ABSTRACT
The aim of this study was to determine suitable extraction solvents for antimicrobial compounds and the antimicrobial activity of two algal species, Plocamium cornutum and Plocamium rigidum collected from the coastline of Namibia. Samples were collected at low tide from the intertidal area of the coastline at Lüderitz and Henties Bay. The samples were collected about 5 to 10 cm under water by hand and placed in a sealable polythene bag and refrigerated at -20°C. Dried algae extracts were reconstituted in distilled water, hexane, dichloromethane, ethanol, methanol and chloroform, respectively and tested in vitro for antimicrobial activity using the Kirby Bauer disc diffusion method against 12 pathogens (Escherichia coli, Staphylococcus aureus, Staphylococcus saprophyticus, Pseudomonas aeruginosa, Streptococcus pyogenes, Proteus mirabilis, Listeria monocytogenes, Shigella sonnei, Salmonella species, Enterococcus faecalis, Candida albicans and Staphylococcus epidermidis). Screening confirmed that water extracts showed no activity against all the pathogens as the extracts were insoluble in water. The Plocamium extracts in the remaining solvents showed varying degrees of antimicrobial activity. Both dichloromethane and methanol extracts reconstituted in chloroform showed the greatest activity amongst the five different solvents that were used. Ampicillin (10 µg/ml) showed no antimicrobial activity against S. epidermidis whilst a zone of inhibition of 6.26±0.07 mm was recorded for 10 µg/ml of P. cornutum extract reconstituted in chloroform. An ethanolic extract of P. rigidum showed a zone of inhibition of 6.35±0.25 mm against L. monocytogenes while the standard ampicillin had no activity. Extracts of P. rigidum in ethanol and P. cornutum in chloroform are evidently potential lead candidate antibiotics in vitro against L. monocytogenes and S. epidermidis, respectively.
Key words: Antimicrobial activity, Plocamium cornutum, Plocamium rigidum, Listeria monocytogenes.
Due to emerging resistance of pathogenic microorganisms to existing antibiotics and the fast spreading of resistant microorganisms, there is an increasing need for new antibiotics. In marine environments, competition for space and nutrients led to the evolution of antimicrobial defence strategies. This includes the production of chemically active metabolites in their surroundings which act as an aid to protect themselves against other settling organisms, maintenance of unfouled surfaces, deterrence of predation, the ability to successfully reproduce, protection from UV radiation and as allelopathic agents (Taskin et al., 2012; Chakraborthy et al., 2010). Marine algae are one of the largest producers of biomass in the marine environments and are a rich source of structurally novel biologically active metabolites (El-Din and El-Ahwany, 2016). Therefore, they offer a rich source of potentially new drug leads.
Algae metabolites have great industrial potential and accessibility, and thus they have attracted attention for health and cosmetic applications. The use of microalgae and their derivatives in applications to combat skin aging, as well as for depigmentation and antimicrobial applications in the cosmetic industry is wide spread (Wang et al., 2015). A wide range of metabolites, such as antioxidants, anti-inflammatory agents, alginates, polysaccharides, and carotenoids, have been investigated for cosmeceutical preparations. The antimicrobial properties of marine algae have been known since ancient times and well documented in recent years (Patra et al., 2008). Algae are thus a source of raw materials for one of the most promising and profitable sectors of the biotechnology industry. Phycocolloid substances from marine algae such as alginate, carrageenan and agar have been used globally for decades in medicine and pharmacy. Thus, they are of interest for potential use in cosmetic products (Patra et al., 2008). Four species of Algerian marine algae were tested for anti-fungi properties and results showed that they had fungi inhibiting effects (Patra et al., 2008). Algal materials collected from the Red Sea Coast of Jeddah inhibited the growth of Enterococcus faecalis (11 mm) but no activity was recorded for water extracts (Al-Saif et al.,2014).
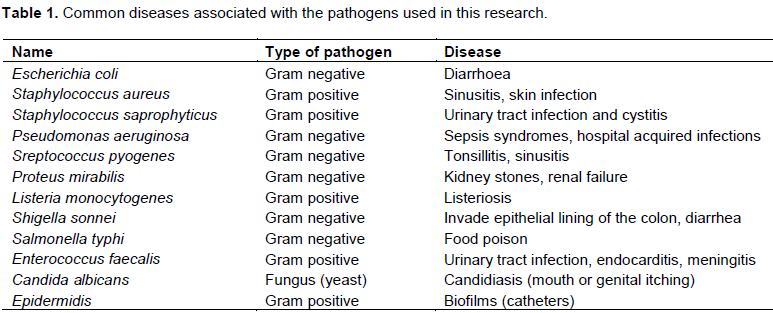
Marine algae provide a rich source of structurally diverse secondary metabolites some of which have marked antimicrobial activity against marine pathogens. The bactericidal agents found in algae include amino acids, terpenoids, phlorotannins, acrylic acid, phenolic compounds, steroids, cyclic polysulphides and fatty acids. Watson and Cruz-Rivera (2003) reported that 54 seaweeds were tested in vitro for antimicrobial activity and a staggering 95% of the extracts showed activity against different pathogens. Bromophenol compounds have been frequently encountered in various marine algae including red and brown algae. Red algae of family Rhodomaceae are especially known as a rich source of bromophenols (Oh et al., 2008). Some of these compounds which were previously isolated from the family exhibited a wide spectrum of pharmacological activities such as enzyme inhibition, cytotoxic, antioxidant, feeding deterrent, anti-inflammatory, and antimicrobial activities (Williamson and Carughi, 2010). Taskin (2012), studied the inhibitory activities of various organic extracts of algae against various fish pathogenic bacteria and their results confirmed the possible use of some marine algae as a source of antimicrobial compounds. In this study, organic crude extracts from Plocamium species, a red marine algae from the coastline of Namibian, were studied for their potential inhibitory activities against common pathogens. Twelve pathogens selected for this study are important in our everyday life as they are common causes of a variety of human diseases (Table 1).
The ability of marine algae to produce metabolites of potential interest has been extensively documented. The antimicrobial activity of marine algae may be influenced by some factors such as the habitat, the season of algal collection, different growth stages of plant and experimental methods (Al-Saif et al., 2014). Although a variety of solvents have been employed in screening marine algae for antimicrobial activity, it is still uncertain what kinds of solvents are most effective and suitable for the extraction of secondary metabolites from marine algae (Manivannan et al., 2011). El-Din and El-Ahwany (2015) also reported that antimicrobial activity depends on the solvents used for extraction. It was found that benzene and diethyl ether were suitable solvents for extracting various antibiotic compounds. However, extracts obtained with acetone, ethyl alcohol and ether showed higher antimicrobial activity than extracts from chloroform (Manivannan et al., 2011). In a similar study, Sasidharan et al. (2009) found that chloroform exhibited the strongest activity, which is consistent with the findings of (El-Din and El-Ahwany, 2015). There is a dearth of information concerning the antimicrobial activities of Namibian marine algae. Antimicrobial potential of Namibian marine algae are unexplored despite the availability of marine algae in the coastal areas of the country. This study therefore aimed to screen for potentially bioactive metabolites from Namibian marine algae and test them for antimicrobial activity. This would be beneficial to the development of Namibian medicine if a novel solution was found to common microbial infections.
Sample collection
Plocamium spp. were collected during low tide along the coastline of Namibia from Henties Bay and Lüderitz, where they are abundant in intertidal, shallow and coastal estuaries. The samples were collected about 5 to 10 cm under water by hand and placed in a sealable polythene bag and refrigerated at -20°C. Collections were done twice from the same areas. The coordinates of Lüderitz and Henties Bay are 26° 38' 53" S, 15° 9' 34" E and 22° 7' 0"S, 14° 17' 0" E, respectively. As the samples co-exist with other settling organisms, they were first washed in seawater and then in fresh water to remove sand, epiphytes and other necrotic parts. The identification of Plocamium cornutum and Plocamium rigidum was done on the basis of their morphological characteristics, including colour and the arrangement of the branchlets. The final identification was done at the Marine and Fisheries Department of the University of Namibia by visual appearance and standard collection guides (Lluch, 2002). The samples were transported to the laboratory in polythene bags under ice and were frozen at -87°C for future analyses.
Test microorganisms
Extracts of P. rigidum and P. cornutum were investigated to evaluate their antimicrobial activity against five Gram positive human pathogens (Staphylococcus aureus, Staphylococcus saprophyticus, Listeria monocytogenes and E. faecalis), 6 Gram negative human pathogens (Escherichia coli, Pseudomonas aeruginosa, Streptococcus pyogenes, Proteus mirabilis, Shigella sonnei and Salmonella) and a fungi, Candida albicans using a disc diffusion method. The 12 pathogens used in this study were obtained from the Department of Biochemistry and Microbiology, School of Medicine, University of Namibia.
Preparation of marine algae extracts
Frozen samples of marine algae (33 g) were soaked in extraction solvent (MeOH:DCM 1:1 v/v) for 48 h at room temperature (±26°C). The extracts were then decanted into a 250 ml beaker. The resulting extracts were filtered through Whatman No. 1 filter paper and concentrated using a rotary evaporator (model RE 100; Bibby Sterilin Ltd). The concentrated extracts were then dissolved in 100 ml of extraction solvent and transferred to a separating funnel. Two layers of immiscible liquids were formed in the separating funnel, of which the upper layer consisted of MeOH extract which form the polar fraction and the lower layer composed of DCM extracts which is the non-polar fraction. The MeOH and DCM layers were carefully decanted into pre-weighed glass vials. The extracts were left to dry in a laminar airflow cabinet. The dried extracts were weighed and placed in a freezer at a temperature of about -80°C. DCM and MeOH extracts of Plocamium spp. were reconstituted in six different solvents to yield solutions of 1 mg/ml in water, hexane, dichloromethane, ethanol, methanol and chloroform (H2O, C6H14, DCM, EtOH, MeOH and CH3Cl) to evaluate the antimicrobial potential of the different solvent extracts.
Microbial inoculum preparation
All media used in the study were supplied from Hi Media laboratories, India. Standard ampicillin antibiotic susceptibility discs were obtained from SRL chemicals Ltd., India. All media were prepared in deionised water and autoclaved at 121°C for 15 min prior to use according to the manufacturers’ instructions. Twelve selected pathogens were cultured on nutrient agar plates. The plates were incubated at 35 to 37°C in an incubator (Scientific series 2000, L. digital incubator; model 286) for 24 h. Colonies of each of the selected twelve bacteria were inoculated into Tryptone Soya Broth in 2 ml culture tubes. The culture tubes were left to incubate at 35 to 37°C for 24 h.
Determination of antimicrobial activity of Plocamium spp.
The antimicrobial activity of the Plocamium extract was carried out using the Kirby Bauer disc diffusion method (Arullappan et al., 2009). The DCM and methanol extracts were tested separately against 12 pathogens and the tests were run in triplicate and average inhibition zones were recorded using Vernier callipers. The negative controls consisted of six different sterile solvents (H2O, C6H14, DCM, EtOH, MeOH and CH3Cl) used to dissolve the extracts. The culture solutions were used to impregnate the diffusion disc used for antimicrobial screening.
Twelve petri dishes with Mueller-Hinton agar were impregnated with a different bacteria and each dish was divided into three sectors. Discs soaked in different extracts in their respective solvents were placed in different sectors of the petri dish along with both negative and positive controls. Three petri dishes with the same extract and controls (triplicate) were incubated at 37°C for 24 h. The zones of inhibition were measured after 24 h.
Determination of minimum inhibitory concentration
Minimum inhibitory concentration (MIC) was determined for all extracts that showed antimicrobial activities in the screening phase. This was carried out using a modified procedure from (Peng et al., 2010). Each algal extract was serially diluted to yield 800, 600, 400, 200, 100, 20 and 10 µg/ml. Filter paper discs were placed in each of the diluted extract to absorb their respective solvent for 15 min and allowed to dry. The discs were respectively placed onto the plates containing each of the twelve selected bacteria. Standard ampicillin antimicrobial susceptibility test discs of 10 µg/ml and blank solvent discs served as the positive and negative controls, respectively. Different discs with different concentrations along with positive and negative controls were placed in their respective divisions and labelled on the petri dishes. The petri dishes were prepared in triplicates. The plates were incubated at 37°C for 24 h. MIC was defined as the lowest concentration at which no visible growth was observed; this indicates the presence of antimicrobial activity.
Statistical analysis
Means of triplicate analysis were calculated and data was expressed as mean ± standard deviation (SD) and analysed using SPSS 22 software. A Shapiro test was used to determine the normality of MIC. Kruskal-Wallis test was then used to identify significant differences in the MIC among the treatment groups (p<0.05). Dun-Bonferroni post hoc statistical test was used for pairwise comparison of the treatment groups.
Antimicrobial screening
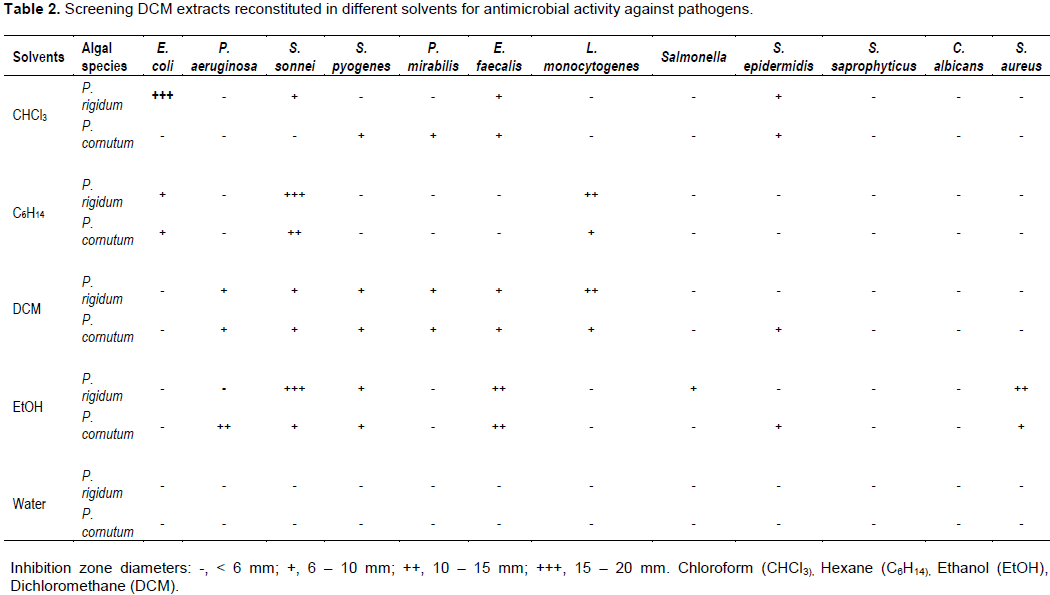
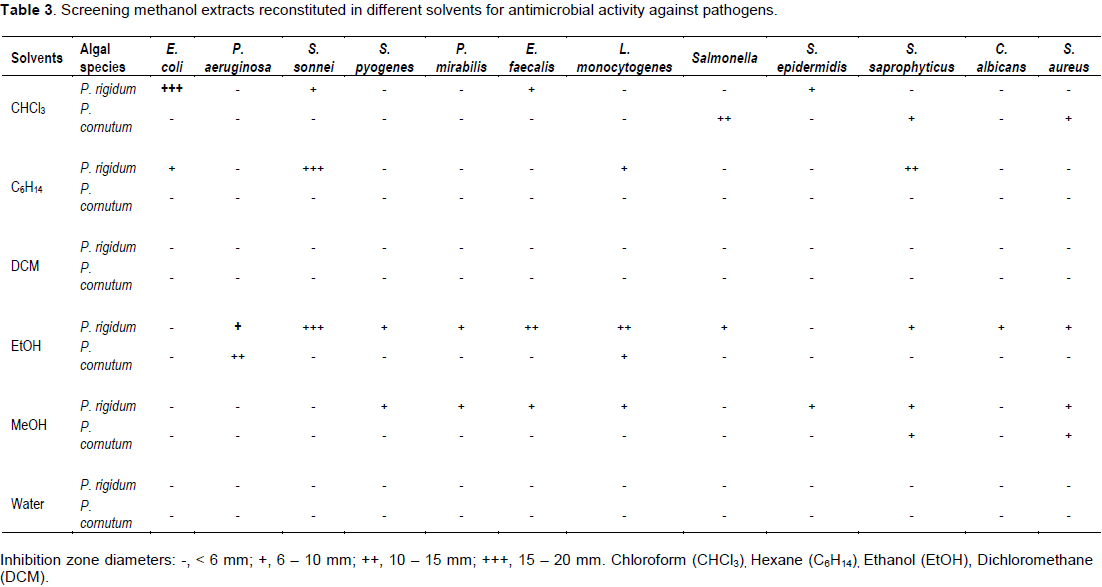
Water extracts showed no antimicrobial activity against all the pathogens. The antimicrobially active algae extracts were probably not soluble in water. The remaining solvents had varying degrees of antimicrobial activity as shown in Tables 2 and 3 for DCM and MeOH extracts, respectively.Table 2 highlights that DCM extracts of P. rigidum and P. cornutum reconstituted in chloroform and ethanol showed high antimicrobial activity (+++) against, E. coli, S. sonnei and S. aureus. Similar extracts only demonstrated moderate antimicrobial activity (++) against P. aeruginosa, E. faecalis, L. monocytogenes and S. sonnei when reconstituted in ethanol, dichloromethane and hexane. DCM extracts of P. rigidum and P. cornutum reconstituted in chloroform, ethanol and other solvents showed only traces of antimicrobial activity (+) or no activity against the pathogens. Table 3 shows that the antimicrobial activity of MeOH extracts of algae reconstituted in chloroform, hexane, dichloromethane and ethanol showed less activity against the selected pathogens, compared to DCM extracts. MeOH extracts of P. rigidum and P. cornutum reconstituted in chloroform, hexane and ethanol showed high antimicrobial activity (+++) against, E. coli and S. sonnei. Similar extracts only demonstrated moderate antimicrobial activity (++) against, E. faecalis, L. monocytogenes, Salmonella and S. saprophyticus when reconstituted in ethanol, chloroform and hexane.
Minimum inhibitory concentration of algal extracts
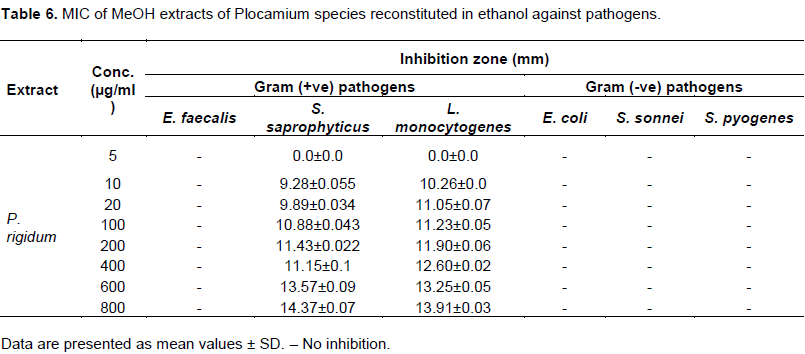
Evaluation of antimicrobial activity of the algal extracts was recorded in Tables 4, 5 and 6. Of the six solvents used to reconstitute extracts from dichloromethane and methanol for antimicrobial activity, water extracts showed no activity against any of the twelve pathogens used in the test. This is in agreement with the results obtained of Al-Saif et al. (2014). The other algal extracts reconstituted in C6H14, DCM, EtOH, and CH3Cl demonstrated varying degrees of inhibitory activity against the test pathogens. Chloroform proved to the solvent of choice, as it was observed to have significantly higher inhibitory activity against tested pathogens (p<0.05). Ampicillin (10 µg/ml) showed no activity against S. epidermidis in DCM extract of P. rigidum reconstituted in chloroform.
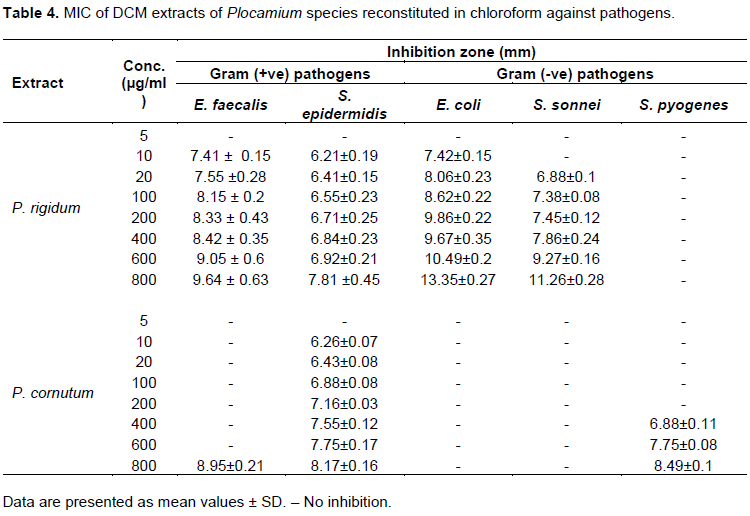
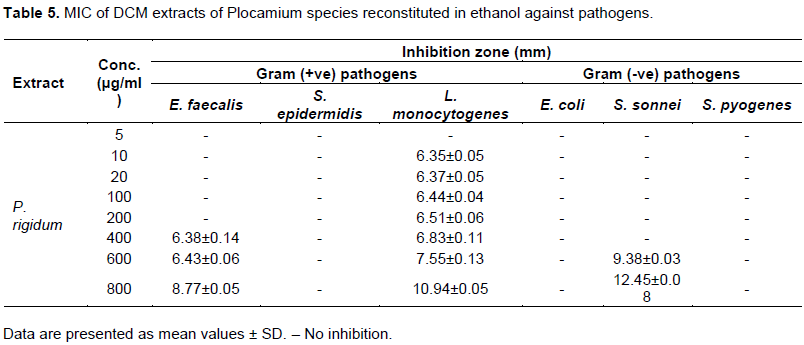
Antimicrobial activity of DCM extracts of algae reconstituted in chloroform
DCM extracts reconstituted in chloroform showed prominent antimicrobial activity (15 to 20 mm) against E. coli but only traces of antimicrobial activity (6 to 10 mm) against E. faecalis, S. Epidermidis and S. sonnei species in DCM extracts of P. rigidum and P. cornutum. S. saprophyticus and C. albicans proved resistant to all the extracts of DCM reconstituted in chloroform and ethanol. P. mirabilis, S. pyogenes and S. sonnei also showed traces of activity (6 to 10 mm) in all DCM extracts. DCM extracts of sample P. rigidum demonstrated a ZOI of 10 to 15 mm against L. monocytogenes. DCM extracts of P. rigidum reconstituted in hexane showed activity of 10 to 15 mm against L. monocytogenes and S. sonnei but only traces of antimicrobial activity against E. coli. DCM extracts of P. rigidum reconstituted in EtOH showed high antimicrobial activity (15 to 20 mm) against S. sonnei and S. aureus but moderate antimicrobial activity (10 to 15 mm) against E. faecalis. The extract showed only traces of inhibition against S. pyogenes and S. epidermidis. Ampicillin (10 µg/ml) showed no activity against L. monocytogenes in DCM extract of P. rigidum reconstituted in ethanol. The results of methanol extracts of Plocamium species reconstituted in EtOH (Table 6) revealed that the algal extracts were potentially active in suppressing microbial growth of S. saprophyticus and L. monocytogenes. It was found that the ZOI of 10 µg/ml ampicillin against L. monocytogenes was 10.05 mm as compared to 10.26 mm recorded for the MeOH extract of P. rigidum reconstituted in EtOH.
The lowest extract concentration that prevents visible bacterial growth was determined for allthree extracts. P. rigidum showed a ZOI of 6.35±0.25 at the lowest MIC of 10 µg/ml. This could be an indication of better antibacterial activity of this extract in comparison to other extracts. DCM extracts of P. rigidum reconstituted in chloroform showed a MIC of 10 µg/ml and a ZOI of 7.41 ± 0.2 mm against E. faecalis. The same extract with MIC of 10 µg/ml showed a ZOI of 6.21 ± 0.08 mm against S. epidermidis which indicates a better growth inhibition of E. faecalis than S. epidermidis. The same algal extract demonstrated the same growth inhibition against E. coli at 10 µg/ml. However, as the algal concentration increases, there is a marked growth inhibition demonstrated in E. coli and S. sonnei than the gram positive E. faecalis and S. epidermidis. DCM extracts of P. rigidum reconstituted in chloroform thus has the characteristics of a broad spectrum antibiotic in vitro. Table 5 shows that DCM extracts of P. cornutum reconstituted in chloroform showed no activity at lower extract concentration against E. faecalis and S. pyogenes. However, weak inhibitions were recorded at 400 µg/ml. There is no activity against E. coli and S. sonnei. DCM extracts of P. rigidum reconstituted in ethanolshowed pronounced activity against L. monocytogenes at low concentration (MIC of 10 µg/ml and ZOI of 6.35±0.25 mm). This result was better than the observed activity of standard ampicillin antimicrobial susceptibility test disc against the same pathogen.
Antimicrobial activity of MeOH extracts of algae reconstituted in ethanol
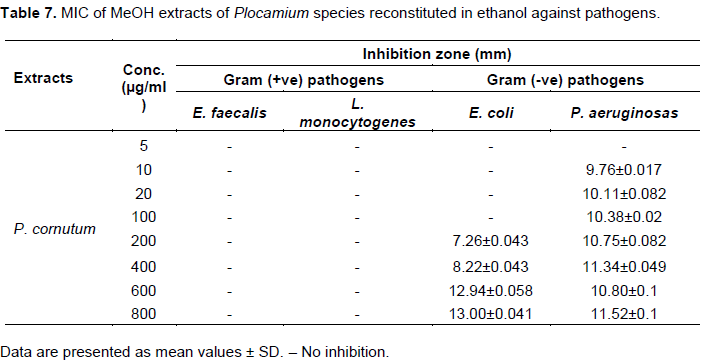
MeOH extracts of P. rigidum reconstituted in ethanol showed no activity against E. faecalis, E. coli, S. sonnei and S. pyogenes. The extracts however demonstrated strong activity against S. saprophyticus and L. monocytogenes. While standard ampicillin antimicrobial susceptibility test disc of 10 µg/ml did not inhibit the growth of L. monocytogenes, 10 µg/ml of P. rigidum reconstituted in ethanol inhibited the growth of L. monocytogenes by 10.26±0.01 mm. MeOH extracts of P. cornutum reconstituted in ethanol only inhibited the growth of E. coli and P. aeruginosa. Table 7 shows that the activity against P. aeruginosa even at low concentration of 10 μg/ml is significant as this pathogen is resistant standard ampicillin.
The data obtained in the present study indicated that chloroform was the most effective solvent for the extraction of bioactive compounds, followed by ethanol. Organic solvents always have a higher efficiency in extracting antimicrobial compounds than water as the solvent for extraction (Kamra and Bhatt, 2012). This result is consistent with literature that extracts from organic solvents give more consistent antimicrobial activity than water extracts (Kamra and Bhatt, 2012; Tiwari et al., 2011). Antimicrobial activity of marine algae could be attributed to combined effects of the high percentage of phenolic content, due to the presence of various phytochemicals (Govindasamy et al., 2011) and the presence of halogenated monoterpenes which are regularly present in marine algae (Cabrita et al., 2010). The type and amount of halogens present in the algal molecule also have a role in the overall defense against pathogenic Gram-positive and Gram-negative bacteria (Andrianasolo et al., 2006). The fats and fatty acids from marine algae also play an important role in the formation of many other bioactive secondary metabolites since some fatty acids have been shown to possess antibacterial activities (Barbosa et al., 2007; Oh et al., 2008). The DCM extract of P. rigidum reconstituted in chloroform demonstrated antimicrobial activity against E. faecalis, E. coli and S. epidermidis.
The ZOI for the extracts are 7. 41 ± 0.2, 7.42 ± 0 .31 and 6.21 ± 0.08 mm, respectively. Although these extracts were not very active in vitro compared to 10 µg/ml of ampicillin that showed activity in the range of 16.84 ± 0.22 mm (E. coli) and 8.94 ± 0.44 mm (E. epidermidis), they offer potential leads in the search for alternative antibiotics that could be active against E. faecalis and S. sonnei. The DCM extract of P. rigidum reconstituted in ethanol showed a noteworthy result. 10 µg/ml of this extract showed trace antimicrobial activity of 6.18 ± 0.56 mm against S. epidermidis, while standard ampicillin of the same concentration showed no activity at all against S. epidermidis. The DCM extract of P. cornutum reconstituted in chloroform showed ZOI of 6.26 ± 007 mm against S. epidermidis while standard ampicillin showed no inhibitory activity against S. epidermidis. Concentrations higher than 10 µg/ml of extracts showed varying degrees of inhibition against all pathogens tested. In addition, DCM extracts of P. rigidum and P. cornutum reconstituted in chloroform showed antimicrobial activity against both Gram positive (E. faecalis and S. epidermidis) and Gram negative (E. coli and S. sonnei) pathogens, which is an indication of a broad spectrum of activity. The DCM extract of P. rigidum reconstituted in chloroform, was the most effective marine algae extract against the various pathogens. The ZOI and MIC obtained in this research are comparable to the results presented in similar work reported (Mostafa et al., 2017).
In this study presenting the first report of antimicrobial activity of Namibian marine alage, chloroform was the most suitable extraction solvent for antimicrobial compound extraction among the solvents used. It can be inferred from this study that the antimicrobial potential of P. cornutum and P. rigidum depend on the solvent medium used for extraction and the type of organism tested. P. cornutum and P. rigidum collected along the coastline of Namibia can be used as agents for the development of new drug leads for bacterial infections. DCM extracts of P. rigidum (in ethanol) and P. Cornutum (in chloroform) are evidently potent antibiotics lead candidates in vitro against L. monocytogenes and S. epidermidis.
The authors have not declared any conflict of interests.
This study was partly funded by the Namibian Commission for Research Science and Technology (NCRST) and the University of Namibia Staff Development Fund.
REFERENCES
|
Al-Saif SSAL, Abdel-Raouf N, El-Wazanani HA, Aref IA (2014). Antibacterial substances from marine algae isolated from Jeddah coast of Red sea, Saudi Arabia. Saudi J. Biol. Sci. 21(1):57-64.
Crossref
|
|
|
|
Andrianasolo EH, France D, Cornell-Kennon S, Gerwick WH (2006). DNA Methyl Transferase Inhibiting Halogenated Monoterpenes from the Madagascar Red Marine Alga Portieria h ornemannii. J. Nat. Prod. 69(4):576-579.
Crossref
|
|
|
|
|
Arullappan S, Zakaria Z, Basri DF (2009). Preliminary screening of antibacterial activity using crude extracts of Hibiscus rosa sinensis. Trop. life Sci. Res 20(2):109-118.
|
|
|
|
|
Mostafa AA, Al-Askar AA, Almaary KS, Dawoud TM, Sholkamy EN, Bakri MM (2017). Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J. Biol. Sci. 25(2):361-366
Crossref
|
|
|
|
|
Barbosa JP, Fleury BG, da Gama BA, Teixeira VL, Pereira RC (2007). Natural products as antifoulants in the Brazilian brown alga Dictyota pfaffii (Phaeophyta, Dictyotales). Biochem. Syst. Ecol. 35(8):549-553.
Crossref
|
|
|
|
|
Cabrita MT, Vale C, Rauter AP (2010). Halogenated compounds from marine algae. Mar. Drugs 8(8):2301-2317.
Crossref
|
|
|
|
|
Chakraborthy K, Lipton AP, Paulraj R, Vijayan KK (2010). Antibacterial Diterpernoids of Ulva fasciata Delile from South-Western coast of Indian peninsula. Food Chem. 119(4):1399-1408.
Crossref
|
|
|
|
|
Govindasamy C, Narayani S, Arulpriya M, Ruban P, Anantharaj K, Srinivasan R (2011). In vitro antimicrobial activities of seaweeds extracts against human pathogens. J. Pharm. Res. 4(7):2076-2077.
|
|
|
|
|
Kamra A, Bhatt AB (2012) Evaluation of antimicrobial and antioxidant activity of Ganoderma luciduma extracts against human pathogenic bacteria. Int J. Pharm. Pharm. Sci. 4(2):359-362.
|
|
|
|
|
Lluch JR (2002). Marine benthic algae of Namibia. Sci. Mar. 66:5-256.
Crossref
|
|
|
|
|
Manivannan K, Anantharaman P, Balasubramanian T (2011). Antimicrobial potential of selected brown seaweeds from Vedalai coastal waters, Gulf of Mannar. Asian Pac. J. Trop. Biomed. 1(2):114-120.
Crossref
|
|
|
|
|
El-Din SMM, El-Ahwany AM (2016). Bioactivity and phytochemical constituents of marine red seaweeds (Jania rubens, Corallina mediterranea and Pterocladia capillacea). J. Taibah Univ. Sci. 10(4):471-484.
Crossref
|
|
|
|
|
Oh KB, Lee JH, Chung SC, Shin J, Shin HJ, Kim HK, Lee HS (2008). Antimicrobial activities of the bromophenols from the red alga Odonthalia corymbifera and some synthetic derivatives. Bioorg. Med. Chem. Lett. 18(1):104-108.
Crossref
|
|
|
|
|
Patra JK, Rath SK, Jena K, Rathod VK, Thatoi H (2008). Evaluation of antioxidant and antimicrobial activity of seaweed (Sargassum sp.) extract: A study on inhibition of Glutathione-S-Transferase activity. Turkish J. Biol. 32(2):119-125.
|
|
|
|
|
Peng Q, Huang B, Hou D, Hua F, Qian Y (2010) Green tea extracts weakens the antimicrobial effects of amoxicillin in methicillin-resistant Staphylococcus aureus infected mice. Phytother. Res. 24(1):141-145
Crossref
|
|
|
|
|
Sasidharan D, Darah I, Noordin MKMJ, Kassim M, Jain M (2009). Screening antimicrobial activity of various extracts of Gracilaria changi. Pharm. Biol. 47(1):72-76.
Crossref
|
|
|
|
|
Taskin E, Taskin E, Ozturk M (2012). Antimicrobial activities of some seaweeds From northern Cyprus against some food- related pathogens. Asian J Biol. Sci. 5(5):250-256.
|
|
|
|
|
Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H (2011). Phytochemical screening and extraction: a review. Int. Pharm. Sci. 1(1):98-106.
|
|
|
|
|
Wang HMD, Chen CC, Huynh P, Chang JS (2015). Exploring the potential of using algae in cosmetics. Bioresour. Technol. 184:355-362.
Crossref
|
|
|
|
|
Watson SB, Cruz-Rivera E (2003). Algal chemical ecology: an introduction to the special issue. Phycologia 42(4):319-323.
Crossref
|
|
|
|
|
Williamson G, Carughi A (2010). Polyphenol content and health benefits of raisins. Nutr. Res. 30(8):511-519.
Crossref
|
|