ABSTRACT
Groundnut (Arachis hypogaea L.) is an important cash crop in the lowland areas of Ethiopia. However, prevalence of Aspergillus invasions and subsequent aflatoxin contamination compromises the quality of groundnut kernels. This study was conducted to evaluate the effect of farm yard manure (FYM) and seed treatments against Aspergillus species pod colonization and aflatoxin accumulation under field conditions. The inhibitory efficacy of Trichoderma species as biocontrol agents was also assessed. A total of 20 treatment combinations including pre-planting applications of FYM at 0, 2.5, 5, and 7.5 tons/ha and seed treatments with carbendazim at 2 g/kg and mancozeb at 3 g kg-1, and Trichoderma harzianum and Trichoderma viride each at 5 g/kg as well as untreated seed as control were used. Treatments were laid out in a randomized complete block design (RCBD) in three replications. The experiment was conducted in two consecutive seasons (2014 and 2015) at Babile Haramaya University sub-Research Station. The highest pod and seed yields (1901.5 and 1281.5 kg/ha, respectively) were recovered from plots treated with T. harzianum at 5 g/kg seed. A. flavus was abundantly recorded in control plots, which could be responsible for the high aflatoxin B1 (5704.4 µg/kg) and B2 (2219.0 µg/kg) contamination. However, plots treated with T. harzianum at 5 g/kg seed and FYM at 5 tons/ha + T. harzianum at 5 g/kg were free from aflatoxin. Integrations of T. harzianum as biocontrol seed treatment and soil amendment with FYM were effective in the pre-harvest management of Aspergillus spp. and aflatoxins contamination.
Key words: Aflatoxin, Aspergillus, carbendazim, farm yard manure, groundnut, mancozeb, Trichoderma species.
Groundnut (Arachis hypogaea L.) is an important monoecious annual legume used as oilseed, food and animal feed all over the world (Upadhyaya et al., 2006). In Ethiopia, groundnut production is adapted to warm climates and predominantly grown under rainfed conditions (Amare and Tamado, 2014). The total groundnut yield in the country was 1,296,364.18 tons for 2016/2017, with the productivity of 1.73 tons/ha (CSA, 2016/2017). Eastern and lowland part of the country, mainly East Hararghe zone, is the leading groundnut production area accounting for 43.4% of the total production. In this area, groundnut is replacing major crops like maize and sorghum (Amare and Tamado, 2014). Despite its ever-increasing importance, groundnut quality and marketability are hampered by pre- and post-harvest aflatoxin contamination in the area (Ayalew et al., 1995; Eshetu, 2010; Chala et al., 2013; Mohammed and Chala, 2014; Mohammed et al., 2016).
Groundnut is one of the legume crops most susceptible to invasion by Aspergillus flavus and Aspergillus parasiticus, which subsequently produce aflatoxin, a mycotoxin that poses serious human and animal health risks (Williams et al., 2004). Aflatoxin is a major constraint to groundnut export and foreign exchange for many countries in sub-Saharan Africa such as Ethiopia and Kenya. Losses from rejected shipments and lower prices for poor quality grain can devastate a developing country’s export markets (IFPRI, 2003). These losses can have a higher impact on sub-Saharan Africa due to the favorable environmental conditions for the growth of mycotoxigenic fungi and the lack of adequate storage infrastructure. For example, in Nigeria, regulatory agencies destroyed mycotoxin-contaminated foods worth more than US$ 200, 000 in 2010 (Hussaini, 2013). Income losses due to aflatoxin contamination cost US producers more than US$ 100 million per year, on average, including US$ 26 million paid to peanut farmers alone (US$ 69.34 ha-1) (Coulibaly et al., 2008).
In Ethiopia, aflatoxin contamination of groundnut is often reported. Ayalew et al. (1995) reported a total aflatoxin contamination in groundnut ranged from 5 to 250 µg/kg, furthermore Eshetu (2010) reported aflatoxin level up to 447 µg/kg in groundnut seed from Eastern Ethiopia. Death cases in Kenya were reportedly caused by ingestion of maize with aflatoxin concentrations up to 4,400 µg/kg (Azziz-Baumgartner et al., 2005). However, Chala et al. (2013) reported aflatoxin levels of about 12,000 µg/kg in groundnut seed from Babile district in Eastern Ethiopia orders of magnitude higher than the levels observed in Kenya; whereas the acceptable limit set for the European Union is 4 µg/kg (OJEU, 2010).
However, there is no acceptable limit of aflatoxin in Ethiopia. Recently, Mohammed et al. (2016) reported aflatoxin B1 concentrations of 2,526 and 158 µg/kg, in groundnut seed and groundnut cake locally known as “Halawa”, respectively, from Eastern Ethiopia. The high aflatoxin levels observed indicate the urgent need for management of Aspergillus and associated aflatoxin contamination in this area.
Management practices that reduces the incidence of aflatoxin contamination at pre-harvest in the field include timely planting, maintaining optimal plant densities, proper plant nutrition, avoiding drought stress, controlling other plant pathogens, weeds and insect pests and proper harvesting (Bruns, 2003). The application of lime, FYM, poultry manure, host plant resistance, and chemical fumigation of the soil was employed earlier in reducing the aflatoxin contamination in the groundnut crops (ICRISAT, 2000). Among which, lime, FYM and cereal crop residues as soil amendments have shown to be effective in reducing A. flavus contamination as well as aflatoxin levels by 50 to 90% (Bruns, 2003). Besides biocontrol agents such as, non-toxigenic bacterial strains, especially Bacillus species (Bottone and Peluso, 2003) and the fungus Trichoderma harzianum (Inglis and Kawchuk, 2002) have also been used. Most of these biocontrol options are not accessible or are difficult to apply on the seeds for smallholder farmers in developing countries. Hence, there is a need for evaluations of effective integrations of biocontrol agents with cultural practices that are affordable for adoption by smallholder farmers.
Fungicide seed treatment is also beneficial for the management of seed-borne pathogens and its application before planting decreases pre-emergence as well as post-emergence damping-off and increases seedling survival rates or establishment and plant vigor in various crops (Elwakil and El-Metwally, 2000). In Eastern Ethiopia, Getnet et al. (2013) conducted field experiments aimed at suppressing aflatoxigenic fungi on groundnut through fungicide seed treatments with mancozeb and carbendazim and reported seed yield increase by 42.1 and 70.9%, respectively. However, the comparison of fungicide and biocontrol seed treatments with cultural practices in reducing the pre-harvest Aspergillus spp. invasion and aflatoxin contamination of groundnut was meager in the area.
Given the negative effects that aflatoxins have on human health and marketability of groundnut, it is imperative to find cultural practices that can help reduce/eliminate aflatoxins from this crop, integration with biological control methods adds the advantage of being eco-friendly and relatively safe. The objectives of this study were to evaluate the effects of FYM and seed treatments with fungicides and biocontrol agents on the development of Aspergillus spp. and aflatoxin production under field conditions.
Field experiment and treatment applications
Experiments were conducted at Babile research sub-station of Haramaya University during 2014 and 2015 major cropping seasons and planted in mid-May each year under rainfed conditions. The site is located in eastern Hararghe zone at 9°13’13.5’’ N and 42°19’20.9’’ E with an altitude of 1647 m above sea level. The growing season starts from mid-April to end of October and the area has an annual average rainfall of 569 mm. The soil at Babile research sub-station is sandy loam soils.
The local groundnut variety Oldhale, commonly grown in Babile, Gursum and Bisidmo areas by smallholder farmers (Bethlehem, 2011) was used as experimental material. Treatments included soil amendments with farm yard manure (FYM) at rates of 0, 2.5, 5, and 7.5 tons/ha applied one week before planting. Fungicides seed treatments with carbendazim at 2 g/kg and mancozeb at 3 g/kg seed; biocontrol agents T. harzianum and Trichoderma viride each at 5 g/kg seed; and untreated seeds used as a control.
Farm yard manure (FYM) was a mixture of cattle and goat manure collected for approximately 10 years and protected from water erosion commonly used by growers as organic fertilizer. It is rich in nutrients, including trace elements necessary for crop growth and inducing resistance in the plants. The amount of FYM required for the experiment was purchased from the growers and spreaded on the soil surface and amended to the soil manually a week prior to planting the seeds. Agronomic practices were performed for manual hand weeding and inter-cultivations in each plot. Groundnut harvesting at physiological maturity was performed manually using human labors in avoiding the mechanical damages of the pods. Fungicide seed treatments were done by first weighing each chemical and placing it in a 100 mL Erlenmeyer flask to which the groundnut seeds were added; this was followed by 1-h shaking (Flask shaker SF1, Stuart Scientific, UK) at speed of 150 rpm to uniformly coat the seeds. A total of 20 treatment combinations (Table 1) replicated three times were set up in a factorial RCBD. The crop was planted 60 cm between rows and 10 cm apart within rows giving a total of 150 plants per plot. Each plot had 5 rows of 30 plants per row 3 × 3 m plots. Plants in the central three rows were used for data collection.
For the Trichoderma species treatment application, pure isolates of T. harzianum and T. viride preserved at Ambo Plant Protection Research Center (Ambo Ethiopia), were used. Isolates were multiplied using PDA medium. Seeds were treated with Trichoderma from 8-day-old PDA culture. Mycelial mat/harvesting (at 5 g/kg seed for each species) with spores and conidia amended with carboxy-methyl cellulose (CMC) (0.5%) placed in 1000 ml Erlenmeyer flask with droplets of sterile distilled water to produce a thin paste. Then the seeds were mixed through rotary shaker at 150 rpm for 6 h. Control seeds were treated with sterile distilled water. Seeds were incubated at 25°C for 24 h to create fungal emergence for further effective adherence.
The variables observed were: days to 50% emergence (D50%E), days to 50% flowering (D50%F), and days to 95% maturity (D95%M) of groundnut. Plant populations were evaluated as stand count at emergence (SCE) and stand count at harvest (SCH). Yield components measured were: number of pods per plant (NPP), seeds per pod (NSP), pod yield (PY), seed yield (SY), the 100 seed weight (HSW), and shelling percentage (SHP) of pods. The crop was harvested at physiological maturity and dried on sterilized materials (rugs). Each treatment was harvested and groundnut seed sample of 1 kg homogeneously produced from each plot and a total of 120 samples in two cropping seasons were taken for further laboratory analysis.
Isolation and identification of Aspergillus spp. from groundnut seeds
Aspergillus spp. were isolated from harvested groundnut seed samples using modified Dichloran Rose Bengal (MDRB) (Horn and Dorner, 1998). Twenty grams of groundnut seeds were weighed from each sample and placed in 50 mL FalconTM tubes containing 25 ml of sterile distilled water. From each sample, 50 and 100 µL suspensions were spread on MDRB medium and incubated at 37°C for 72 h. Colonies of Aspergillus spp. were counted on MDRB medium. Fungal species load per treatment was derived from plate counts, expressed as the logarithm of the number of colony forming unit (CFU) and were presented as Log10 CFU/g.
Spores from the individual colony were aseptically transferred to fresh MDRB plates using a sterile needle. Small pieces of agar containing hyphal tip were transferred to Czapek Dox Agar (CDA; OXOID Ltd, Hampshire, England), slant medium prepared according to Horn et al. (1996) and incubated at 30°C for 10 to 14 days for identification. Isolates were identified using taxonomic systems of Aspergillus (Klich, 2002) and confirmation was done by comparison with reference cultures of Dr. Bruce Horn’s collection (USDA National Peanut Research Laboratory, Dawson, Georgia, USA).
Aflatoxin analysis from groundnut seed
About 1 kg of groundnut sample from each plot was taken and totally produces 120 samples, and representative of 50 g of seed was suspended in 1 L glass jar (Waring Products Div., Torrington, CT, USA) with 100 mL of methanol/distilled water (80/20 v/v, respectively) and blended at high speed (13, 000 rpm) for 1 min, for aflatoxin extraction. A pre-pleated filter paper Whatman No. 4 was inserted in the mixture, 500 μL of filtrate was transferred to a disposable glass test tube followed by addition of 500 μL of acetonitrile to the same tubes and mixed thoroughly (Mohammed et al., 2016). Then, 500 μL of the mixture was pipetted into the 1.5-mL columns prepared for cleaning. The eluate containing aflatoxins was collected into 500 μL-ultra performance liquid chromatography (UPLC) glass vials and immediately closed with caps with septa. The limit of detection (LOD) for aflatoxins was 1 µg/kg for B1 and G1 and 0.05 µg/kg for aflatoxin B2 and G2. Aflatoxin standards of B1, B2, G1, and G2 were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Stock and spike solutions of each aflatoxin B1, B2, G1, and G2 were prepared according to the protocol developed by Sobolev and Dorner (2002).
Aflatoxin detection was carried out by UPLC Acquity, using column ACQUITY UPLC ® BEH C18 1.7 µm, 2.1 × 50 mm (Waters, Milford, MA, USA), at a temperature of 40°C, and with fluorescence detection. The mobile phase was methanol/water/acetonitrile (20/70/10%, v/v/v, respectively), and the flow rate of 0.25 mL/min with an injection volume of 1 µL was used. All instrument control, analysis, and data processing were performed using Waters® Empower 3® Chromatography Data Software (CDS). The concentration of each class of aflatoxin was computed as µg/kg.
Data analysis
Data collected from the field and laboratory experiments were subjected to analysis of variance (ANOVA) using general linear models (GLM) of SAS (2002) for Windows 9 (SAS Institute Inc., Cary, NC, USA). Means were compared using the least significant difference (LSD) at the p≤0.05 level of significance. Correlation analysis was done using Pearson correlation coefficient method (Pelosi and Sandifer, 2003).
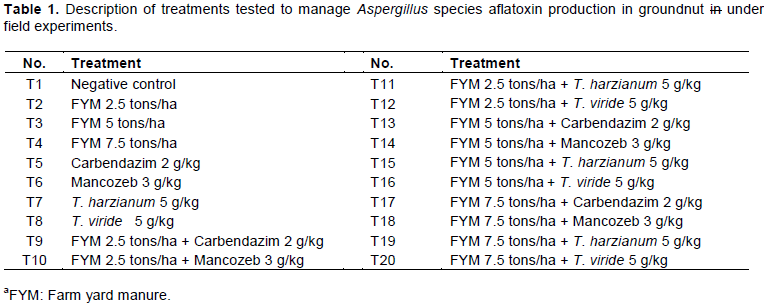
Efficacies of treatments on Aspergillus spp. contamination in groundnut
The experiments were employed for the comparison of fungicides and Trichoderma spp. seed treatment as biological control agent integration with FYM soil application against pre-harvest Aspergillus spp. invasion and subsequent aflatoxin production. In the 2014 experiment, the highest seed invasion of A. flavus (1.3 log CFU/g) and A. parasiticus (0.48 log CFU/g) was found in samples from plots treated with FYM at 5 tons/ha followed by control plot samples infected with A. flavus of 1.08 log CFU/g (Table 2). Among plots that received sole FYM treatment, the lowest seed invasion (0.69 log CFU/g) of A. flavus was recorded at the highest rate of FYM at 7.5 tons/ha soil application. However, plots sown with seeds treated with carbendazim at 2 g/kg and T. harzianum 5 g/kg were devoid of Aspergillus spp. invasion, which is 100% reduction in seed invasion compared with the control plots in 2014 season. On the other hand, A. niger occurrences were relatively less with the highest (0.78 log CFU/g) of seed invasion in plots treated with FYM 2.5 tons/ha + T. harzianum 5 g/kg. The samples obtained from plots treated with FYM 2.5 tons/ha integrated with mancozeb 3 g/kg and T. viride 5 g/kg and FYM 5 tons/ha + T. harzianum 5 g/kg also had less contamination by Aspergillus spp.
In the samples from the 2015 experiment, the highest seed invasion of A. flavus (1.63 log CFU/g) was found in control plots, followed by plots sown with seeds treated with mancozeb 3 g/kg which had 1.36 log CFU/g (Table 2). Among the plots sown with seed treatments, T. harzianum 5 g/kg managed 100% seed invasion by Aspergillus spp., except Aspergillus niger, which was recorded at 0.3 log CFU/g of seed. Plots treated with T. harzianum 5 g/kg in single and combination with FYM at 5 tons/ha had no or relatively less invasion with Aspergillus spp., which is consistent with the preceding year results.
Effects of treatments on aflatoxin contamination of groundnut seed
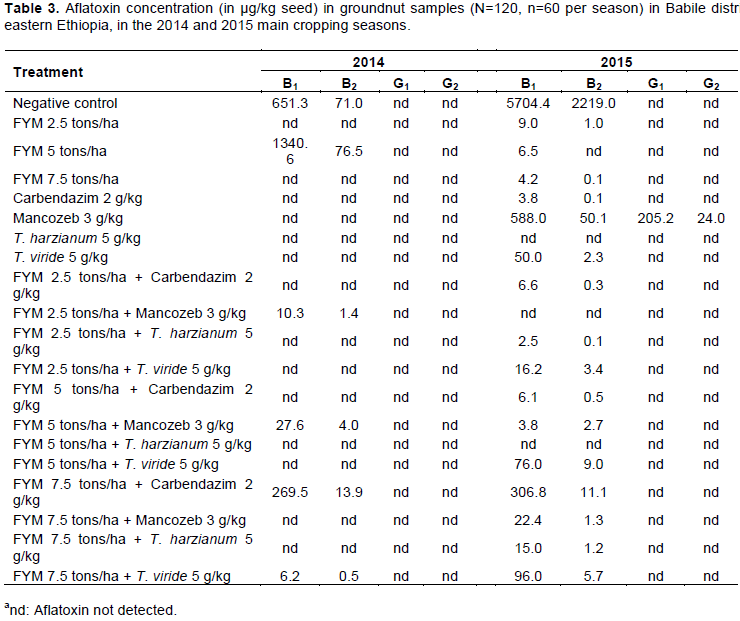
In 2014 cropping season, samples (n=60), 10% (n= 6) were positive for aflatoxin contaminations, while 90% (n= 54) were negative (Table 3). Among positive samples, 33% had aflatoxin B1 below 10 µg/kg, which is the tolerable limit of East African Commission. However, the highest aflatoxin concentration of B1 (1340.6 µg/kg) was detected in samples from plots treated with FYM at 5 tons/ha, followed by the control plots which had aflatoxin B1 in excess of 600 µg/kg. The high aflatoxin levels from plots with a low rate of FYM came from seeds that were not treated with fungicides or bio agents. Concomitantly, there were high incidences of A. flavus and A. parasiticus isolates from these samples that might have accounted for high aflatoxin concentrations. None of the aflatoxin G types were detected in the present samples in spite of isolation of Aspergillus spp. were employed. All plots subjected to T. harzianum as a seed treatment singly and in combinations with different levels of FYM applications were devoid of aflatoxin contaminations in the samples. Among plots subjected to T. viride singly and in combinations, only samples from plots treated with FYM 7.5 tons/ha + T. viride 5 g/kg had aflatoxin B1 and B2 that were 99% less aflatoxin contamination than plots with the highest aflatoxin levels.
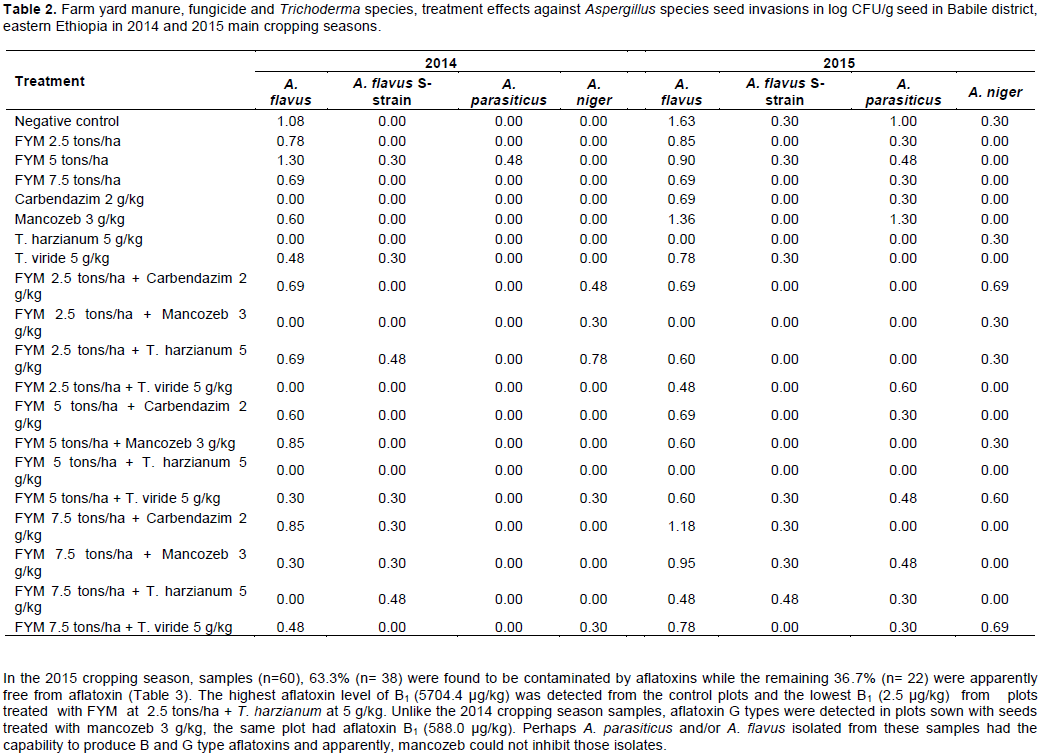
In the 2015 cropping season, samples (n=60), 63.3% (n= 38) were found to be contaminated by aflatoxins while the remaining 36.7% (n= 22) were apparently free from aflatoxin (Table 3). The highest aflatoxin level of B1 (5704.4 µg/kg) was detected from the control plots and the lowest B1 (2.5 µg/kg) from plots treated with FYM at 2.5 tons/ha + T. harzianum at 5 g/kg. Unlike the 2014 cropping season samples, aflatoxin G types were detected in plots sown with seeds treated with mancozeb 3 g/kg, the same plot had aflatoxin B1 (588.0 µg/kg). Perhaps A. parasiticus and/or A. flavus isolated from these samples had the capability to produce B and G type aflatoxins and apparently, mancozeb could not inhibit those isolates.
In both cropping seasons, plots treated with T. harzianum at 5 g/kg seed and FYM 5 tons/ha + T. harzianum at 5 g/kg were not contaminated by aflatoxins. In the later year (2015), plots that received FYM 2.5 tons/ha + T. harzianum 5 g/kg decreased both aflatoxins B1 and B2 by 99.9% as compared to the untreated control plots. In 2014, apart from control plots, only samples from plots treated with FYM 5 tons/ha were positive for aflatoxin with maximum aflatoxin B1. In the latter year (2015), all single treatment applications were positive for aflatoxins except plots sown with T. harzianum 5 g/kg seed. Among plots treated with sole FYM in 2015 cropping season, maximum (9.0 µg/kg) aflatoxin B1 concentration was estimated in samples taken from plots treated with FYM 2.5 tons/ha, whereas plots that received FYM 7.5 tons/ha had aflatoxin B1 (4.2 µg/kg), which revealed that the highest rate of FYM application significantly decreased aflatoxin contaminations and vice versa.
Effects of treatments on yield and yield components of groundnut
Yield and yield components varied across treatments but the variation was not significant in most cases regardless of the experimental year (Table 4). In the 2014 experiment, the highest mean of SCE (90.0 plants/plot) recorded from plots treated with FYM 2.5 tons/ha + mancozeb at 3 g/kg, FYM 2.5 tons/ha + T. viride at 5 g/kg, FYM 5 tons/ha + T. viride 5 g/kg, and FYM 7.5 tons/ha + T. harzianum 5 g kg-1. On the other hand, control plots had the lowest number of plants (74.0 plants/plot). The highest mean SCH (33.7 plants/plot) was obtained from plots treated with FYM 5 tons/ha and FYM 7.5 tons/ha + T. harzianum 5 g/kg. The highest means PY (670.9 kg/ha), NSP (1.8), and the second highest mean SY (601.0 kg/ha) and NPP (34.9) were harvested from plots treated with FYM at 7.5 tons/ha + mancozeb 3 g/kg. These results were achieved due to the integration of soil amendment with a high amount of FYM and seed treatment with fungicides.
In the 2015 season, 10 different treatments led to the highest (90 plants/plot) SCE (Table 4), the same levels of performances. The lowest (80.3 plants/ plot) SCE was recorded from untreated plots and plots treated with FYM at 5 tons/ha + T. harzianum 5 g/kg. In case of SCH, the highest mean (47.7 plants/plot) was obtained from plots treated with FYM at 7.5 tons/ha. The highest PY and SY (1901.5 and 1281.5 kg/ha, respectively) were obtained from pots treated with T. harzianum at 5 g/kg. The highest NPP (27.9) was recorded from plots treated with FYM at 5 tons/ha + T. harzianum 5 g/kg and the lowest (20.0) was from the control plots.
Days to 50% emergence in experimental years, viz. 2014 and 2015, showed non-significant (p> 0.05) differences among treatments (Table 5). Days to 50% flowering showed slight differences among treatments and all plots attained flowering with the mean intervals of 37.0 to 46.0 days. Days to 95% physiological maturity exhibited significant differences among treatments from 146.0 to 152.0 days. Evidently, Tegene et al. (2013) reported an unnamed local variety reaching physiological maturity from 148 to 154 days in eastern Ethiopia.
Pearson correlation coefficients evaluated among the agronomic and yield parameters suggested differential relationships (Table 6). In the 2014 experiment, SY showed highly significant and positive (r=0.42, P= 0.01) correlation with NPP; correspondingly PY had significant positive (r= 0.94 and 0.40, P= 0.01) correlations with SY and NPP. Similarly, Vaithiyaungan et al. (2010) found that SY of groundnut had highly significant and positive association with NPP and harvest index. In the 2015, PY (r= 0.43, P= 0.01) and SHP (r= 0.41, P= 0.01) were significantly and positively correlated with SY.
Plots sown with groundnut seed treatments with fungicides (carbendazim and mancozeb) or biocontrol (T. harzianum and T. viride) had a minimum invasion by Aspergillus species compared with sole FYM application. Fungicide seed treatment is effective in reducing losses caused by A. niger in crops like groundnut which are vulnerable at the seedling stage (Manju et al., 2017), thereby enhancing the crop resistance and contributing for harvesting of healthy seed. Biocontrol agents of T. viride and T. harzianum have also shown benefits in managing the collar rot of groundnut (Pratibha et al., 2012; Gangwar et al., 2014). The effectiveness of integrated soil organic amendments of FYM with biocontrol agent (T. harzianum) and fungicide (carbendazim) against Aspergillus invasion was evaluated (Manju et al., 2017). Presently, the samples obtained from plots treated with FYM 2.5 tons/ha integrated with mancozeb 3 g/kg and T. viride 5 g/kg and FYM 5 tons/ha + T. harzianum 5 g/kg had less invasion by Aspergillus spp. Particularly, the samples from plots treated with Trichoderma spp. in single and integrated with FYM showed lower invasions of Aspergillus spp. Mohamed (2015) reported that groundnut seed treated with bioagents produced healthier and higher yielding plants than control. Trichoderma spp. are widely used in agriculture as biopesticides, bioprotectants, biostimulants, and biofertilizers on a wide variety of plants (Ranasingh et al., 2006).
The plots treated with carbendazim at 2 g/kg seed had less invasion when compared with samples from control plots. These results are in agreement with the observation of Reddy et al. (2008) who reported a significant reduction in the growth of Aspergillus spp. and aflatoxin contaminations of rice that received seed treatment with carbendazim at 3 g/kg. In contrast, the prevalence of A. flavus and A. parasiticus was high in samples collected from plots sown with mancozeb treatment at 3 g/kg seed. However, Getnet et al. (2013) reported that mancozeb 3 g/kg and carbendazim 2 g/kg seed treatment were effective in suppression of seed invasion by A. flavus and A. parasiticus than A. niger, where as plots sown with carbendazim 2 g/kg or mancozeb 3 g/kg seed treatment were found to be free of A. niger in our study. Treatments were also evaluated on the aflatoxin contamination reduction. In the first year (2014) experiment, 90% samples were negative for aflatoxins, while 10% had detectable aflatoxin concentrations, of that 33% had aflatoxin B1 below 10 µg/kg. Studies have shown that aflatoxin B1 concentration in food above 10 µg/kg is considered hazardous and a threat to food security (Lewis et al., 2005). Plots treated with FYM at 5 tons/ha had aflatoxin B1 of 1340.6 µg/kg, and the same plots were infected with A. flavus and A. parasiticus which might have been responsible for the accumulated aflatoxin level. It has been reported that high aflatoxin concentration accumulates in the soils and if microorganisms do not rapidly degrade it, it will be absorbed by the roots of crops in the subsequent years and translocated to other plant parts like seeds and fruits (Mertz et al., 1980). Samples harvested from the plots planted with T. harzianum alone and integrated with different rates of FYM had aflatoxin below the detectable levels. Perhaps the antagonistic effect of T. harzianum coated to planted seeds likely inhibited the Aspergillus spp. and inhibited aflatoxin production in those plots. The current finding is in agreement with the report of Benizri et al. (2001).
In the second year (2015), the highest aflatoxin B1 (5704.4 µg/kg) and B2 (2219.0 µg/kg) were detected in samples harvested from the control plots. Recently Mohammed et al. (2016) detected aflatoxin B1 2526.3 µg/kg in groundnut seed collected from growers’ storages. These results indicated that, the groundnut seed produced in eastern Ethiopia was badly contaminated with aflatoxin. However, of the contaminated samples, 55% had aflatoxin B1 below acceptable levels (10 µg/kg) set by some countries like China which is 20 µg/kg (Xiaoxia et al., 2015); 15 µg/kg for Taiwan (Chen et al., 2013), and 10 µg/kg for Korea and Uganda (Kaaya et al., 2006; Ee et al., 2007). This indicates that pre-harvest management decrease aflatoxin accumulation. Plots subjected to FYM 7.5 tons/ha + T. harzianum 5 g/kg had aflatoxin B1 levels of 15.0 µg/kg. Plots sown with seeds treated with T. harzianum 5 g/kg, FYM 2.5 tons/ha + mancozeb 3 g/kg, and FYM 5 tons/ha + T. harzianum 5 g/kg showed no aflatoxin contamination. The findings affirmed that the use of T. harzianum as seed treatment or in combinations with FYM significantly reduced seed invasion by Aspergillus spp. and aflatoxins contamination. Choudhary (1992) also reported that, Trichoderma spp. inhibited aflatoxin B1 by 73.5% and G1 by 100% produced by A. flavus. Soil amendment with FYM significantly reduced aflatoxin contamination. Waliyar et al. (2007) reported that treatment with FYM 2.5 tons/ha reduced aflatoxin contamination by 42%. However, in the current study the same rate reduced aflatoxin concentration by 99.8% in 2015 cropping season and 100% in 2014.
The proportion of aflatoxin contaminated samples and concentrations were higher in 2015 cropping season than samples harvested in 2014. The results were in agreement with the prevalence of Aspergillus spp. Perhaps, the amount of rainfall received in 2015 cropping season (446.2 mm) contributed to higher aflatoxin levels compared to the preceding year (2014) which received 596 mm of rainfall during the growing season. In fact, drought stress is the principal factor contributing to Aspergillus spp. occurrences and aflatoxin production under field conditions. Researchers (Waliyar et al., 2003; Craufurd et al., 2006) investigated drought stress as the main factors that predispose seeds to aflatoxigenic fungi and aflatoxin contamination in the field. Groundnut seed samples harvested from rainfed conditions under moisture stress had a maximum (10,240 µg/kg) concentration of aflatoxin, while traces amount were detected in samples from well irrigated plots (Mehan et al., 1988). In the current finding, the highest total aflatoxin (B1+B2+G1+G2= 7, 923.4 µg/kg) was observed in the control plot samples in the 2015 crop season, while 1,417.1 µg/kg aflatoxin levels were detected in the 2014 samples. This is in agreement with the variable amount of rainfall received during 2014 and 2015 crop seasons.
The PY and SY obtained from plots treated with FYM 2.5 tons/ha + T. viride 5 g/kg indicated that low rate of FYM application resulted in low yields, while the highest corresponding mean PY and SY were recorded from plots subjected to treatment with FYM at 7.5 tons/ha + mancozeb 3 g/kg and FYM at 7.5 tons/ha, respectively. These findings are in accordance with the result of Waliyar et al. (2006), who obtained an increase in yield with FYM supplement at different cropping stages contributing to increased groundnut yield. The estimated national and global yield of groundnut in 2014/2015 was about 1600 kg/ha (FAOSTAT, 2014; CSA, 2014, 2015). However, in the current study the highest yield of 1901.5 kg/ha was achieved in plots treated with T. harzianum 5 g/kg which was higher than the yields reported at the national level and globally. This could be due to growth and yield enhancement in addition to the antagonistic effects of bioagents against groundnut pathogens. CSA (2009) reported that, groundnut yields with good management practiced could rise to 3 tons/ha, supporting the present finding. The increase in yield is also attributed to increased vigor of healthy plants through growth regulators produced by Trichoderma spp., which improved plant photosynthesis according to Govindappa et al. (2011). Albeit, Trichoderma spp. seed treatments contribution for photosynthesis improvement should not be ruled out in the current study.
Application of FYM at 10 to 15 tons/ha has been reported to increase the pod and haulm yields and improved the yield parameters compared to inorganic fertilizers (Subrahmaniyan et al., 2000). In the current study, plots treated with FYM at 7.5 tons/ha had the highest average mean of PY (1029.2 kg/ha) and SY (844.4 kg/ha). This indicated that the highest rate of FYM applications could produce better yields. The present finding is in agreement with the report of Lokanath (2010), who confirmed heavy application of FYM at 75 tons/ha producing higher dry pod yield (3510 kg/ha) of groundnut in better moisture conditions. Higher soil moisture in organically amended plots generally leads to poor aeration and decreased activity of soil microorganisms and affects the nutrient availability in the soils and subsequently reduces the yield components of the crop (Lokanath, 2010). In the current study, the amount of rainfall received during 2014 experimental year was 596 mm and PY of 596.4 kg/ha was obtained, while in 2015 when the rainfall was less (446.2 mm) a PY of 1462.0 kg/ha was obtained from plots treated with FYM 7.5 tons/ha. Generally, the important yields of PY and SY were higher in 2015 than 2014 experimental year.
Among the agronomic data, the required days for 50% emergence, 50% flowering, and 95% maturity were 18.0 to 20.3, 37 to 46.0, and 146.0 to 152.0 days, respectively in the current study. Likewise, the same variety “Oldhale” used by Bethlehem (2011) reported that, it took 17, 36.4, and 140.3, days to attain 50% emergence, 50% flowering and 95% physiological maturity, respectively, in Babile district. MoARD (2009) reported that an improved variety, Fetene, took 27 to 35 to attain 50% flowering and 115.9 days to 95% physiological maturity; while “Oldhale” took relatively longer time to reach the specified stages. However, Jeyaramraja and Fantahun (2014) reported that Tole 2 variety required 157 days to reach 95% physiological maturity, much later than “Oldhale”. In case of the Pearson correlation coefficient, the experiment indicates that PY and SY contributed a great deal to the total yields of groundnut. Bethlehem (2011) reported that NPP was significantly and positively correlated with SY. NPP and NSP were significant components of groundnut yield and could be utilized as yield indicators. Parameswarappa et al. (2008) revealed that NPPs are an important yield component of the crop.
This study has demonstrated the effects of FYM on the management of pre-harvest aflatoxins in groundnuts. As a conclusion, in the eastern part of Ethiopia farmers apply FYM for maintaining soil fertility in some cash crops but the awareness of its effects on plant disease management is quite less. However, in the current study, FYM effects on the groundnut yields, fungal invasions and aflatoxin contamination reductions in single and integrated with seed treatment produced important information for small-scale growers. Therefore, the practice of using FYM for soil fertility improvement by farmers in Eastern Ethiopia should be encouraged. The study also affirmed that, T. harzianum as seed treatment had tremendous effects in reducing Aspergillus spp. invasion and subsequent aflatoxin contaminations in groundnut. Therefore, the future research should be focused on either development of resistant varieties against aflatoxin contaminations, looking for non-toxigenic biocontrol strains, or alternatively developments of simple and easy formulations of T. harzianum for small-scale farmers.
The authors have not declared any conflict of interests.
The authors gratefully thank USAID-FtF and Peanut and Mycotoxin Innovation Laboratory at the University of Georgia, USA, under the terms of award No. AID-ECG-A-00-07-0001 and USD-ARS- National Peanut Research Laboratory for offering their laboratory. They also acknowledge Mr. Solomon Debele for his excellent assistance during the field experiment and Haramaya University Plant Protection Laboratory team members for their unreserved assistance.
REFERENCES
|
Amare K, Tamado T (2014). Genotype by environment interaction and stability of pod yield of elite breeding lines of groundnut (Arachis hypogaea L.) in eastern Ethiopia. Science, Technology and Arts Research Journal 3(2):43-46.
Crossref
|
|
|
|
Ayalew A, Dawit A, Mengistu H (1995). Mycoflora, aflatoxins and resistance of groundnut cultivars from eastern Ethiopia. SINET Ethiopian Journal of Science 18(1):117-131.
|
|
|
|
|
Azziz-Baumgartner E, Lindblade K, Gieseker K, Schurz Rogers H, Kieszak S, Njapau H, Schleicher R, McCoy LF, Misore A, DeCock K, Rubin C, Slutsker L (2005). Case-control study of an acute aflatoxicosis outbreak – Kenya-2004. Environmental Health Perspectives 113(12):1779-1783.
Crossref
|
|
|
|
|
Benizri E, Baudoin E, Guckert A (2001). Root colonization by inoculated plant growth promoting rhizobacteria. Biocontrol Science Technology 11(5):557-574.
Crossref
|
|
|
|
|
Bethlehem M (2011). Seed yield and quality of groundnut (Arachis hypogaea L.) as influenced by phosphorus and manure application at Babile, eastern Ethiopia. MSc Thesis. Haramaya University P 97.
|
|
|
|
|
Bottone EJ, Peluso RW (2003). Production by Bacillus pumilus (MSH) of an antifungal compound that is active against Mucoraceae and Aspergillus species. Preliminarily Report. Journal of Medical Microbiology 52:69-74.
Crossref
|
|
|
|
|
Bruns HA (2003). Controlling aflatoxin and fumonisins in maize by crop management. Journal of Toxicology- Toxin Reviews 22(2-3):153-173.
Crossref
|
|
|
|
|
Chala A, Mohammed A, Ayalew A, Skinnes H (2013). Natural occurrence of aflatoxins in groundnut (Arachis hypogaea L.) from eastern Ethiopia. Food Control 30(2):602-605.
Crossref
|
|
|
|
|
Chen YC, Liao CD, Lin HY, Chiueh LC, Shih DC (2013) Survey of aflatoxin contamination in peanut products in Taiwan from 1997 to 2011. Journal of Food Drug and Anal 21(3):247-252.
Crossref
|
|
|
|
|
Choudhary AK (1992). Influence of microbial co-inhabitants on aflatoxin synthesis of A. flavus on maize kernels. Letter of Applied Microbiology 14(4):143-147.
Crossref
|
|
|
|
|
Coulibaly O, Hell K, Bandyopadhyay R, Hounkponou S, Leslie JF (2008). Mycotoxins: Detection methods and management. Public Health and Agricultural Trade, CAB International.
|
|
|
|
|
Craufurd PQ, Prasad PVV, Waliyar F, Taheri A (2006). Drought, pod yield, pre-harvest Aspergillus infection and aflatoxin contamination on peanut in Niger. Field Crops Research 98:20-29.
Crossref
|
|
|
|
|
Central Statistical Agency (CSA) (2009). Estimation of area production and yield of crops for 2007/2008 and2008/2009 Meher season. Addis Ababa, Ethiopia.
|
|
|
|
|
Central Statistical Agency (CSA) (2014/2015). Agricultural Sample Survey for the 2014/2015 Crop Season. Volume I. Report on Area and Production of Crops for Private Peasant Holdings (Meher Season). Statistical Bulletin, Addis Ababa, Ethiopia. Available at: View
|
|
|
|
|
Central Statistical Agency (CSA) (2016/2017). Agricultural Sample Survey for 2016/2017 Crop Season. Volume I. Report on Area and Production of Crops for Private Peasant Holdings (Meher Season). April, 2017, Addis Ababa, Ethiopia. Available at: View
|
|
|
|
|
Ee OKH, Kim HJ, Bo SW, Lee H, Bae DH, Chung DH, Chun HS (2007). Natural occurrence of aflatoxin B1 in marketed foods and risk estimates of dietary exposure in Koreans. Journal of Food Protection 70(12):2824-2828.
Crossref
|
|
|
|
|
Elwakil MA, El-Metwally MA (2000). Hydroquinone a promising antioxidant for managing seed-borne pathogenic fungi of peanut. Pakistan Journal of Biological Science 3(3):374-375.
Crossref
|
|
|
|
|
Eshetu L (2010). Aflatoxin content of peanut (Arachis hypogaea L.) in relation to shelling and storage practice of Ethiopian farmers. MSc Thesis. Addis Ababa University P 81.
|
|
|
|
|
Food and Agricultural Organization and Statics (FAOSTAT) (2014). Production data. In. FAO, Promoting regional trade in pulses in the Horn of Africa. 2016. www.ecdpm.org. Available online at:
View
|
|
|
|
|
Gangwar RK, Rathore SS, Sharma RK (2014). Integrated management of collar rot disease of groundnut. Pestology 38(1):63-66.
|
|
|
|
|
Getnet Y, Amare A, Nigussie D (2013). Management of aflatoxigenic fungi in groundnut production in eastern Ethiopia. East African Journal of Sciences 7(2):85-98.
|
|
|
|
|
Govindappa M, Lokesh S, Naik VR, Raju SG (2011). Induction of systemic resistance and management of safflower Macrophomina phaseolina root-rot disease by biocontrol agents. Arches Phytopathology Plant Protestation 43(1):26-40.
Crossref
|
|
|
|
|
Horn BW, Greene RL, Sobolev VS, Dorner JW, Powell JH, Layton RC (1996). Association of morphology and mycotoxin production with vegetative compatibility groups in Aspergillus flavus, A. parasiticus, and A. tamarii. Mycologia 88(4):574-587.
Crossref
|
|
|
|
|
Horn BW, Dorner JW (1998). Soil populations of Aspergillus species from section Flavi along a transect through groundnut-growing regions of the United States. Mycologia 90(5):767-776.
Crossref
|
|
|
|
|
Hussaini AM (2013). Mycotoxin and food safety in developing countries. Published by In Tech Janeza Trdine 9, 51000 Rijeka, Croatia.
View
|
|
|
|
|
International Crops Research Institute for the Semi-Arid Tropics (ICRISAT) (2000). Aspergillus and aflatoxin in groundnut. Proceeding of International Workshop, April, 2005, ICRISAT, Patancheru, India.
|
|
|
|
|
International Food Policy Research Institute (IFPRI) (2003). African agriculture: Past performance and future imperatives. Background paper No. 2 presented at the conference "Successes in African Agriculture: Building for the Future". Pretoria, South Africa, 1-3 December 2003. IFPRI, Washington, D.C.
|
|
|
|
|
Inglis GD, Kawchuk LM (2002). Comparative degradation of oomycete, ascomycete, and basidomycete cell walls by mycoparsitic and biocontrol fungi. Canadian Journal of Microbiology 48(1):60-70.
Crossref
|
|
|
|
|
Jeyaramraja PR, Fantahun W (2014). Characterization of yield components in certain groundnut (Arachis hypogaea L.) varieties of Ethiopia. Journal of Experimental Biology and Agricultural Sciences 2:2320-8694.
|
|
|
|
|
Kaaya AN, Harris C, Eigel W (2006). Peanut aflatoxin levels on farms and in markets of Uganda. Peanut Science 33(1):68-75.
Crossref
|
|
|
|
|
Klich MA (2002). Identification of common Aspergillus species. New Orleans (LA): USDA, Agricultural research service, Southern Regional Research Center.
|
|
|
|
|
Lewis L, Onsongo M, Njapau H, Schurz-Rogers H, Luber G, Kieszak S, Nyamongo J, Backer L, Dahiye AM, Misore A, DeCock K (2005). Aflatoxin contamination of commercial maize products during an outbreak of acute aflatoxicosis in eastern and central Kenya. Environmental health perspectives 113(12):1763-1767.
Crossref
|
|
|
|
|
Lokanath HM (2010). Effect of organics on the productivity of groundnut and its residual effects on succeeding safflower under rainfed farming situations. World Congress of Soil Science, Soil Solutions for a Changing World. Brisbane, Australia. Published on DVD.
|
|
|
|
|
Manju K, Sharma OP, Mahabeer S (2017). Collar rot (A. niger) a serious disease of groundnut, its present status and future prospects. International Journal of Chemical Study 5(4):914-919.
|
|
|
|
|
Mehan VK, Nageswara R, McDonald RC, Williams JH (1988). Management of drought stress to improve field screening of groundnut for resistance to Aspergillus flavus. Phytopathology 78(6):659-663.
Crossref
|
|
|
|
|
Mertz D, Lee D, Zuber M, Lillehoj E (1980). Uptake and metabolism of aflatoxin by Zea mays. Journal of Agriculture and Food Chemistry 28(5):963-966.
Crossref
|
|
|
|
|
Ministry of Agriculture and Rural Development (MoARD) (2009). Animal and plant health regulatory directorate. Crop variety register issue No. 12. Addis Ababa, Ethiopia.
|
|
|
|
|
Mohamed A (2015). Efficiency of some bioagents and nemastop compound in controlling damping off and root rot diseases on peanut plants. International Journal of Advanced Research in Biological Sciences 2(11):77-86.
|
|
|
|
|
Mohammed A, Chala A (2014). Incidence of Aspergillus contamination of groundnut (Arachis hypogaea L.) in eastern Ethiopia. African Journal of Microbiology Research 8(8):759-765.
Crossref
|
|
|
|
|
Mohammed A, Chala A, Mashilla D, Fininsa C, Hoisington A, Sobolev S, Arias S (2016). Aspergillus and aflatoxin contamination of groundnut (Arachis hypogaea L.) and groundnut cake in Eastern Ethiopia. Food Additives and Contaminants - Part B.
|
|
|
|
|
OJEU (Official Journal of the European Union) (2010). Regulations, commission regulation (EU) N0. 165/2010. Official Journal of the European Union 27.2.2010. L50/8-12.
|
|
|
|
|
Parameswarappa KG, Malabaari TA, Lingaraju BS (2008). Analysis of correlation and path effects among yield contributing traits in two crosses of large seeded groundnut (Arachis hypogaea L.). Journal of Oil Seeds Research 25(10):4-7.
|
|
|
|
|
Pelosi KM, Sandifer MT (2003). Elementary Statistics: From Discovery to Decision. John Wiley and Sons Inc.
|
|
|
|
|
Pratibha S, Mahesh KS, Swati D, Vignesh K (2012). Biological control of groundnut root rot in farmer's field. Journal of Agricultural Sciences 4(8):48-59.
|
|
|
|
|
Ranasingh N, Saturabh A, Nedunchezhiyan M (2006). Use of Trichoderma in disease management, Orissa Review pp. 68-70.
|
|
|
|
|
Reddy CS, Reddy KRN, Muralidharan K, Mangala UN (2008). Aflatoxigenic Aspergilli and aflatoxin contamination of rice and its management in India. Available at:
View
|
|
|
|
|
Statistical Analysis System (SAS) (2002). SAS Institute Inc., Administrator Guide for the SAS® System Version 9 for Microsoft®Windows®, Cary, North Carolina 27513, USA.
|
|
|
|
|
Sobolev VS, Dorner JW (2002). Cleanup procedure for determination of aflatoxins in major agricultural commodities by liquid chromatography. Journal of Association of Official Analytical Chemists International 85(3):642-645.
|
|
|
|
|
Subrahmaniyan K, Kalaiselvan P, Arulmozhi N (2000). Studies on the effect of nutrient spray and graded level of NPK fertilizers on the growth and yield of groundnut. International Journal of Tropical Agriculture 18(3):287-290.
|
|
|
|
|
Tegene S, Taddesse F, Abduselam F, Legesse Z (2013). Effect of Parthenium compost on the incidence and severity of groundnut root rot caused by Sclerotium rolfsii in eastern Hararghe. Canadian Journal of Plant Protection 1(1):7-14.
|
|
|
|
|
Upadhyaya HD, Reddy LJ, Gowda CL, Singh S (2006). Identification of diverse groundnut germplasim: Sources of early maturity in a core collection. Field Crops Research 97(2-3):261-271.
Crossref
|
|
|
|
|
Vaithiyalingan M, Manoharan V, Ramamoorthi N (2010). Association analysis among the yield and yield attributes of early season drought tolerant groundnut (Arachis hypogaea L.). Electronic Journal of Plant Breeding 1(5):1347-1350.
|
|
|
|
|
Waliyar F, Traore A, Fatondji D, Ntare BR (2003). Effect of irrigation interval, planting date, and cultivar on Aspergillus flavus and aflatoxin contamination of peanut in a sandy soil of Niger. Peanut Sciences 30(2):79-84.
Crossref
|
|
|
|
|
Waliyar F, Craufurd P, Padmaj KV, Reddy RK, Reddy SV, Nigam SN, Kumar PL (2006). Effect of soil application of lime, crop residue and biocontrol agents on pre-harvest A. flavus infection and aflatoxin contamination in groundnut. In International Conference on Groundnut Aflatoxin Management and Genomics, November 2006, Guangzhou, China.
|
|
|
|
|
Waliyar F, Ntare BR, Diallo AT, Kodio O, Diarra B (2007). On-farm management of aflatoxin contamination of groundnut in West Africa. A Synthesis Report. ICRISAT. 13.
|
|
|
|
|
Williams JH, Phillips TD, Jolly PE, Stiles JK, Jolly CM, Aggarwal D (2004). Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. American Journal of Clinical Nutrition 80(5):1106-1122.
Crossref
|
|
|
|
|
Xiaoxia D, Linxia W, Peiwu L, Zhaowei Z, Haiyan Z, Yizhen B, Xiaomei C, Jun J (2015). Risk assessment on dietary exposure to aflatoxin B1 in post-harvest peanuts in the Yangtze River Ecological Region. Toxins 7(10):4157-4174.
Crossref
|
|