Full Length Research Paper
ABSTRACT
The quality and quantity of secondary metabolites produced by plants can be influenced by biotic and abiotic factors, since the biochemical and physiological processes that coordinate the synthesis of these compounds are influenced by these. Plant regulators can also interfere with plant growth and terpene biosynthesis in aromatic species, which is why they are widely used in agriculture. Lippia origanoides H.B.K is an aromatic shrub that has glandular trichomes on the leaf, which secrete and accumulate essential oil used in the production of culinary seasonings and in the cosmetic and pharmaceutical industry. The objective of this research was to evaluate the effects of plant regulators associated with different levels of light availability in L. origanoides exposed to levels of 100, 50, 35 and 25% of irradiance. The increase in irradiance in plants treated with plant regulator caused changes in the structure and leaf dry biomass, density of glandular trichomes and essential oil content in the 100% full sun treatment. The treatments did not influence the chemical profile of the essential oil, which presented thymol as the major component. However, the application of bioregulators in full sun increased the production of essential oil demonstrating an excellent option for increasing the production.
Keywords: Aromatic plant, shading, biorregulador vegetable, leaf anatomy.
INTRODUCTION
Plants are important sources of bioactive substances with high demand and interest in exploration for new drug discovery (Newman and Cragg, 2016). Aromatic plants the quantity and concentration of secondary metabolites produced suffer interference from environmental factors, which may influence the course of physiological processes by changing the plant metabolism (Bourgaud et al., 2001; Rezaie et al., 2020).
Acclimation related plasticity in situations differentiated luminous intensity availability can change photosynthetic apparatus, in order to increase the ability of carbon assimilation and promote growth (Alvarenga et al., 2005). Growth is changed according to the availability of light radiation (Miralles et al., 2011) and in aromatic plants can significantly alter the production of essential oil (Fernandes et al., 2013).
Vegetable regulators, synthetic substances similar to plant hormones influence the response of plants to different environmental conditions, interfering in the morphology, physiology, growth and production (Shukla and Faoroqi, 1990). The use of plant regulators is a practice widely used agricultural (Khan et al., 2020). Seeking the development of new agricultural management techniques vegetable regulators have been used for the purpose of increasing production and improving the quality of the oil. The responses to the effects of these plant regulators on essential oil production have been highly variable between aromatic species.
Lippia origanoides H.B.K are aromatic shrubs native to Central America and the northern region of South America. They are raw materials for the production of culinary seasonings and essential oils enriched in phenylpropanoids that are useful in cosmetic and pharmaceutical industries (Hernandes et al., 2017; Ortiz et al., 2017; Stashenko et al., 2018). In the ontogeny of the vegetable organs, phytohormones as the cytokinins have key role in the regulation of cell division and the formation of the photosynthetic apparatus (Zubo et al., 2008). This phytohormones may still suffer influence of physiological responses of plant interfered in the accumulation of monoterpenes are typical of essential oil (Cappelari et al., 2019).
The aim of this study was to determine the effects of a biostimulant made up of 90 mg, 50 mg of kinetin indole butyric acid and gibberellic acid 50 mg per litre of the product, with the availability of light radiation on biomass in foliar morphology, anatomy and in the production of essential oil in L. origanoides.
MATERIALS AND METHODS
Plant material and growing conditions
The experiment was conducted on the campus of the Universidade Estadual de Santa Cruz (UESC), Ilhéus, Bahia, Brazil (14°47′00″S, 39°02′00″W). Meteorological data were obtained through the Meteorological Database of the National Institute of Meteorology (INMET), presenting temperature averages of 26°C, 41 mm of precipitation and 86% of relative humidity.
Plants obtained by vegetative propagation from cuttings, were transplanted to pots containing substrate ratio 4:1 (soil: sand). In the luminous radiation treatments were used black plastic screens of type 'sombrite', set out in individual frames of wood, constituting individual environments for each level of light, under field conditions. The values of photosynthetically active radiation (PAR) were obtained at intervals of 1 h of 07:00 am to 06:00 pm through a quantum sensor BQM-SUN (Apogee Instruments, Inc., USA) in full sun and in every environment.
Three applications were held biostimulant made up of 90 mg of kinetin (Kt), 50 mg of indole butyric acid (IBA) and 50 mg of gibberellic acid (GA3) per liter of product. The sprays were carried out by foliar route at 30, 50 and 70 days after transplanting. The treatments were constituted by plants treated and not treated with plant regulators, submitted to different levels of light. These being: full sun + biostimulant (T1), full sun without biostimulant (T2), 50% of full sun + biostimulant (T3), 50% of full sun without biostimulant (T4), 35% of full sun + biostimulant (T5), 35% of full sun without biostimulant (T6), 25% of full sun + biostimulant (T7), 25% of full sun without the biostimulant (T8).
Growth measurements
The growth variables were evaluated in five plants per treatment, at the end of the experiment, totaling 40 plants. Number of leaves (NL) was quantification; the dry biomass of root (RDB), stems (SDB), leaves (LDB) and total biomass (TB) were obtained by drying at 75°C in a circulating air oven until constant biomass was reached. Leaf area (LA) was obtained using an LI-3100 Area Meter (Li-Cor) and 30 milimeter ruler was used to determine the height of the plants (HP) and stem diameter (SD), just above ground level, with aid of digital caliper.
Production and chemical composition of essential oil
The essential oil was extracted from the dried leaves by the hydrodistillation using a Clevenger apparatus, with all dry biomass of the plant in 1500 mL of distilled water for 90 min, with five repetitions for treatment. The essential oil was separated from the high grade using dichloromethane and then dried with anhydrous sodium sulphate (in excess) and concentrated. The content was determined based on the volume extracted per 100 g dry biomass leaf (% w/v). Essential oil production was obtained by total biomass of oil extracted from the leaves of each treatment (g plant-1).
The chemical composition of essential oil was analyzed performed by gas chromatography using Varian Saturn 3800 (Varian Palo Alto, CA) equipped with a flame ionization detector (GC-FID) and VF-5ms capillary column (30 m × 0.25 mm × 0.25 μm film thickness), using helium as the carrie gas at a flow rate of 1.0 mL min-1. The injector and detector temperatures were 250 and 280°C, respectively. The column temperature program began at 60°C, increased by 8°C mm-1 to 240°C for 5 min. One milliliter of a 10% solution of oil in chloroform was injected in the 1:10 split mode. The concentration of the chemical constituents was calculated through the area of their respective peaks in relation with the total area of all the constituents of the sample. Qualitative analysis of the oils was performed out using a mass spectrometer (Agilent 5975C) triple quadruple and ZB-624 capillary column (20 m × 0.18 mm × 1.00 μm). The injector temperature was 250°C. The column temperature program began at 50°C, increased by 8°C min-1 to 240°C for 10 min. The operation mode were eletronic impact at 70 eV, using helium as gas the carrie, with electronic injection of 0.1 µL. The chemical constituents were identified by computer comparing with the apparatus library and literature, and linear retention indices of Kováts (IK) were calculated by injecting a series of n-alkanes (C8-C26) under the same chromatographic conditions as used for the samples.
Leaf anatomy
Fragments of leaves third node, with three repetitions per treatment, were fixed in 2.5% glutaraldehyde (v/v), embedded in resin, and transverse cross-sections 5 μm thick were obtained with a microtome, stained with Toluidine Blue O and imaged under light microscopy Leica, model ICC50HD. Were measurements thickness of the adaxial (EDA) and abaxial (EBA) surface of the epidermis, palisade (PP) and spongy parenchyma (SP) and mesophyll using the Leica application suite software V3.
For analysis of surfaces fragments of leaves fixeds in 2.5% glutaraldehyde (v/V), were dehydrated in ethanol series, covered with gold and observed in scanning electron microscope Quanta 250 model. Density of trichomes and stomata were determined quantified the number of structures for mm2.
Experimental design
The experimental was conducted following a completely randomized design, in split-plot array with two conditions: Plants treated and not treated with plant regulators, submitted to different levels of light. These being: Full sun + biostimulant (T1), full sun without biostimulant (T2), 50% of full sun + biostimulant (T3), 50% of full sun without biostimulant (T4), 35% of full sun + biostimulant (T5), 35% of full sun without biostimulant (T6), 25% of full sun + biostimulant (T7), 25% of full sun without the biostimulant (T8), and five replications per tratament, (n = 40 plants). The data were examined by analysis of variance (ANOVA, F-test). For the treatment averages, polynomial regression models were adjusted using Sisvar 5.3 (Ferreira, 2010). Tukey tests were used to assess the average values within qualitative treatments. Significance was defined as P ≤ 0.05.
RESULTS AND DISCUSSION
Photosynthetically active radiation (PAR) daily average for the environment to full sun (T1 and T2) and other treatments presented maximum values of 1600, 800, 561 and 400 µmol m-² s-¹, respectively to 11:00 h (Figure 1).
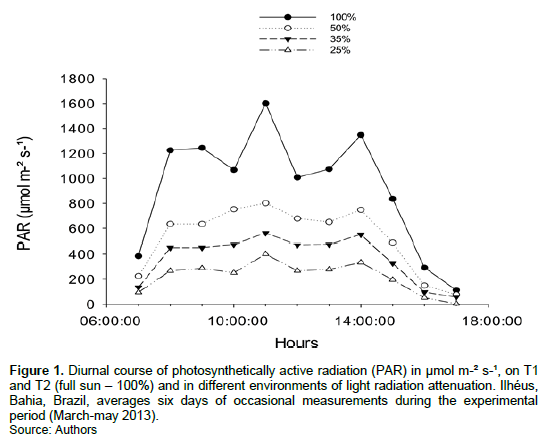
L. origanoides cultivated under different light levels and application of biostimulant presented changes in growth. The average values dry biomass of root and aerial part suffered influence only of light radiation levels. Plants exposed to full sun condition accumulated more biomass in root and those cultivated to 25% of full sun presented increased in dry biomass of stem (Figure 2A and B).
Plant species grown at different intensities and quality of the incident light spectrum may undergo changes related to biomass production, biometric characteristics and metabolite production (Kunz et al., 2020), which occur as result of changes in carbon allocation to the different organs (Valladares and Niinemets, 2008).
Dry biomass of plants that received the application of growth regulators to 100% of full sun was about 40% larger than plants that received 25% of full sun (Figure 3A to B).
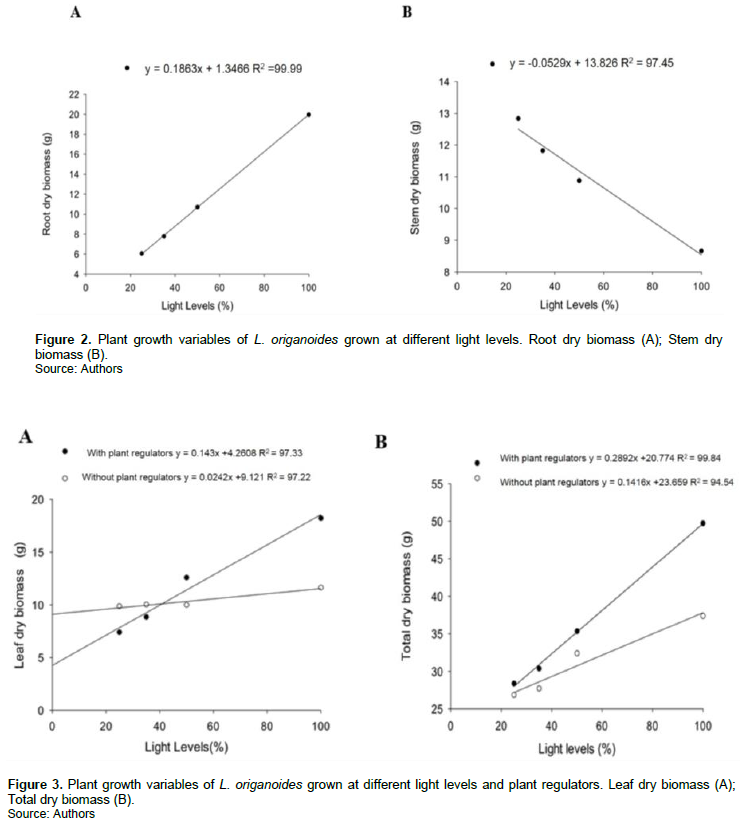
The levels and proportions of hormones in the composition of growth regulators influence the efficiency of these in the plant physiology, also as in carbon allocation (Castro and Vieira, 2001; Andrade et al., 2018), which may explain the influence only of the observed light radiation availability and production of biomass and essential oil. The ability to grow rapidly when shaded is a mechanism of acclimatization of the plants with a strategy to escape shading, can be attributed to a greater investment in cell elongation (Osunkoya and Ash, 1991; Moraes Neto et al., 2000).
Number of leaves increased linearly with increasing light levels, however, leaf area, plant height and stem diameter decreased linearly (Figure 4A to D).
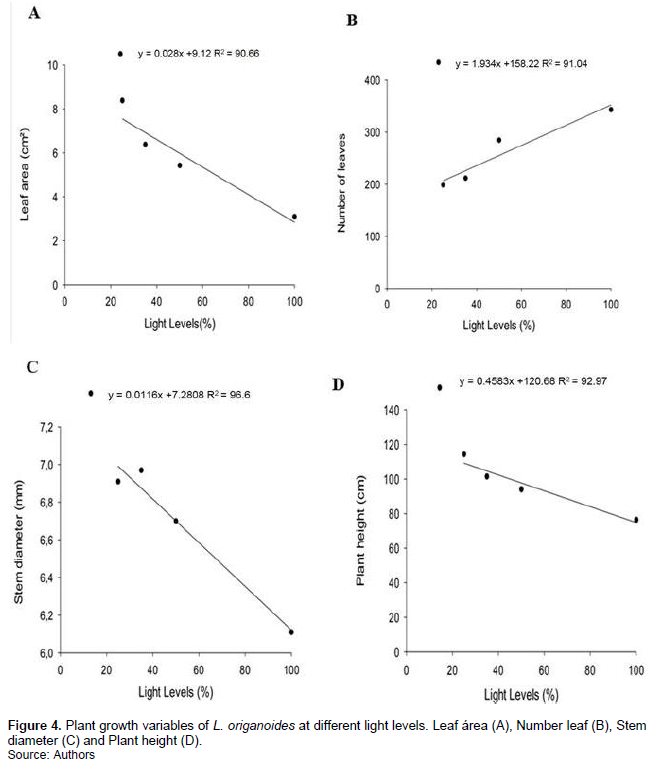
The increase in leaf area with add to shading is one ajuste to increase the photosynthetic surface and ensure greater use of low light intensities (Lambers et al., 2008; Liu et al., 2019). According to Passos (1997), the higher the number, the bigger the leaf CO2 assimilation and consequently greater production of essential oil. O. basilicum plants treated with growth regulators increased the total number of leaves (Shedeed et al., 1990), differed from results observed in this study where there was no significant variation in the number of leaves in plants treated with growth regulators.
Due its composition (90 mg Kt, 50 mg IBA and 50 mg GA3 per liter of the product) the biostimulant used can increased growth and development plant by stimulating cell division and increase the uptake of water and nutrients by plants, resulting in increment total dry biomass (Vieira and Castro, 2004). The increased dry biomass of leaves is thus related the elongation of the palisade parenchyma cells when exposed to full sun with application of the biostimulant. According to Romero et al. (2002), this occurs by increased cell divisions and variation in the pattern of cell expansion. The application of GA3 increased the dry weight of leaves from Cymbopogon jwarancusa (Ansari et al., 1988), dry biomass of leaves and consequently total dry biomass of Citrus amblycarpa (Leonel et al., 1994) and increased fresh (85.85%) and dry biomass (82.84%) in Basil (Shedeed et al., 1990). In the Ocimum basilicum treated with kinetine was verified the largest total dry biomass values when compared to plants that did not receive this plant hormone (Barreiro et al., 2006).
The essential oil production in leaves was significantly affected by the interaction between biostimulant and light levels. In plants treated with the plant regulator at full sun condition the essential oil production was significantly superior of to the other treatments (R2= 99.89) (Figure 5A). Only light levels showed significant effects on the essential oil content of L. origanoides. Plants grown in full sun presented highest essential oil content (0.93%), a positive linear trend in relation to the increase in the availability of light radiation, whereas plants subjected to 25% of full sun reached 0.71% (Figure 5B). The highest essential oil content occurred under of full sun with biostimulant application conditions (Figure 5B).
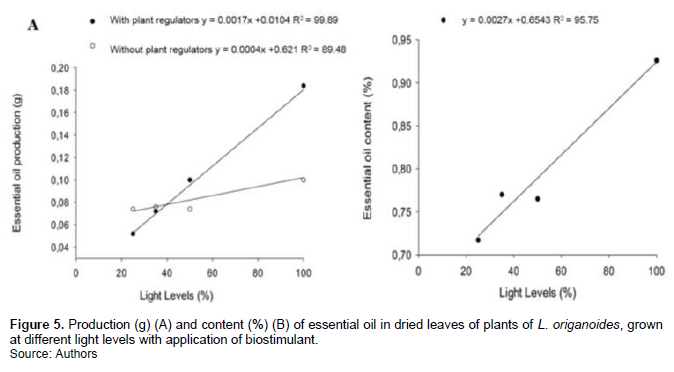
The effects of plant regulators on essential oil production through leaf spraying are variable; one of the most common responses is the influence on the production of essential oil, through increased biomass production (Shukla and Farooqi, 1990; Prins et al., 2010), which was observed in this study. Cytokinins can stimulate the global accumulation of typical monoterpenes of the essential oil (El-keltawi and Croteau, 1987; Ivanova et al., 2014). In Mentha piperita, application of growth regulators increased the production of biomass and essential oil (Çoban and Baydar, 2016). The condition of full sun is more stressful (low relative humidity, higher temperature and incidence of winds) and can raise the production of essential oil (Tavares et al., 2005).
The different light conditions associated to application of the biostimulant no affected on the chemical profile of the essential oil. Nine chemical components were identified in the essential oil; totaling about 97% of the chemical composition of the essential oil; thymol was the major component (Table 1).
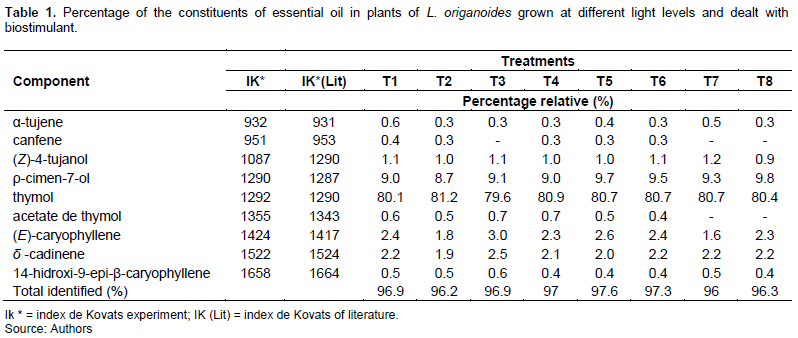
The significant changes in the chemical composition of the essential oil in O. basilicum resultent of the decrease of luminous radiation were attribuite for Chang et al. (2008) as a result of a strategy of acclimatization to this condition, where they are usually checked higher transpiration rates by increased incidence of wind and solar radiation and, consequently, higher water requirement and other environmental agents. The analyses of leaf cross sections revealed significant differences in leaf anatomical characteristics. The thickness of the adaxial and abaxial surface of epidermis and spongy parenchyma were suffered only influence of irradiance treatments (Figure 6).
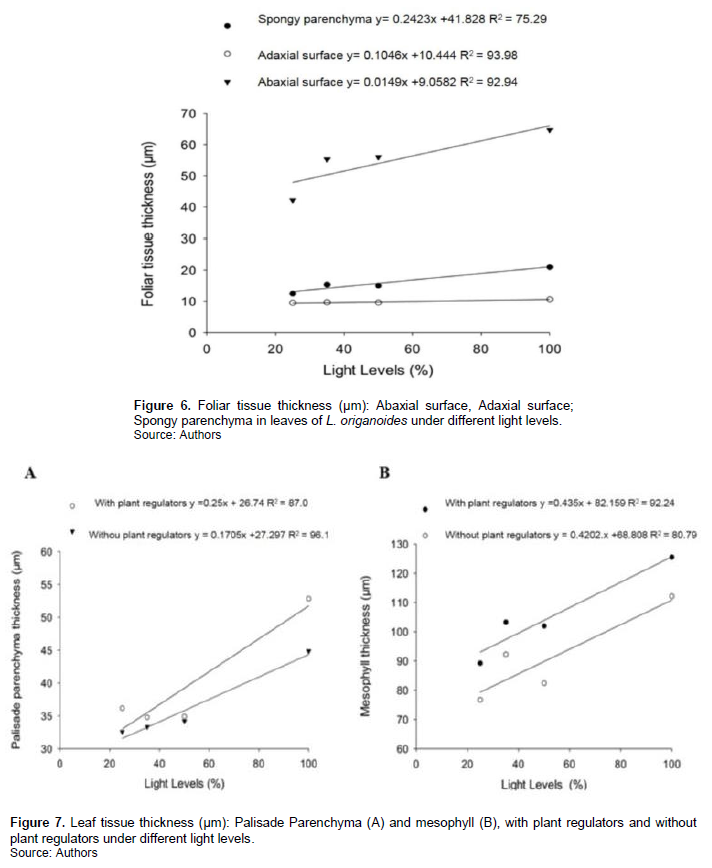
The greatest thickening of the epidermis in sun leaves minimizes the effect of high irradiation under the sensitive tissues of the mesophyll (Markesteijn et al., 2007). The direct contact of the different faces of the epidermis with the environment, it may cause changes due to various environmental factors, mainly the luminosity, favoring the even distribution of light in the interior of the leaf (Volgelmann; Martin, 1993). The palisade parenchyma increased of the treatments with application of the biostimulant associated with the increased availability of luminous radiation resulting from the elongation of the cells that tissue, since there was no change in the number of layers of the even (Figure 7).
To optimize the absorption of photosynthetically active radiation, the leaves of shade in general have smaller thickness of the mesophyll (Gorton et al., 1999; Brodersen et al., 2008), which results from the difference in the pattern of cell division in sun and shade leaves (Yano and Terashima, 2004). The periclinal and anticlinal divisions occur at the same time in sun leaves, while shadow leaves the periclinal division happens later. The cytokinins are hormones associated with the growth and development of plants, participating in the control of cell division (Nishimura et al., 2004), while the gibberellins are involved with regulation and foliar expansion by stimulating elongation and division (Scavroni et al., 2006). These being two of the three hormones present plant regulator used, associated with the cell divisions.
The different levels of light influenced densitythe glandular and tectores trichomes in both surface of the leaf of L. origanoides. The density of tectores and glandular trichomes presented quadratic adjustment in the surface abaxial with increased light radiation (Figure 8A to B). Glandular trichomes presented the highest means values of density for both surface of leaf with increased of the light radiation with application of biostimulant (Figure 8A). Tector trichome presented a maximum mean value of 43.18 mm-2 in adaxial surface and 129.56 mm-2 in abaxial surface to light levels of 88 and 100% full sun with application of biostimulant, respectively (Figure 8B). Stomatal density also showed interaction between treatments applied with significant difference for the interaction of the factors: Light levels × biostimulant application (Figure 8C).
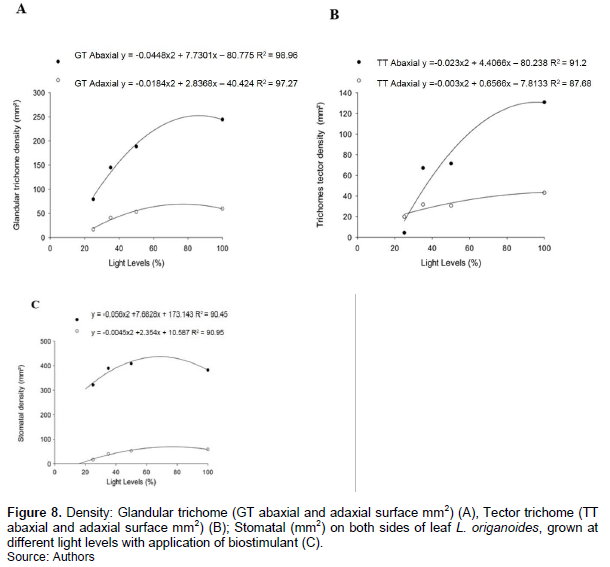
The density of glandular trichome may vary according to leaf age, species and environmental conditions. Gomes et al. (2009) found that the density of glandular trichomes in the full sun, 70 and 50% of the full sun did not show significant differences among the species Lippia citriodora. However, in plants of O. gratissimum presented higher density of tectonic trichomes when kept in full sun (Martins et al., 2008). The increase in the density of trichomes is a strategy of acclimatization to the reduction in the rate of perspiration, increased reflection of light, which decreases the temperature of the leaf (Gomes et al., 2009). As a result of the increased density of glandular trichomes, a production of essential oils has been increased because it is secretly stored in the glandular trichomes. It was observed an increase in density of glandular trichomes with application of plant regulators in Mentha arvensis (Bose et al., 2013), and not available in the present study.
The stomatal density could be also related to the photosynthetic capacity of the leaves, because the greater the number of stomata mm-2, lower resistance to diffusion of gases on the sheet. Increased stomatal density is usually connected with a higher stomatal conductance (Justo et al., 2005). In this work treated plants the 25% of full sun showed lower stomatal density, which may contribute to lower growth variables such as dry biomass and foliar biomass total fresh leaves under shade (Lima Junior, 2006). Increased leaf area of shaded plants without applying the biostimulant may explain the lower density of stomata observed. In Arabidopsis thaliana the increased availability of luminous radiation promoted increased stomatal density of leaves showing be mechanisms responsible for stomatal density dependent adjustment of light (Shuluter et al., 2003). Gondim et al. (2008) studying plants of Colocasia esculenta (taro), found that the stomatal density decreases with reduced brightness.
CONCLUSION
L. origanoides demonstrated structural and metabolic plasticity to the effects of applying a biostimulant made up of three plant growth regulators associated with different levels of light radiation. Significant effect occurred biostimulant and light levels in the production of essential oil, showing the interaction of these treatments. The cultivation in 100% of full sun with biostimulant application significantly increased the production of biomass and essential oil keeping the chemical profile.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Alvarenga AA, Castro EM, Lima Júnior EC, Magalães MM (2005). Effects of different light levels on the initial growth and photosynthesis of Croton urucurana Baill. in southeastern Brazil. Revista Árvore 27(1):53-57. |
|
|
Andrade CLL, Silva AG, Melo GB, Ferreira RV, Moura ICS, Siqueira GGC (2018). Bioestimulantes derivados de Ascophyllum nodosum associados ao glyphosate nas características agronômicas da soja RR. Revista Brasileira de Herbicidas 17(3):e592. |
|
|
Ansari SHl, Qadry JS, Jain VK (1988). Effect of plant hormones on the growth and chemical composition of volatile oil of Cymbopogon jawarancusa (SCHULT). Indian Journal of Forestry 11(2):143-146. |
|
|
Barreiro AP, Zucareli V, Ono EO, Rodrigues JD (2006). Análise de crescimento de plantas de manjericão tratadas com reguladores vegetais. Bragantia 65(4):563-567. |
|
|
Brodersen CR, Vogelmann TC, Williams WE, Gorton HL (2008). A new paradigm in leaf level photosynthesis: direct and diffuse lights are not equal. Plant, Cell Environment 31(1):159-164. |
|
|
Bourgaud F, Gravot A, Milese S, Gontier E (2001). Production of plant secondary metabolites: a historical perspective. Plant Science 161(5):839-85. |
|
|
Castro PRC, Vieira EL (2001). Aplicações de reguladores vegetais na agricultura tropical. Guaíba: Livraria e Editora Agropecuária P 588. |
|
|
Cappelari LDR, Santoro MV, Schmidt A, Gershenzon J, Banchio E (2019). Induction of essential oil production in Mentha x piperita by plant growth promoting bacteria was correlated with an increase in jasmonate and salicylate levels and a higher density of glandular trichomes. Plant Physiology and Biochemistry 141:142-153. |
|
|
Chang X, Alderson PG, Wright CJ (2008). Solar irradiance level alters the growth of basil (Ocimum basilicum L.) and its content of volatile oils. Environmental and Experimental Botany 63(3):216-223. |
|
|
Çoban Ö, Baydar NG (2016). Brassinosteroid effects on some physical and biochemical properties and secondary metabolite accumulation in peppermint (Mentha piperita L.) under salt stress. Industrial Crops and Products 86:251-258. |
|
|
Fernandes VF, Almeida LB, Feijó EVRS, Silva DC, Oliveira RA, Mielke MS, Costa LCB (2013). Light intensity on growth, leaf micromorphology and essential oil production of Ocimum gratissimum. Revista Brasileira Farmacognosia 23(3):419-424. |
|
|
Gondim ARO, Puiatti M, Ventrella MC, Cecon PR (2008). Plasticidade anatômica da folha de taro cultivado sob diferentes condições de sombreamento. Bragantia 67(4):1037-1045. |
|
|
Gomes PA, Souza MFS, Souza Júnior IT, Carvalho Júnior WGO, Figueiredo LS, Martins ER (2009). Influência do sombreamento na produção de biomassa, óleo essencial e quantidade de tricomas glandulares em cidrão (Lippia citriodora Lam.). Revista Biotemas 22(4):9-14. |
|
|
Gorton HL, Willians WE, Volgelmann TC (1999). Choroplast movement in Alocasia macrorrhiza. Plant Physiology 106(4):421-428. |
|
|
Hernandes C, Pina ES, Taleb-Contini SH, Bertoni BW, Cestari IM, Espanha LG, Varanda EA, Camilo KFB, Martinez EZ, França SC, Pereira AMS (2017). Lippia origanoides essential oil: an efficient and safe alternative to preserve food, cosmetic and pharmaceutical products. Journal of Applied Microbiology 122(4):900-910. |
|
|
Ivanova K, Manov M, Iliev L, Stefanov B (2014). Effect of cytokinins on the essential oil composition of in vitro produced peppermint plants. Biotechnology & Biotechnological Equipment 10(3):44-48. |
|
|
Justo CF, Soares AM, ML, Gavilanes ML, Castro EM (2005). Leaf anatomical plasticity of Xylopia brasiliensis Sprengel (Annonaceae). Acta Botânica Brassileira 19(1):111-123. |
|
|
Khan N, Bano AMD, Babar A (2020). Impacts of plant growth promoters and plant growth regulators on rainfed agriculture. Plos One 15(4):e0231426. |
|
|
Kunz LY, Redekop P, Ort DR, Grossman AR, Cargnello M, Majumdar A (2020). A phytophotonic approach to enhanced photosynthesis. Energy & Environmental Science 13(9):1-14. |
|
|
Lambers H, Chapim FS, Pons TL (2008). Plant Physiological Ecology. Springer: New York (2a Ed.) P 604. |
|
|
Leonel S, Modesto JC, Rodrigues JD (1994). Influência de fitorreguladores e nitrato de potássio na germinação de sementes e no crescimento de porta-enxerto de Citrus amblycarpa. Scientia Agricola 52(2):252-259. |
|
|
Lima Júnior EC (2006). Aspectos fisioanatômicos de plantas jovens de Cupania vernalis Camb. Submetidas a diferentes níveis de sombreamento. Revista Árvore 30(1):33-41. |
|
|
Liu Q, Dong L, Li F (2019). Modification of a photosynthetic light-response (PLR) model for modeling the vertical gradient in the response of crown PLR curves. Canadian Journal of Forest Research 48(8):1-43. |
|
|
Markesteijn L, Pooter L, Bongers F (2007). Light-dependent leaf taint variation in tropical dry forest tree species. American Journal of Botany 94(4):515-525. |
|
|
Martins JR, Alvarenga AA, Castro EM, Pinto JEBP, Silva APO (2008). Avaliação do crescimento e do teor de óleo essencial em plantas de Ocimum gratissimum L. cultivadas sob malhas coloridas. Revista Brasileira de Plantas Medicinais 10:102-107. |
|
|
Miralles J, Martinez Sanchez JJ, Franco JA, Banon S (2011). Rhamnus alaternus growth under four simulated shade environments: Morphological, anatomical and physiological responses. Scientia Horticulturae 127(4):562-570. |
|
|
Moraes Neto SP, Gonçalves JLM, Takaki M, Gonçalves JC (2000). Crescimento de mudas de algumas espécies arbóreas que ocorrem na mata atlântica, em função do nível de luminosidade. Revista Árvore 24(1):35-45. |
|
|
Newman DJ, Cragg GM (2016). Natural Products as Sources of New Drugs from 1981 to 2014. Journal of Natural Products 79:629-661. |
|
|
Nishimura C, Ohashi Y, Sato S, Kato T, Tabata S, Uequchi C (2004). Histidine kinase homologs that acts as cytokinin receptors possess overlapping functions in the regulation of shoot and root growth in Arabidopsis. Plant Cell 16:1365-1377. |
|
|
Ortiz RE, Afanador G, Vásquez DR, Ariza-Nieto C (2017). Efecto del aceite esencial de orégano sobre el desempeño productivo de ponedoras y la estabilidad oxidativa de huevos enriquecidos con ácidos grasos poli-insaturados. Revista de la Facultad de Medicina Veterinaria y de Zootecnia 64(1):61-70. |
|
|
Osunkoya OO, Ash JE (1991). Acclimation to a change in light regime in seedlings of six Australian rainforest tree species. Australian Journal of Botany Botany 39:591-605. |
|
|
Passos EEM (1997). Ecofisiologia do Coqueiro, In: Warwick DRN, Siqueira LA. (Ed.) Cultura do coqueiro no Brasil. Aracaju: Embrapa-SPI, pp. 65-72. |
|
|
Prins CL, Vieira IJC, Freitas SP (2010). Growth regulators and essential oil production. Journal of Plant Physiology 22(2):91-102. |
|
|
Rezaie R, Abdollahi Mandoulakani BA, Fattahi M (2020). Cold stress changes antioxidant defense system, phenylpropanoid contents and expression of genes involved in their biosynthesis in Ocimum basilicum L. Scientific Reports 10:5290. |
|
|
Romero MEM, Martinez S, Atkins S, Rotman AD (2002). Morfologia de lãs inflorescência em verbenaceae, verbenoideae. Darwiniana 40(3-4):1-15. |
|
|
Scavroni J, Vasconcellos MC, Valmorbida J, Ferri AF, Marques MOM, Ono EO, Rodrigues JD (2006). Rendimento e composição química do óleo essencial de Mentha piperita L. submetida a aplicações de giberelina e citocinina. Revista Brasileira Plantas Medicinais 8(4):40-43. |
|
|
Shedeed MR, Gamassy KM, Hashim ME, Kandeel AM (1990). Physiological studies on the growth, oil yield and chemical constituents in basil plant, Ocimum basilicum L. Annals of Agricultural Sciences 35(2):971-979. |
|
|
Shukla A, Farooqi AHAE (1990). Review: utilization of plant growth regulators in aromatic plant production. Current Research on Medicinal and Aromatic Plants 12:152-177. |
|
|
Stashenko E, Martínez J, Arias J, Córdoba Y, Durán D, Mejia J, Tavera C (2018). Method for making full use of Lippia origanoides. WO2018122654A1. |
|
|
Tavares ES, Julião LS, Lopes D, Bizzo HR, Lage CLS, Leitão SG (2005). Análise do óleo essencial de folhas de três quimiotipos de Lippia alba (Mill.) N. E. Br. (Verbenaceae) cultivados em condições semelhantes. Revista Brasileira Farmacognosia 15(1):1-5. |
|
|
Valladares F, Niinemets U (2008). Shade tolerance, a key plant feature of complex nature and consequences. Annual Review of Ecology, Evolution, and Systematics 39:237-257. |
|
|
Vieira EL, Castro PRC (2004). Ação de bioestimulante na cultura da soja (Glycine max L. Merrill). Stoller do Brasil, São Paulo, Brasil P 47. |
|
|
Vogelmann TC, MARTIN G (1993). The functional significance of palisade tissue: penetration of directional versus diffuse light. Plant, Cell Environment 16:65-72. |
|
|
Yano S, Terashima I (2004). Developmental process of sun and shade leaves in Chenopodium álbum L. Plant, Cell & Environment 27(6):781-793. |
|
|
Zubo YO, Yamburenko MV, Selivankina SY, Shakirova FM, Avalbaev AM, Kudryakova NV, Zubkova NK, Liere K, Kulaeva ON, Kusnetsov VV, Börner T (2008). Cytokinin stimulates chloroplast transcription in detached barley Leaves. Plant Physiology 148(2):1082-1093. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0