ABSTRACT
The seed germination test under lead (Pb) stress could be a quick test to understand plant tolerance to this heavy metal. The aim of this study was to compare the germination behavior of Raphanus sativus L. germinated in solution with different increasing concentration of lead, also to assess its accumulation and toxicity. The test was carried out in an incubator at 25 ± 1°C for 7 days and in a greenhouse for 11 weeks. Lead caused significant germination behavioral disturbances by changing the velocity coefficient and germination kinetics, with reducing the rate of early and final germination, however, the duration of germination was lengthened. Pb reduced the levels of the chlorophyll, delta-aminolevulinic acid dehydratase (ALAD) activity and growth. It increased lipid peroxidation and induced a significant accumulation of proline, positively correlated with Pb accumulation. Pb has a depressive effect on germination and causes disruptive disturbances of R.. sativus L. revealed by changes in non-enzymatic antioxidants, ALAD activity and growth. Radish has a capacity to accumulate Pb. The present results provide a model for detecting natural compounds able to improve seed germination of radish and counteract the harmful effects of lead.
Key words: Radish, concentration, rate, accumulation, proline, lipid peroxidation, chlorophyll.
Lead is one of nature's most polluting heavy metals; it has harmful consequences on human and animal health and also on plants. It is the most encountered and the most toxic among plant pollutants. The plant accumulates lead in its various parts after absorption, Pb is not essential for its growth and its effect on the plant is different depending on the nature of the soil, the plant species and also its concentration (Sharma and Dubey, 2005; Patra et al., 2004).
In the plant cell when lead successfully penetrates, it increases the production of reactive oxygen species (ROS), which causes oxidative stress in different parts of the growing plant and leads to cell damages (Verma and Dubey, 2003). Lead has negative effects on the germination and growth of plants; it inhibits the photosynthesis process and reduces mineral nutrition and enzymatic activity (Mishra and Choudhari, 1998; Sharma and Dubey, 2005).
The leaves are the most important parts of the plant due to their role in producing food for the plant by capturing solar energy in the photosynthesis process. One of the first committed enzymes in the chlorophyll biosynthetic pathway is δ-aminolevulinic acid dehydratase (ALAD), which catalyzes the union of two δ-aminolevulinic acid (ALA) molecules into the monopyrrole porphobilinogen (PBG) (Jordan and Seehra, 1980). The cotyledons and leaves have a high chlorophyll content, they were used to measure the δ-aminolevulinic dehydratase activity and the chlorophyll content in order to determine the effect of lead on these two parameters. The purpose of our work was to evaluate the effects of lead on radish submitted at different concentrations, by analyzing plant tolerance, plant development, lead accumulation capacity and biochemical responses.
The biological material that we used during the experiment is radish (Raphanus sativus L). The test was carried out in an incubator at 25 ± 1°C for 7 days for a seed germination test in laboratory of experimental bio-toxicology, bio-depollution and phytoremediation, then in the greenhouse of the University of Oran 1 Ahmed Ben Bella, Algeria for 11 weeks for a plant test.
Seed germination and plant growth
To prepare the seeds for germination, they were sterilized with 2% sodium hypochlorite for 10 min, then they were washed several times with distilled water to remove all traces of sodium hypochlorite. Seed germination was tested in Petri plates containing two Whatman filter papers. Ten seeds of radish were placed in each dish and moistened with 5 ml of either 0, 100, 250, 500 or 1000 mg l-1 of Pb solution. Eight replications were used in the experimentation for each concentration. The Petri plates were closed and maintained at 25 ± 1°C for 7 days in the incubator. Seed germination in each group was noted daily and the observations were performed for 7 days and germination rate was calculated. Two of the seedlings from each previous replication were planted in clean plastic pots filled with 400 g of sandy soil (2 V of sand / 1 V of compost) in a greenhouse.
Every week the replicates of each treatment were watered twice with lead acetate solution at different concentration and one time with Hoagland's solution for 11 weeks. Eight replications were used in the experimentation in the greenhouse for each concentration.
Determination of seedling growth and biomass
The length of seedlings was measured with a ruler (data expressed in millimeters) and the seedlings biomass was determined using a digital balance (the results expressed in grams).
Germination parameters
Early germination
This is the rate of first sprouts observed in the time interval between planting the seeds and their germination.
Estimation of germination rate (GR)
GR = (NI / NT) × 100
where NI: the total number of germinated seeds, NT: the number of seeds used in our experiment for each treatment (Li, 2008).
Mean daily germination
MDG = FGP / D
where FGP: final germination percent, D: test time -days (Osborne et al., 1993).
Kinetics of germination
Kinetics of germination is the evolution of the germination rate curve over a period of 7 days calculated according to the number of newly germinated seeds in each observation (Hajlaoui et al., 2007).
Mean time of germination (MTG) and coefficient of velocity (CVG)
It is an index of seed germination speed and velocity calculated by the following formula of Kotowski (1926).
CVG = (S1 + S2 + ... + Sn) /〠(S1T1) + (S2T2) +…. + (SnTn) ã€‘× 100
MTG =〠(S1T1) + (S2T2) + …. + (SnTn) 】/ (S1 + S2 + …. +Sn)
where S1: Number of seeds germinated at time T1 and Sn: at time Tn.
Biochemical estimations
Determination of lead concentration
One to two grams of dry samples (plant and soil) was calcined at 450°C in a muffle oven for 4 h and they were digested with aqua regia (25% HNO3 and 75% HCL) in the beakers. The beakers were heated until the contents had evaporated. The drops that remain in the beakers were dissolved in 10 ml of 5% HCL, filtered and then completed at 20 ml with 5% HCL. Lead was determined by atomic absorption spectroscopy (AAS) using SHIMADZU AA6600 apparatus (Tauzin and Juste, 1986).
Determination of total chlorophyll, chlorophyll a, chlorophyll b and carotenoids
100 mg of leaves cut into small fragments were placed in glass test tubes containing 10 ml of 95% acetone, then the tubes were placed in the cold at 4°C and in the dark for 48 h. The values of the assimilatory pigments levels were calculated according to the following formula (Lichtenthaler, 1987).
1) Total Chlorophyll: (μg. ml-1) = 7.15 DO663 + 18. 71. DO647
2) Chlorophyll a: (μg. ml-1) = 12.25. DO663 – 2. 79. DO647
3) Chlorophyll b: (μg. ml-1) = 21.5. DO647 – 5. 10. DO663
4) Carotenoids: (μg. ml-1) = (1000 DO470 – 1.82 Chl a – 85.02 Chl b) / 198.
Determination of ALAD activity
100 mg of leaves were ground in a volume of 100 ml of Tris-HCl buffer (0.05 M, pH 8.75) which contains 10 mM MgCl2 and 0 to 10 mM β-mercaptoethanol. The resulting mixture was filtered and centrifuged at 15000 g for 20 min.
The resulting supernatant was precipitated with 55% ammonium sulfate (NH4)2 SO4 and then centrifuged at 15000 g for 30 min. The protein pellet was then dissolved in a minimum volume of the grinding buffer and used to measure the ALAD activity (Hault et al., 1987). The porphobilinogen (PBG) first monopyrole, the precursor of tetrapyrroles was determined using the Balance protocol (Balange, 1982). The activity of the ALAD was expressed in arbitrary units (DO at 560 nm).
Determination of lipid peroxidation
The accumulation of lipid peroxides in tissues was presented by the malondialdehyde (MDA) content using the term of thiobarbituric acid reactive substances (TBARS) (Velikova and Loreto, 2001). 1 g of samples was ground in 1 ml of ice-cold trichloroacetic acid (TCA), then it was centrifuged at 12000 g for 20 min. The supernatant was used for the determination of the TBARS in the samples using extinction coefficient of 155 mM-1 cm-1 (at wavelength of 532 nm).
Extraction and proline assay
100 mg of dry samples was ground with 1.25 ml of 95% ethanol, followed by three rinses with 1.25 ml of 70% ethanol each time, and then taken in test tubes. After 1 h, 2.5 ml of the upper phase was mixed with 1 ml of chloroform and 1.5 ml of distilled water. After stirring, the solution was placed in the cold for 48 h to obtain a good separation. The upper phase was used to determine the proline levels using the protocol of Bergman and Loxley (Bergman and Loxley, 1970). A standard curve was produced to evaluate proline concentration at 515 nm.
Statistical analysis
Data were subjected to one-way analysis of variance (ANOVA), a statistical package available from SPSS. Post-hoc testing was carried out using the LSD test. A significant level of 0.05 was used for all statistical tests. Results for each measured parameter were expressed as Mean ± Standard of Error (SE), n = 8 replicates.
The root cells are responsible for the transport of lead from the external medium to the interior of the cell using the cation channels of the plasma membrane, in particular the Ca2+ channels (Seregin and Kozhevnikova, 2008). Penetrated lead can be accumulated or transferred to different parts of the plant. The results showed that the radish had a capacity to accumulate lead in these parts after exposure; this accumulation was significant when the Pb concentration was high. The accumulation was higher in the fibrous roots and taproot compared to the aerial parts (Figure 1A and B). These results concurred with many previous researches as has been reported Nicotiana tabacum (Gichner et al., 2008).
The effects of lead on seedling growth were not similar between plant species and between different parts of the same plant (Sharma and Dubey, 2005). The toxic effects of Pb depended on the dose and time of exposure; the development and germination of seedlings were severely limited by exposure to metal (Gichner et al., 2008).
The present results, as have been mentioned earlier, indicate that exposure to lead has reduced the fresh weight of radish at two periods (Figure 2A and B), which reveal a depressive effect of lead on plant development as compared to the control. Indeed, the biomass was reduced when the level of lead increased. Similar observations (Figure 2) have also been reported by Kibria et al. (2010) who noticed that a high concentration of Pb reduced the fresh and dry weight of the roots and even the aerial parts. The reduction of biomass could be the result of a direct effect of Pb on photosynthesis or on the physiological processes of the plant (Sharma and Dubey, 2005). Eun et al. (2000) reported that decreased root growth was the main effect of Pb, this inhibition can be correlated with the higher content of lead (Liu et al., 2008). This is in agreement with our results on the important decrease of roots length compared to the aerial parts for treatment of 7 days with a strong accumulation of lead.
For treatment of 11 weeks, root elongation was significantly affected only at the dose of 1000 mg l-1 and the lengthening of the aerial part was not affected at the dose of 500 mg l-1 compared to the control (Figure 2C and D).
In Figure 3, at the macroscopic scale, lead caused adverse effects on plants at the germination stage, Pb inhibited germination and growth of seedlings and reduced length and the dry mass (Mishra and Choudhuri, 1998).
The results showed that tested seeds presented significant germination disturbances by reducing the rate of early germination (Figure 3A), mean daily germination (Figure 3B), coefficient of velocity of germination (Figure 3C) and final germination rate (Figure 3D) compared to controls. This depressive effect on the germination behavior was significant statistically, with increasing lead levels in the medium.
MTG was affected by lengthening when the medium of lead acetate concentration increases, 4 days for the control and 5 days for group treated with 1000 mgl-1 of Pb. For all groups that were treated with Pb, the rate of germination increased statistically not significant for the treated group at 100 mgl-1 in the 5, 6 and 7th day, but statistically highly significant for the groups receiving a treatment of 250, 500, or 1000 mgl-1 for all days compared to the control (Figure 3E). Our results coincided with Sengar et al. (2009) who observed a decline in seed germination rate.
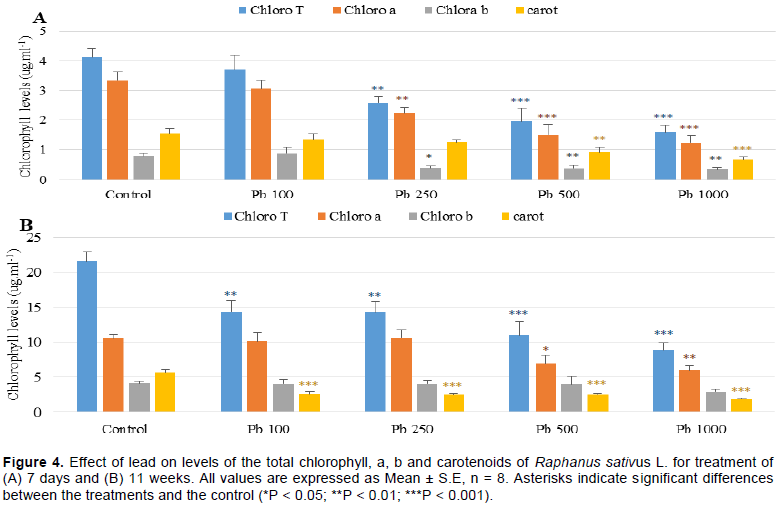
It was noted that the process of photosynthesis was altered by Pb, explained by the decrease in the levels of
chlorophylls and carotenoids. Chlorophyll a was more affected compared to chlorophyll b for treatment of 7 days. For treatment of 11 weeks, the carotenoid levels were significantly reduced for all treated groups; however, the chlorophyll b was not affected (Figure 4A and B). This reduction could be explained by the negative effect of Pb on the absorption of essential nutrients for plants such as Magnesium (Mg) and Iron (Fe) which results in the inhibition of photosynthesis or the closure of the stomata which results in a reduction in the amount of CO2 (Burzynski, 1987; Sharma and Dubey, 2005). Pb could also alter the enzymatic activity such as the delta-aminolevulinic acid dehydratase which is the essential enzyme for photosynthesis (Cenkci et al., 2010).
The level of peroxidation content increased significantly in leaves, stem, fibrous roots and tap root during two periods with increasing lead concentration. The proline content was also significantly increased and ALAD activity was not affected for 7 days period, while it was significantly decreased for the 11 weeks period (Table 1). The absorption of heavy metals by plants has led to the generation of oxidative stress (Clemens, 2006b) and to the product of a large amount of ROS. MDA content is considered as an indicator of lipid peroxidation after abiotic stress (Ding et al., 2004) and biomarker of oxidative stress. Our results show that Pb causes increased level of lipid peroxidation in different parts of a plant after exposure. These results are similar to other published results, where an accumulation of lipid peroxidation content after exposure to Pb was also reported (Souza et al., 2012).
One of the first enzymes involved in the chlorophyll biosynthetic pathway is δ-aminolevulinic acid dehydratase (ALAD). At a high Pb concentration, our results showed a weak ALAD activity correlated with a decrease in the chlorophyll content, this disturbance could be caused by the accumulation of lead in the leaves.
At different concentrations of lead, ALAD activity was reduced. It seems to be more sensitive at the period of 11 weeks, while at the period of 7 days it is less sensitive.
Similar results that indicate inhibition of ALAD activity by Cd in soybean which were obtained by Noriega et al. (2007).
Proline accumulation is one of the methods by which plants can combat the toxic effect of heavy metals. The accumulation of proline contributes to the protection of enzymes, to the detoxification and chelation of metals, to the trapping of reactive oxygen species and to the stabilization of the protein synthesis (Sharmila and Pardha, 2002). Proline is synthesized from glutamic acid via 1-pyrroline-5-carboxylic acid (P5C), but also via arginine and ornithine (Lignowski and Splittstoesser, 1971). Moreover, glutamate and 2-oxoglutarate are the precursors of ALA in higher plants. Our results showed a large accumulation of proline correlated with a decrease in chlorophyll pigments (Table 2). This correlation is negative one for these two parameters. These results suggest there is a link between the biosynthetic pathways of chlorophyll pigments and proline. The competition between these two compounds on their common precursor, glutamate, can be at the origin of this response.
It is clear from the current study that lead has inhibitory effects on radish seed germination capacity, seedlings and plants growth and leads to the generation of oxidative stress. Radish can withstand high concentrations of lead and it has the power to accumulate it in its different parts. Also, we suggest using natural compounds that can improve the germination and development of radish and counteract the harmful effects of lead to increase its capacity to resist lead buildup.
The authors have not declared any conflict of interests.
REFERENCES
|
Balange AP (1982). Photoregulation de l'alfa-aminolévulinate déshydratase de radis sous lumière rouge lointain. Thèse d'Etat. Rouen.
|
|
|
|
Bergman I, Loxley R (1970). New spectrophotometric method for the determination of proline in tissue hydrolysates, Analytical Chemistry 42(7):702-706.
Crossref
|
|
|
|
|
Burzynski M (1987). The uptake and transpiration of water and the accumulation of lead by plants growing on lead chloride solutions. Acta Societatis Botanicorum Poloniae 56:271-280.
Crossref
|
|
|
|
|
Cenkci S, Cigerci IH, Yildiz M, Özay C, Bozdag A, Terzi H (2010). Lead contamination reduces chlorophyll biosynthesis and genomic template stability in Brassica rapa L. Environmental and Experimental Botany 67(3):467-473.
Crossref
|
|
|
|
|
Clemens S (2006b). Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88(11) 1707-1719.
Crossref
|
|
|
|
|
Ding HD, Wan YH, Qi NM, Zhu WM, Yang XF, Shao YC (2004). Effects of Cd+2 and Zn+2 stress on on antioxidant enzyme system of tomato seedlings. Acta Agriculturae Shanghai 20:79-82.
|
|
|
|
|
Eun S, Youn H, Lee Y (2000). Lead disturbs microtubule organization in the root meristem of Zea mays. Physiologia Plantarum 44:5-9.
Crossref
|
|
|
|
|
Gichner T, Znidar I, Száková J (2008). Evaluation of DNA damage and mutagenicity induced by lead in tobacco plants. Mutation Research/Genetic Toxicology and Environmental Mutagenesis 652(2):186-190.
Crossref
|
|
|
|
|
Hajlaoui H, Denden M, Bouslama M (2007). Étude de la variabilité intraspécifique de tolérance au stress salin du pois chiche (Cicer arietinum L.) au stade germination. Tropicultura 25(3):168-173.
|
|
|
|
|
Hault C, Aoues A, Colin P (1987). Immunological study of alfa- aminolevulinate deydratase in etiolated radish cotyledons. Plant Physiology and Biochemistry 25(6):723-728.
|
|
|
|
|
Jordan PM, Seehra JS (1980). Mechanism of action of 5-aminolevulinic acid dehydratase: Stepwise order of addition of the two molecules of 5- aminolevulinic acid in the enzyrnic synthesis of porphobilinogen. Journal of the Chemical Society, Chemical Communications pp. 240-242.
Crossref
|
|
|
|
|
Kibria M, Maniruzzaman M, Islam M, Osman K (2010) Effects of soilapplied lead on growth and partitioning of ion concentration in Spinacea oleracea L. tissues. Soil Environment 29:1-6.
|
|
|
|
|
Kotowski F (1926). Temperature Relations to Germination of Vegetable Seeds. American Society of Horticulture Science Proceedings 23:176-184.
|
|
|
|
|
Lichtenthaler HK (1987). Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods in Enzymology 148:350â€382.
Crossref
|
|
|
|
|
Lignowski EM, Splittstoesser WE (1971). Arginine synthesis, proline synthesis and related process. In John & Thompson (Eds): The Biochemistry of Plants 25:225-229.
Crossref
|
|
|
|
|
Liu D, Li T, Jin X, Yang X, Islam E, Mahmood Q (2008). Lead induced changes in the growth and antioxidant metabolism of the lead accumulating and non-accumulating ecotypes of Sedum alfredii. Journal of Integrative Plant Biology 50(2):129-140.
Crossref
|
|
|
|
|
Li Y (2008). Effect of salt stress on seed germination and seedling growth of three salinity plants. Pakistan Journal Biological Sciences 11:1268-1272.
Crossref
|
|
|
|
|
Mishra A, Choudhari MA (1998). Amelioration of lead and mercury effects on germination and rice seedling growth by antioxydants. Biologia Plantarum 41:469-473.
Crossref
|
|
|
|
|
Noriega GO, Balestrasse KB, Batlle A, Tomaro ML (2007). Cadmium induced oxidative stress in soybean plants also by the accumulation of δ-aminolevulinic acid. Biometals 20:841-851.
Crossref
|
|
|
|
|
Osborne JM, Fox JED, Mercer S (1993). Germination responseunder elevated salinities of six semi-arid blue bush species (Western Australia). In: Lieth H, Al Masoom A (Eds), Towards the Rational Use of High Salinity Plants 1:323-338. Dordrecht: Kluwer Academic Publishers, p. 521.
Crossref
|
|
|
|
|
Patra M, Bhowmik N, Bandopadhyay B, Sharma A (2004). Comparison of mercury, lead and arsenic with respect to genotoxic effects on plant systems and the development of genetic tolerance. Environmental and Experimental Botany 52:199-223.
Crossref
|
|
|
|
|
Sengar RS, Gautam M, Sengar RS, Sengar RS, Garg SK, Sengar K, Chaudhary R (2009). Lead stress effects on physiobiochemical activities of higher plants. Review of Environmental Contamination and Toxicology 196:1-21.
Crossref
|
|
|
|
|
Seregin IV, Kozhevnikova AD (2008). Roles of root and shoot tissues in transport and accumulation of cadmium, lead, nickel, and strontium. Russian Journal of Plant Physiology 55:1-22.
Crossref
|
|
|
|
|
Sharma P, Dubey R (2005). Lead toxicity in plants. Brazilian Journal of Plant Physiology 17:35-52.
Crossref
|
|
|
|
|
Sharmila P, Pardha SP (2002). Proline accumulation in heavy metal stressed plant: an adaptative strategy. In physiology and biochemistry of metal toxicity and tolerance in plants. Prasad MNV, Strzalka K (Eds), pp. 179-199.
Crossref
|
|
|
|
|
Souza LA, Andrade SAL, Souza SCR, Schiavinat MA (2012). Arbuscular mycorrhiza confers Pb tolerance in Calopogonium mucunoides. Acta Physiologiae Plantarum 34:523-531.
Crossref
|
|
|
|
|
Tauzin J, Juste C (1986). Évolution du contenu en métaux lourds d'un sol de limon maintenu en jachère nue après 56 années d'application continue de divers engrais et amendements. L'Académie d'Agriculture de France 72:739-746.
|
|
|
|
|
Velikova V, Loreto F (2001). Isoprene produced by leaves protects the photosynthetic apparatus against zone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiology 27:1781-1787.
Crossref
|
|
|
|
|
Verma S, Dubey RS (2003). Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in grow ing rice plants. Plant Sciences 164: 645-65.
Crossref
|
|