Parthenium hysterophorus is an alien annual herb that belongs to the Asteraceae family (Meena et al., 2017). Currently, it is aggressively threatening the biodiversity of Queen Elizabeth National Park (QENP) in Uganda. It originated from Southern United States of America (Thapa et al., 2018)and due to its high colonization vigour (Steven and Shabbir, 2014), it has reached as far as East Africa in general and Uganda, in particular (Wabuyele et al., 2014). In Uganda, P. hysterophorus has been recorded in the Eastern districts of Jinja and Busia, Central districts of Kampala and Masaka, Western districts of Mbarara and Kasese and the Northern district of Pader (Wabuyele et al., 2014).
P. hysterophorus is an erect plant that attains a mean height of 2 m (Sahrawat et al., 2018). Its life cycle exhibits rosette and reproductive distinct growth stage. In rosette stage, the plant develops large simple leaves that create a wide cover which prevents undergrowth (Kaur et al., 2014). They later become multi-branched, forming bipinnate leaves at reproductive stage (Sahrawat et al., 2018). A single mature Parthenium plant produces a minimum of 25000 (Kaur et al., 2014)and a maximum of 100,000 seeds (Bobo and Abdeta, 2016). The seeds are then dispersed by vehicles, wind, water, machinery, animals, and along with fodders and grains (Meena et al., 2017; Birhanu and Khan, 2018). Seed germination takes place throughout the year since it is not limited by moisture availability (Kaur et al., 2014). Soon after the seedlings’ emergence, the plant forms a dense canopy and then flowers within a month (Bobo and Abdeta, 2016). P. hysterophorus requires an average of 180-240 days to complete its life cycle (Kaur et al., 2014; Meena et al., 2017).
The negative effects of P. hysterophorus are not limited to plant species diversity but also impacts on animals (Bobo and Abdeta, 2016;Meena et al., 2017)that inhabit that same locality. Direct contact with the dry parts of P. hysterophorus, causes asthma and skin irritations (Kumar et al., 2012). Body dehydration, inflammation of soft body membranes, miscarriage and headache have also been reported (Shrestha et al., 2019). An amount of 10-50% of P. hysterophorus in the animals’ fodder kills livestock and buffaloes (Sahrawat et al., 2018). P. hysterophorus’ invasion reduces plant species diversity within national parks (Etana et al., 2015)which propels game animals to search for food beyond park boundaries, creating food insecurity in neighbouring communities (Abdulkerim-Ute and Legesse, 2016;Horo et al., 2020).
Although, P. hysterophorus is spreading rapidly (Thapa et al., 2018)and its occurrence impacting negatively on plant composition of rangelands (Khaket et al., 2015; Hassan et al., 2018), no documentation exists on how it has influenced the vegetation composition in Queen Elizabeth National Park (QENP). Thus, the examination of abundance for P. hysterophorus and how it impacts on plant species diversity, can help in monitoring of the habitat of QENP as an important aspect of wildlife and biological conservation (Pilliod and Arkle, 2013). It will also inform on detection of vegetation changes (Phillippoff and Cox, 2017), especially when such changes become detrimental to species (Nkoa et al., 2015). In this study, therefore, the occurrence and abundance of P. hysterophorus and its impact on plant species diversity of QENP were documented.
Study area
The study was carried out between June 2014 and December 2015 in Mweya peninsula and along the Kazinga channel track located in QENP. The Park is the largest protected area that lies within the Albertine rift valley in Western Uganda (Figure 1). QENP directly spans the equator line (00° 15'S, 30° 00'E) between lakes Edward and George. These two lakes are connected by a 35 km Kazinga channel at an oval land mass, the Mweya peninsula (4.22 km2), located at (0° 11' 40ʺS 29° 53ʹ57ʺ E and 890 meters above sea level. The park which is under the management of Uganda Wild Authority (UWA), covers an area of 1978 km2. QENP receives mean maximum precipitation of 1390 mm and minimum of 750 mm annually. The mean annual maximum temperature is 28°C and minimum is 18°C (UWA, 2012). The original vegetation of the study area is typically savanna grassland, predominated by herbs, grasses, shrubs and trees. However, UWA (2012)reported that the park also inhabited other invasive species such as Lantana camara, Dichrostachys cinerea, Imperata cylindrica, Opuntia vulgaris. Thus, in QENP, adverse impacts of P. hysterophorus are aggravated by the existence of other invasive plant species.
QENP is Uganda’s most popular tourist destination centre, which provides a rich habitat for 95 mammalian and 619 avian species. It is a haven for antelope species like Uganda kobs, duikers, Topis and sitatunga, as well as big mammals like lions, elephants, buffaloes and hippopotamuses. According to UWA (2012), an estimated human population of 30,000 people lives within the park in 11 fishing villages; while 50,000 people live in 52 parishes bordering it. This human population together with the UWA staff and tourists crisscross the park on a daily basis.
Study sites selection
Study sites were selected using two methods. These were based on preliminary information provided by QENP-invasive species management team on P. hysterophorus invasion, and guidelines given by Nkoa et al. (2015). Using the QENP-invasive species management team, hot spots of P. hysterophorus occurrence were located, and the clumpy spatial distribution pattern exhibited by P. hysterophorus within the located study sites necessitated stratification of the hot spots (herein termed as sampling sites), according to Nkoa et al., (2015).
Data collection methods
Occurrence, hotspots and spread of P. hysterophorus in QENP
An observational inspection survey was conducted to assess the occurrence of P. hysterophorus as an indicator of its hotspots and spread in the park. Guided by the presence of at least a single Parthenium plant, occurrence of P. hysterophorus was measured in a 1m2 randomly thrown quadrat within (50 x 50) m plots of each hot spot following survey procedure by Maszura et al. (2018). The number of spots for a specific hotspot inhabited by P. hysterophorus were recorded, and P. hysterophorus occurrence computed as percentage presence in each hot spot for comparisons (Bhusal et al., 2014).
Plant species abundance in P. hysterophorus invaded and uninvaded sites of QENP
A quadrat method was employed to generate the plant species abundances, for examination of P. hysterophorus’ impact on species diversity (Arne et al., 2018; Maszura et al., 2018; Zereen et al., 2018). Three spots per hotspot with > 50% P. hysterophorus area cover, were considered for sampling (Bhusal et al., 2014).
Nine pairs of (10 m X 10 m) plots at 2 meters apart were demarcated for each hotspot; in a manner that nine plots were demarcated in P. hysterophorus invaded and other nine in uninvaded sites. A 1-m2 quadrat was then randomly thrown in triplicate in each plot. Thus, a total of fifty-four (54) quadrats were placed in invaded and an equal number placed in uninvaded plots. All plant species within each quadrat were counted, recorded, collected and taken to Makerere University herbarium for identification. Plant species abundance was calculated as per a formula by Mahajan and Fatima (2017):
Plant species diversity indices in P. hysterophorus invaded and uninvaded sites of QENP
Determination of plant species diversity indices; Species Simpson’s index of diversity, dominance, and evenness for P. hysterophorus invaded and uninvaded sites were computed from plant species abundance using PAST computer software version 4.03, 2020 while species richness was compiled based on the number of plant species collected.
Data analyses
We analyzed data using Student’s t-test for independent samples which compared the mean differences of Simpson’s index of diversity, dominance, evenness, and richness between P. hysterophorus invaded and uninvaded sites while One Way ANOVA compared the computed indices between sampling sites at 0.05 level of significance using IBM SPSS Statistics 21, 2020. A Principal Component Analysis (PCA) was run to correlate the plant species with P. hysterophorus abundance. Effects of P. hysterophorus abundance on species diversity indices were correlated by linear regression models.
Occurrence, spread and hotspots of P. hysterophorus in QENP
From the survey with the invasive species management committee, the hot spots for P. hysterophorus were found to be motor-mechanical workshops, water drainage trenches, campsites, homesteads, trash burning sites located in Mweya Peninsula and spots along Kazinga channel track. P. hysterophorus percentage occurrences were generally high and ranged from 59.1 to 100%. Occurrence was highest in motor-mechanical workshops (100%), followed by burnt sites (85.7%), homesteads (77.1%), campsites (75.0%) then water drainage trenches (60.0%), and lastly, spots along Kazinga channel track (59.1%) as presented in Figure 2. It is evident that incoming vehicles were the most probable means of introduction of P. hysterophorus in these areas and its spread was further enhanced by crisscrossing of more vehicles. Mweya Peninsula once hosted the management headquarters for QENP and it is where vehicle washing and mechanical repairing of the vehicles took place. Thus, water from vehicle washing could have spread and facilitated the seed germination of this noxious weed. The study findings are in line with CLIMEX simulation results that recorded P. hysterophorus in drainage trenches, dumpsites, abandoned buildings, construction sites, residential areas, rangelands and crop fields (Wabuyele et al., 2014 ; Horo et al., 2020). The results also agree with a report from the distribution survey made in Nepal, where P. hysterophorus dispersal was directly associated with vehicle movements (Shrestha et al., 2019). Water channels were highly infested with Parthenium weed in Awash National Park in Ethiopia; thus, water was one of the dispersing egents of this weed (Etana et al., 2015). Tracks regularly used by animals to the peninsula were also observed to be lined by P. hysterophorus. These tracks included the visible ones made by hippopotamuses and elephants as they leave the waters of Kazinga channel. Therefore, animals’ movements especially the big mammals such as elephants, buffaloes and hippopotamus are likely to be playing a big role in spreading the weed by carrying its seeds in their hooves, which can cause rapid spread of the weed and difficulty in its management.
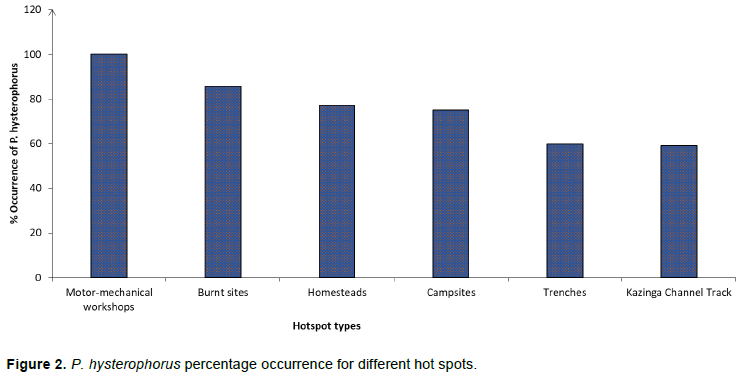
Consistent with the findings of this study, Kuldip et al. (2011) noted that increased tourism and transportation intensified the spread of P. hysterophorus in North-Western Indian Himalaya. P. hysterophorus invasion was found in internally displaced people’s camps in Pader district of Uganda, and near residential and worshipping places in Kampala (Wabuyele et al., 2014) and in Malaysia (Maszura et al., 2018). It is highly likely that the spread of this weed is facilitated by human movements accidentally or due to its ornamental value such as wreath making and wedding decorations in churches. It is possible that P. hysterophorus has spread in the same ways into the neighbouring communities of the QENP.
Plant abundance in P. hysterophorus invaded and uninvaded sites
In this study, plants species collected belonged to 27 genera and 16 families. Members of family Asteraceae were the most abundant (83.2%) in invaded sites, followed by Poaceae (11.6%) while members of other families that included Verbenaceae, Tiliaceae, Solanaceae composed of 5.4% are shown in Table 1. The table shows only plant species with percentage abundance ≥ 0.01. Conversely, members of other plant families (48.8%) dominated the uninvaded sites followed by Poaceae (46.6%), whereas those of Asteraceae (4.2%) were the least abundant. In other studies, Poaceae (14.28%) and Asteraceae (9.52%) were among top three rich families in P. hysterophorus invaded sites (Gebrehiwot and Berhanu, 2015; Gadisa et al., 2019). Annual herbs of Asteraceae family have a high fecundity (Chauhan et al., 2019), that could have enabled them to compete favourably with members of other families for growth requirements. Moreover, invaded sites were characterized with bare soil patches due to disturbance, that could have allowed sunlight for secondary colonizers of Asteraceae family to grow.
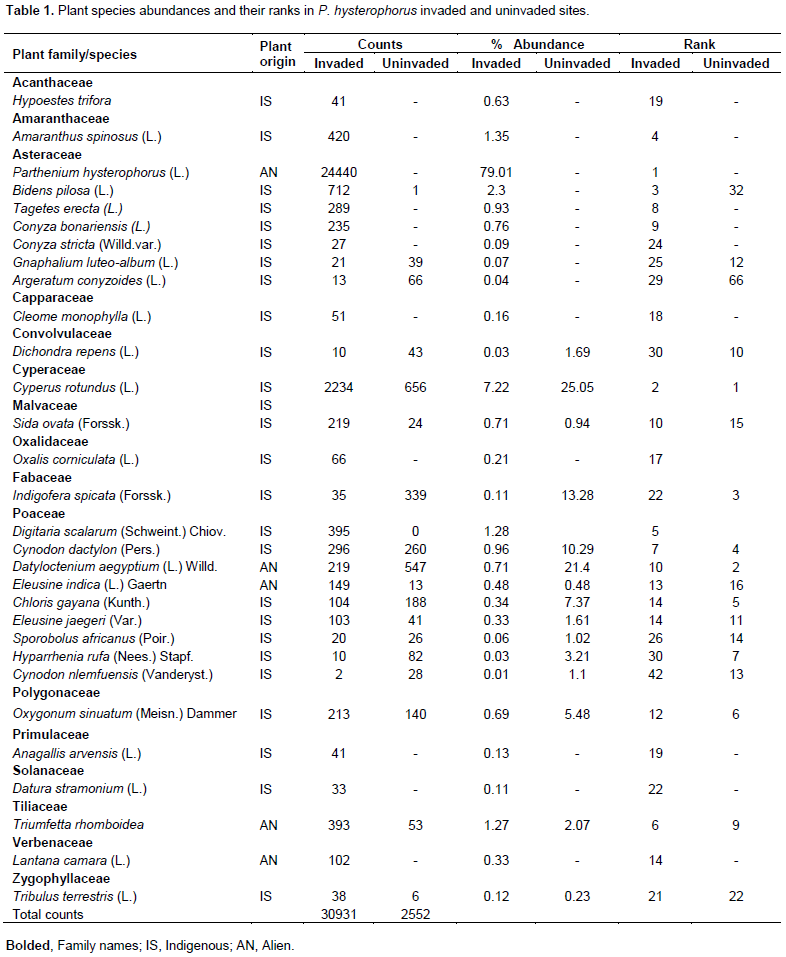
We found that P. hysterophorus was the most abundant (79.0 %) among the flora in invaded sites followed by Cyperus rotundus (7.2%). Cynodon dactylon was recorded in all P. hysterophorus invaded sites, but with a low abundance (0.96%). Similarly, a report on weed survey done in Khyber Pakhtunkhwa in Pakistan, revealed that P. hysterophorus dominated the study sites with the highest abundance (63.4%) among the weeds and was followed by C. dactylon (11.37%) at most locations (Ali and Khan, 2017). P. hysterophorus abundance was also the highest (85.2%) at Kuala Muda in Malaysia (Maszura et al., 2018). In another related study, highest mean important value index (IVI) of (76.15%) was recorded for P. hysterophorus (Gebrehiwot and Berhanu, 2015)in Ethiopia. Musese et al. (2020)recorded the highest abundance (43.0%) of the same alien invasive species in Tanzania. High abundances of P. hysterophorus have been attributed to its allelopathic effects on native species (Meena et al., 2017; Birhanu and Khan, 2018), short life span and high fecundity (Bobo and Abdeta, 2016;Meena et al., 2017), vegetative regeneration (Rwomushana et al., 2019), absence of natural enemies (Meena et al., 2017)and diverse means of dispersal; which could have enhanced its rapid spread and fast colonization (Abdulkerim-Ute and Legesse, 2016).
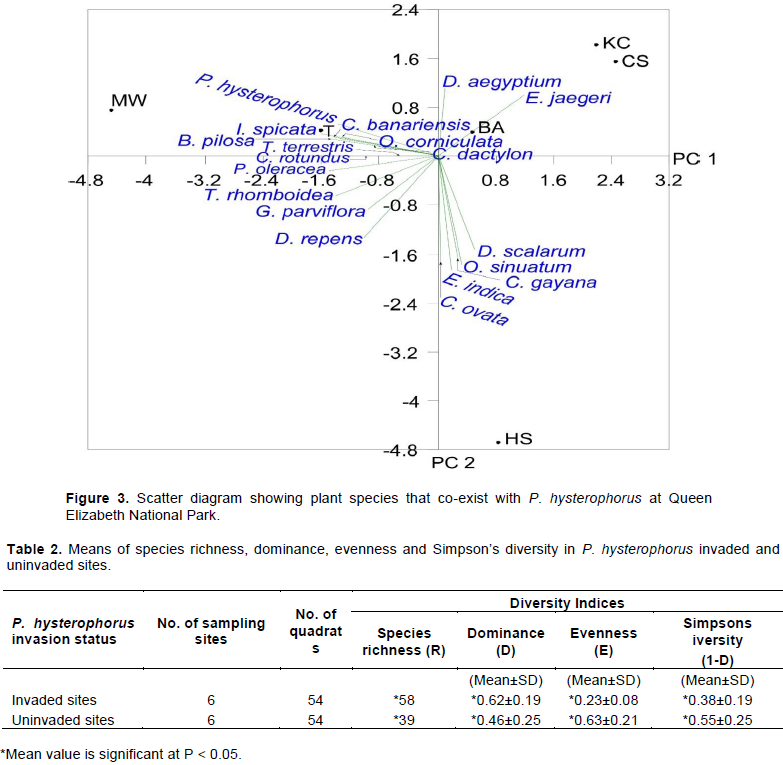
Co-existence of plant species with P. hysterophorus at different sampling sites
Analysis of plant species that co-existed with P. hysterophorus at different sampling sites revealed a strong association of P. hysterophorus abundance with Cyperus rotundus and Tribulus terrestris. They clustered more closely with P. hysterophorus on negative axis of PC1 in Trenches (T). Other plant species such as Bidens pilosa, Indigofera spicata, Cynodon dactylon, Oxalis corniculate, Portulaca oleracea, and Triumfetta rhomboidea also showed a strong association and clustered close to negative axis of PC1 in motor-mechanical workshops (MW). Digitaria scalarum, Cida ovata, Oxygonum sinuatum, Eleusine indica, Chloris gayana clustered close to negative axis of PC2 around homesteads (HS), showing a weak association with P. hysterophorus abundance. Datyloctenium aegyptium and Eleusine jaegeri clustered on positive axis of PC2 at burnt sites (BA), also showing a weak association with abundance of the invading weed. Galinsoga parviflora, Dichondra repens clustered close to negative axis of PC2 showing a weak relationship (Figure 3). C. rotundus, which inhabits wet environments, co-existed in trenches where the Parthenium seeds could have dispersed by water currents. Secondary colonizers such as B. pilosa could have existed in motor-mechanical workshops following soil disturbance. T. terrestris was also recorded in high and low P. hysterophorus infested clusters of Simanjaro Rangeland in Tanzania (Musese et al., 2020). The co-existence can also be explained from morphological perspective. For example, C. dactylon develops stolons and rhizomes which enhance its rapid vegetative growth that enables it to compete favourably with P. hysterophorus. Also, the stolons and rhizomes already in the soil could have been spread into fresh areas by a grader while making drainage systems. Furthermore, a study by Kruk and Satorre. (2008), reported that C. dactylon reproduces by seeds that have a low primary seed dormancy. Thus, seeds emerge from the soil faster than seedlings of other plants, which makes them strong competitors (Donato et al., 2019). Therefore, C. dactylon is a potential candidate for suppressing P. hysterophorus and is recommended for vegetation restoration in P. hysterophorus invaded sites. In other studies, C. dactylon was also found in P. hysterophorus invaded sites (Kumari et al., 2014)in India and Woreda in Ethiopia (Gadisa et al., 2019).
Plant species diversity indices in P. hysterophorus invaded and uninvaded sites in QENP
Generally, the study results showed a significant difference in species richness, dominance, evenness and diversity between P. hysterophorus invaded and uninvaded sites with P < 0.05 (Table 2). Although invaded sites were richer (P = 0.043, R = 58) in plant species than uninvaded (R = 39), uninvaded sites had more evenly distributed plant species (P = 0.04, E = 0.63 ± 0.02) than invaded (E = 0.23 ± 0.08). Species dominance was also found to be higher in invaded (P = 0.04, D = 0.62 ± 0.19) than uninvaded sites (D = 0.46 ± 0.25). Consequently, species diversity became significantly less (P = 0.039, 1-D = 0.38 ± 0.19) in invaded than uninvaded (1-D = 0.55 ± 0.25). Reviewed impacts of P. hysterophorus on species diversity in India revealed a decrease (46.1%) in grass cover and a loss (398.1) g m-2 in dry biomass of Poaceae family (Birhanu and Khan, 2018). Assessment of P. hysterophorus impact on herbaceous plant diversity of Awash National Park in Ethiopia (Etana et al., 2015), attributed the difference in species richness to high regeneration of plant species that follow soil disturbance.
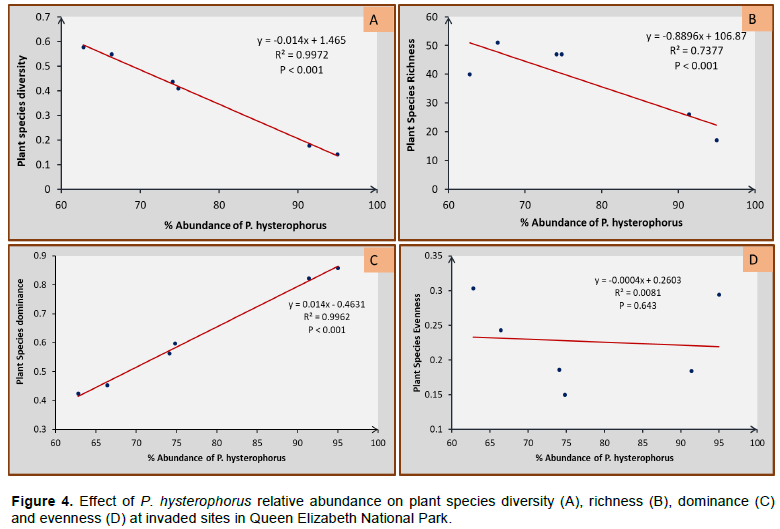
Effect of P. hysterophorus abundance on plant species diversity, dominance, richness and evenness in invaded sites of QENP
It was found that increasing relative abundance of P. hysterophorus causes a significant decline in plant species diversity (R² = 0.9972; P < 0.001), and plant species richness (R² = 0.7377; P < 0.001), but increases dominance in invaded sites (R2 = 0.9962; P <0.001). However, it does not contribute to species evenness in invaded sites (R2 = 0.0081; P = 0.643) as indicated in Figure 4A to D. Furthermore, extrapolation of the generated linear regression models, based on linear equations (y = -0.014x + 1.465) in 4A, and (y = -0.8896x + 106.87) in 4B, the abundance at which P. hysterophorus starts to significantly reduce species diversity and richness is at very low percentage of 4.6 and 7.7% respectively. However, its effect on dominance according to (y = 0.014x - 0.4631) in 4C, was exerted at relatively higher percentage abundance (40.2%).
This confirms that P. hysterophorus reduced the species diversity and richness of plant species and this agrees with the documented impact of P. hysterophorus that also revealed a reduction of 80-90% carrying capacity of pasture in Central West Asia (Rwomushana et al., 2019). In addition, P. hysterophorus frequency was inversely proportional to species diversity and a significant shoot growth reduction (47.9%) and root growth inhibition (59.3%) was recorded on grassland species of Australia (Birhanu and Khan, 2018). The dominating effect of P. hysterophorus was attributed to allelopathic nature of monoterpenes it contains (Belgeri and Adkins, 2015). Over 73.7% reduction in species richness in invaded areas as shown in Figure 4B, was accounted to P. hysterophorus. The same trend was reported by Gadisa et al. (2019), further confirming the detrimental effect of the weed especially in protected areas like QENP. Similarly, a decline (90%) in forage production was attributed to inhibitory potentials of P. hysterophorus on plant germination (Birhanu and Khan, 2018).