2004). Hepatoprotective activity of Trichilia roka on carbon tetrachloride-induced liver damage in rats. J Pharm Pharmacol. 53(11):1569-74.
Crossref
|
|
|
|
|
Graham GG, Scott KF, Day RO (2004). Alcohol and paracetamol. Aust. Prescribe. 27:14-15. Available at:
View
Crossref
|
|
|
|
|
Huong NTT, Matsumoto K, Kasai R, Yamasaki K, Waranabe H. (1998). In vitro antioxidant activity of Vietnamese ginseng saponin and its components. Biol Pharmaceut Bull. 21(9):978-983.
Crossref
|
|
|
|
|
Kouakou-Siransy G, Sahpaz S, N'guessan GI, Datté JY, Brou JK, Gressier B, Bailleul F (2010). Effects of Alchornea cordifolia on elastase and superoxide anion produced by human neutrophils. Pharm. Biol. 48(2):128-133.
Crossref
|
|
|
|
|
Madhu KP, Vijaya RA, Ganga RB (2012). Investigation of hepatoprotective activity of Cyathea gigantea (Wall. ex. Hook.) leaves against paracetamol-induced hepatotoxicity in rat. Asian. Pac. J. Trop. Biomed. 2(5):352-356.
Crossref
|
|
|
|
|
Manga HM, Brkic D, Marie DEP, Quetin-Leclercq J (2004). In vivo anti-inflammatory activity of Alchornea cordifolia. J. Ethnopharmacol. 92(2):209-214.
Crossref
|
|
|
|
|
Nkosi CZ, Opoku AR, Terblanche SE. (2005). Effect of pumpkin seed (Cucurbita pepo) protein isolate on the activity levels of certain plasma enzymes in CCl4-induced liver injury in low protein fed rats. Phytother. Res. 19(4):341-345.
Crossref
|
|
|
|
|
Nolan CM, Goldberg SV, Buskin SE (1999). Hepatotocixity associated with isoniazid preventive therapy. Jama 281:1014-1018.
Crossref
|
|
|
|
|
OCDE (1998) Système intégré harmonisé de classification des dangers pour la santé humaine et les effets environnementaux des substances chimiques. Organisation de coopération et de développement économiques, Paris, France.
|
|
|
|
|
Olaleye MT, Adegboye OO, Akindahunsi AA (2006). Alchornea cordifolia extract protects wistar albino rats against acetaminophen-induced liver damage. Afr. J. Biotechnol. 5(24):2439-2445
|
|
|
|
|
Olaleye MT, Kolawole AO, Ajele JO (2007). Antioxidant Properties and Glutathione S Transferases Inhibitory Activity of Alchornea cordifolia Leaf Extract in Acetaminophen-Induced Liver Injury. Iran. J. Pharmacol. Ther. 6:63-66.
|
|
|
|
|
Osadebe PO, Okoye FBC, Uzor PF, Nnamani NR, Adiele IE, Obiano NC (2012). Phytochemical analysis, hepatoprotective and antioxidant activity of Alchornea cordifolia methanol leaf extract on carbon tetrachloride-induced hepatic damage in rats. Asian Pac. J. Trop. Med. 5(4):289-293 289-293
Crossref
|
|
|
|
|
Parthasarathy R, Nivethetha M, Brindha P (2007). Hepatoprotective activity of Caesalpinia bonducella seeds on paracetamol induced hepatotoxicity in male albino rats. Ind. Drugs 44(5):401-404.
|
|
|
|
|
Prabakan M, Anandan R, Devaki T (2004). Protective effect of Hemidesmus indicus against rifampicin and isoniazid-induced hepatotoxicity in rats. Fitoterapia 71(1):55-59.
Crossref
|
|
|
|
|
Pramod K, Deva RG, Lakshmayya, Ramachandra SS (2008). Antioxidant and hepatoprotective activity of tubers of Momordica tuberose Cogn. against CCl4 induced liver injury in rats. Ind. J. Exp. Biol. 46(7):510-513.
|
|
|
|
|
Reitman S, Frankel S. (1957). A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 28(1):56-63.
Crossref
|
|
|
|
|
Repetto MG, Llesuy SF (2002). Antioxidant properties of natural compounds used in popular medicine for gastric ulcers. Braz. J. Med. Biol. Res. 35(5):523-534.
Crossref
|
|
|
|
|
Ronchetti F, Russo G (1971). A new alkaloid from Rauvolfia vomitoria. Phytochem. 10(6):1385-1388.
Crossref
|
|
|
|
|
Santhosh S, Sini TK, Anandan R, Mathew PT (2007). Hepatoprotective activity of chitosan against isoniazid and rifampicin-induced toxicity in experimental rats. Eur. J. Pharmacol. 572(1):69-73.
Crossref
|
|
|
|
|
Saraswathy SD, Suja V, Prema G, Shyamala DC (1998). Effect of Liv. 100 against antitubercular drugs (isoniazid, rifampicin and pyrazinamide) induced hepatotoxicity in rats. Ind. J. Pharmacol. 30:233-238.
|
|
|
|
|
Shakya R, Rao BS, Shrestha B (2004). Incidence of hepatotoxicity due to antitubercular medecines and assessment of risk factors. Ann. Pharmacother. 38:1074-9
Crossref
|
|
|
|
|
Singh K, Singh N, Chandy A, Manigauha A (2012). In vivo antioxidant and hepatoprotective activity of methanolic extracts of Daucus carota seeds in experimental animals. Asian Pac. J. Trop. Biomed. 2:385-388.
Crossref
|
|
|
|
|
Wagner H, Bladt S, Zgainski EM (1983). Drogen analyse, Dünschicht chromatographische Analyse von Arzneidrogen. Springer Verlag Berlin Heidelberg New York. 522p.
|
|
|
|
|
Wu SJ, Wang JS, Lin CC, Chang CH (2004). Evaluation of hepatoprotective activity of legumes. Phytomedicine 8(3): 213-219.
Crossref
|
|
|
|
|
Yee D, Valiquette C, Pelletier M, Parisien I, Rocher I, Menzies D (2003). Incidence of serious side effects from first line antituberculosis drugs among patients treated for active tuberculosis. Am. J. Respir. Crit. Care Med. 167:1477.
Crossref
|
|