Review
ABSTRACT
Pumpkin has high nutritional value essential for food security. Despite many benefits, it still considered an orphan crop in Africa. Very little information is available on the potential and production of pumpkin in Africa due to neglect by researchers and improvement program. Classical research, both theoretical and empirical, was used to conduct a systematic review of the various result trends obtained by researchers in relation to the topic. Using Google Scholar, relevant literature was selected, analyzed and summarized. In Africa, the fruit, seeds and leaves of the pumpkin is consumed. This dietary diversity of pumpkin could contribute to the improvement of people's livelihoods. Countries such as Morocco, South Africa and Tunisia are making considerable profits from the export of pumpkin. However, its production is very low because its potential is not fully exploited. In this context, research and development strategies must be put in place so that pumpkin becomes part of the African diet. The diversity, origins and distribution, utilization patterns, relative importance, production and bottlenecks of pumpkin in Africa are reviewed. These results can be used as a basis for further research on yield improvement and pest management.
Key words: Africa, bottlenecks, food security, orphan crops, pumpkin.
INTRODUCTION
In 2019, global production of Cucurbita species (Cucurbita moschata, Cucurbita maxima, Cucurbita pepo) was estimated at 22,900,826 mt (FAOSTAT, 2019). According to the same source, African production is estimated at 2,793,530 mt. China (8,427,676 mt), India (5,655,994 mt), Ukraine (1,346,160 mt), and Russia (1,195,611 mt) are the largest global producers while Algeria (420,135 mt), Egypt (406,778 mt), Malawi (368,025 mt) and South Africa (270,486 mt) hold the largest production in Africa (FAOSTAT, 2019). Thus, pumpkin production in Africa is very low when compared with other continents, there is need to popularize this underutilized crop but potentially rich in nutrients in order to feed the growing population in a sustainable manner.
The consumption of plant-based foods has increased in recent years, due to public awareness of their benefits. Several epidemiological studies have shown that such a diet significantly reduces degenerative diseases such as cardiovascular events and some types of cancer (Adebayo et al., 2013). In traditional medicine, pumpkin is being used to cure the following diseases: hyperglycemia in diminishing the blood glucose concentration, hepatitis, macular dystrophy, abdominal cramps and distension due to intestinal worms, diabetes, hypertension, cancer, immunomodulation, bacterial and microbial infections, hyperon, hypercholesterolemia, intestinal parasites, digestive parasites, inflammation, obesity, and analgesic diseases (Chen, 2005; Yadav et al., 2010, Adam et al., 2011). Moreover, traditionally, it helps in increasing fertility immune system, eyesight, tackling convulsion, and promoting heart and skin health (Rahman et al., 2019; Hosen et al., 2021). Pumpkin is generally grown for its fruit and sometimes for its oil seeds (Fu et al., 2006). It is a low-calorie vegetable that fulfills many dietary requirements (PROTA, 2018). González et al. (2001) reported that pumpkin is an important source of vitamin A (4 ± 20 mg / g). This abundance of pumpkin in nutrients will be greatly beneficial for pre-school children (FAO/WHO, 2007). It is in this context that the works of Mbogne et al. (2015) in Cameroon have shown that pumpkin can thus play an important role in the fight against vitamin A, deficiency which affects more than 250 million children under the age of five worldwide. Pumpkin is a good source of ascorbic acid (22.9 mg/100 g) and inhibits the development of degenerative diseases such as cancer, diabetes, cardiovascular and neurological diseases (Roura et al., 2007).
Despite its dietary and economic advantages, pumpkin is not efficiently exploited and valorized in Africa. In Africa, production is still lower than that of China and India and almost the same with Ukraine and Russia. There is a need to value C. moschata as a source of nutrients to combat hunger and malnutrition and to boost its production in Africa through research and along the production of high added value products. Traditional crops are insufficient to feed the growing population in Africa, thus complementary underutilized food sources like C. moschata could be an important addition to the African diet. African researchers and improvement programs can no longer continue to ignore this valuable crop.
Aruah et al. (2010)conducted a study on the agro-morphological variations of local accessions of Cucurbita in Nigeria. The floral biology and breeding systems of C. moschata varieties in Nigeria were studied by Agbagwa et al. (2007). Kiramana and Isutsa (2017)conducted a detailed study on the quality characteristics of pumpkin in Kenya. Pumpkin was considered in the general vegetable census in Benin (Achigan?Dako et al., 2010). But no in-depth study has been conducted on C. moschata in Benin. The objective of this review was to (i) synthesize the most important information on morphological, agronomic, nutritional, ethnobotanical and economic traits of pumpkin; and (ii) elucidate the diversity, origins and distribution, utilization patterns, relative importance, production and bottlenecks of pumpkin in Africa.
METHODOLOGY
The relevant literatures were selected from Google Scholars and then analyzed and summarized. The readings of those literatures gave us enough crossed views allowing to substantiate our remarks in a mega-analysis. A systematic review was used to achieve the goals of the paper, which was fundamentally based on classy research from both theoretical and empirical discoveries. The key results generated from update literature and deductive logical reasoning significantly contributed to understanding the crucial role that farmers play in the management and exploitation of the diversity of the Cucurbita spp. in Africa. The organization of this diversity with a viewpoint of varietal improvement and for future perspective in terms of research and policies making is important.
BOTANY AND KNOWLEDGE OF THE PUMPKIN (C. MOSCHATA)
Origin, domestication and geographic distribution of the species
All species of Cucurbita are native to the Americas (OECD, 2016). According to Jeffrey (1990), America is the center of origin of the different species of Cucurbita. C. moschata is native to North America (Mexico).
C. moschata was domesticated in Colombia (Whitaker and Davis, 1962). There are divergent opinions on the precise area where domestication probably occurred. Scientific studies on the natural distribution and domestication of C. moschata have been conducted by Merrick (1990)and Whitaker (1974). It was found that C. moschata had undergone two domestications (Mexico and the northern South America). These assertions were supported by linguistic evidence (Lira et al., 1995; Robinson and Decker-Walters, 1997). After Europe, C. moschata adapted to different ecological conditions across the globe (OECD, 2016). It is the most heat tolerant and well-known Cucurbita spp. in tropical Africa (PROTA, 2018).
Taxonomy
Cucurbitales, Cucurbitaceae and Cucurbitoideae are respectively the order, the family and the subfamily of the genus Cucurbita (Jeffrey, 1990). It is distantly related to other genus in the family Cucurbitaceae (OECD, 2016). It is considered a different genus with 20 to 27 species (Cutler and Whitaker, 1968; Esquinas-Alcazar and Gulick, 1983). The genus Cucurbita is divided into two groups considering the ecological characteristics and life cycle length of the different cultivated species (OECD, 2016). Xerophytic species are perennial plants adapted to drought conditions with tuberous storage roots. Mesophytic species are determinate (annual) or indeterminate (perennial) plants adapted to humid climates, short-lived with fibrous roots. The genus Cucurbita includes 18 species among which 5 species are the most cultivated in the world. They are Cucurbita argyrosperma, Cucurbita ficifolia, Cucurbita maxima, C. moschata and C. pepo. These five species belong to the group of mesophytic species. Many names and grouping systems of which these species have been the subject are due to the morphological variation of the fruits and seeds (Table 1).
Despite its importance in many parts of the world in general and particularly in Africa, fewer scientific studies to elucidate the taxonomy of C. moschata have been conducted (OECD, 2016). The different relationships between 31 genotypes of landraces obtained in Malawi and Zambia were studied using random amplified polymorphic DNA analysis (Gwanama et al., 2000). It revealed that C. argyrosperma is the wild ancestor of C. moschata.
Morphological characteristics
C. moschata is an annual herbaceous plant, very branched, creeping or climbing by lateral tendrils with 3 to 4 branches. The stems are angular with obtuse angle, very running, pubescent at the beginning and often rooting at the tendrils. These leaves are green or green mottled with white, they are alternate without stipules. The length of the petiole varies from 9 to 24 cm. The leaf blade has a broadly oval outline with 5 to 7 shallowly lobed palms of 10 to 35 cm in diameter. It has a toothed edge, soft hairs with sometimes white spots disappearing at senescence and 3 veins starting from the base.
The pumpkin is an allogamous plant (Lira et al., 1995; Whitaker and Robinson, 1986). They grow in the axils of the leaves (Figure 1). They have a color that varies between lemon yellow and dark orange. The length of the sepals ranges from 1 to 3 cm, these sepals are free. The corolla is campanulate with widely spread lobes. The lengths of the pedicels of the male flowers extend up to 16 cm. They are 3 staminate with free filaments and the anther is usually supported by a long twisted organ. The female flowers have a short pedicel (up to 3.5 cm). Their ovaries are inferior, ellipsoid with a thick style and 3 stigmas. The fruit is a large berry with a multiform (globular, cylindrical and ovoid). It weighs up to 10 kg. The fruits are covered with green spots and gray streaks. The flesh of the fruit varies from yellow to orange. The fruits have many seeds. The fruit stalk is angular with five ribs clearly widened at the apex.
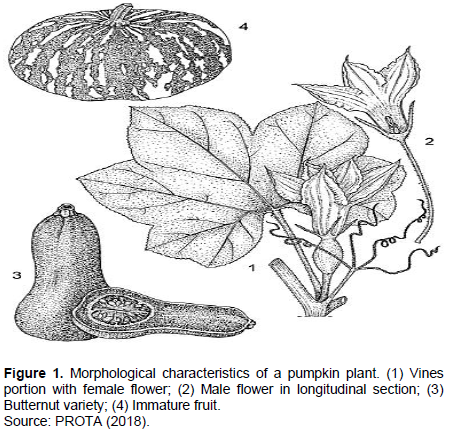
The seeds are obovoid and flattened. The length and width of the seeds vary between 1-2 and 0.5-1 cm, respectively. The color of the seeds can be white or tan or sometimes dark. The surface of the seeds is smooth or a little rough. The seedlings have an epigeous germination. The cotyledons have an elliptical shape with a length of 2 to 4 cm. Despite these morphological characters, it is often very difficult to differentiate C. moschata from C. maxima. Differences in fruit peduncle, stems and leaves are the best indicators for easy differentiation (Table 2).

Reproduction
Cucurbita spp. possess male and female reproductive flower organs in one single plant, thus they are monoecious plants (Lira et al., 1995; Whitaker and Robinson, 1986). Flowering of Cucurbita spp. is asynchronous, meaning male flowers appear well before female flowers (OECD, 2016). The near-synchronization at anthesis of both sexes is due to the early development of female flowers (Janick and Paull, 2008). Their lifespan is half a day (Nepi and Pacini, 1993). According to the same authors, the viability of pollen in a male flower decreases with time.
Cross-pollination of Cucurbita spp. is favored by both their monoecious natures and the size of the male flowers than the female flowers on the same plant (OECD, 2016). The proportion of male and female organs on a plant varies according to abiotic factors (Janick and Paull, 2008). This variation is due to temperature and light (Whitaker and Davis, 1962). Cucurbita pollen grains are large (80 to 150 μm in diameter), sticky and well adapted to transport by insects (OECD, 2016). For this reason, pollination is not carried out by wind in Cucurbita spp. Apis mellifera are the pollinators of these flowers (Canto-Aguilar and Parra-Tabla, 2000). A very large number of live pollen grains (500-1000) on the stigma of the female flower will allow for proper fruit development (Stephenson et al., 1988; Vidal et al., 2010). The flowering constitutes the favorable period or the ovules are fertile (OECD, 2016). In the process of pollinating female flowers, bees are guided by the olfactory signals of some species and probably also by visual or auditory sensors (OECD, 2016). According to these authors, nectar is collected by pollinators from female flowers, but pollen and nectar are collected from male flowers. The pollen adheres to the body of the bee when it visits a male flower in search of nectar. This pollen is then passed on to the stigmas which are covered with a somewhat sticky substance that allows the pollen to attach itself when visiting the female flowers (Zomlefer, 1994). Each pollen grain on the sigma of the female reproductive organ sprouts and a tiny (almost microscopic) rootlet develops, moves down the pistil, reaches the ovary of the flower which is then fertilized (PROTA, 2018).
After pollination, the fruit evolves from the mature ovary resulting from fertilization between the ovum and the spermatozoon, and the zygote becomes the seed in the ovary. The shape of the mature fruit depends on the shape of the ovary. The pulp around the seeds comes from the inner parts of the ovary (mesocarp and endocarp) (OECD, 2016). There is no means of asexual reproduction either by cuttings or stolons or budding in Cucurbita spp. level (OECD, 2016).
Genetic diversity
Diversity inventor
The chromosome number of C. moschata is 2n=2x=40 (OECD, 2016). The chromosomes are small and difficult to differentiate (Weeden, 1984). This complicates the description of their morphologies. Annual Cucurbita spp. evolved from perennial species (Wilson et al., 1992). Sanjur et al. (2002)and Wilson et al. (1992)suggested that C. argyrosperma is the wild ancestor of C. moschata (cultivated species).
Center of diversity and biotechnology developments
Cucurbita spp. are well represented in the Cucurbitaceae genetic resource collections of several institutions around the world (PROTA, 2018). The National Seed Storage Laboratory (NSSL) in Fort Collins, Colorado, USA and the Vavilov Institute (VIR) in St. Petersburg, Russia, maintains substantial core collections. Large collections of C. moschata are conserved at the NBPGR genebank in New Delhi (India), NPGRL in Los Baños (Philippines), NIAS in Ibaraki (Japan), INIA in Celaya (Mexico), and CATIE in Turrialba (Costa Rica). Insubstantial attention has been paid to African landraces. C. moschata is one of the species for which the Southern African Developing Countries Plant Genetic Resources Center (SPGRC) in Lusaka, Zambia, is responsible. The introduction of improved cultivars threatens the disappearance of old landraces and the collection of plant genetic resources of these African landraces must be a priority.
The different cultivated Cucurbita spp. play a primary role in the food and nutritional security of the population (OECD, 2016). However, these species are subject to viral diseases that can cause considerable economic losses (Provvidenti, 1990). Insect vector control is the key means of controlling these diseases, but not without limitations (OECD, 2016). The use of advanced and biotechnological techniques has boosted and quickened the development of resistant varieties. Indeed, the insertion in the genome of the species of DNA sequences coding for the gene of the envelope protein of the virus will allow the protection of plants against these viruses (OECD, 2016).
Cucurbita production in the world
The world production of fruits of Cucurbita including all species was about 22.900.826 t in 2019 (FAOSTAT, 2019). The main producing countries are: the People's Republic of China with 8.427.676 t, Ukraine with 1.346.160 t and Russia with 1.195.611 t (FAOSTAT, 2019).
According to FAOSTAT (2019), Algeria (420.135 mt), Egypt (406.778 mt) and Malawi (368.025 mt) are the major producers of pumpkin in Africa 2019. The major producers in West Africa are: Niger (192.581 mt), Mali (80.915 mt) and Côte d'Ivoire (19.316 mt).
PHYSICOCHEMICAL, NUTRITIONAL AND ETHNOBOTANICAL COMPOSITIONS
Physical properties, chemical compositions and nutritional values
The fruits of pumpkin range in weight from 0.59 to 8.75 kg, while fruit length and width range from 13.21 to 91.99 cm and 11.69 to 42.97 cm, respectively (Jacobo-Valenzuela et al., 2011). The thickness of the fruits varies from 1.80 to 6.95 cm. The pulp represents 71.75 to 86.06% of the fruit weight while the seeds represent 2.7 to 5.89% of the same weight.
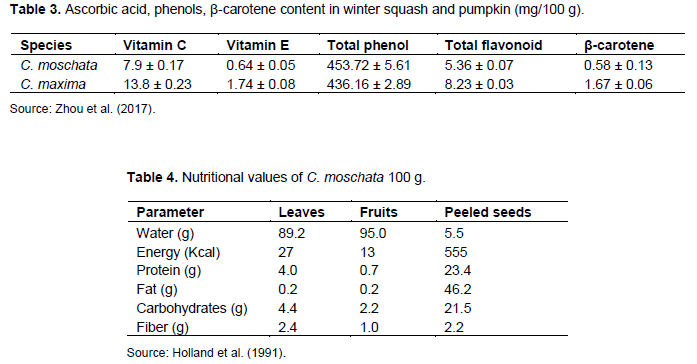
C. moschata has a very important nutritional value for human health (Rakotovao, 1999). But this species of Cucurbita can express anti-nutritional compounds called cucurbitacins which are toxic and deadly to the humans (US Environmental Protection Agency, 1999). Carotenoids are essential group of biologically active elements with a wide range of health benefits (Kulczy?ski and Gramza-Micha?owska, 2019a). The key carotenoids present in pumpkin are: zeaxanthin, lutein, β-carotene and retinol equivalent. According to the work of Norshazila et al. (2014), β-carotene is the dominant carotenoid in pumpkin. Lutein was the most predominant carotenoid in pumpkin fruits. Several internal and external factors could affect the concentrations of lutein and beta-carotene in pumpkin namely the type of variety, the growing conditions, the maturation period, and the storage time. In storage, lutein content rises while beta-carotene decline in pumpkin fruits (Jaswir et al., 2014). Indeed, beta-carotene concentration is also influenced by sun exposure, temperature, water availability, and the chemical composition of the soil. High beta-carotene levels are recorded during the early stages of fruit formation. The content of this compound decreases when the pumpkin fruits reach maturity (Kulczy?ski and Gramza-Micha?owska, 2019a). The β-carotene content of fruits of the genus Cucurbita varies among species (Table 3). The dietary value of these species is mainly due to their organic (carbohydrate and protein) and especially mineral (calcium, phosphorus, and iron) contents (Rakotovao, 1999)(Table 4). The description of bioactive compounds in the pumpkin pulp is diverse and depends on the species and cultivar (Kulczy?ski and Gramza-Micha?owska, 2019b).
Pumpkin is a vegetable used in human food which presents several derivatives (fruit, seeds and leaves). Pumpkin fruits from a nutritional point of view can be compared to tomato. Dietary diversification leads to better nutrition and food security. According to Dar and Sharma (2011), tomato fruits are rich in β-carotene (1.64 mg/100 g) and ascorbic acid (27.04 mg/100 g). The seeds of Lagenaria siceraria have high values of protein (28.2 g), lipids (49.8 g) while the seeds of C. moschata hold the high values of carbohydrates and fiber. The two-dimensional aspects of pumpkin could help improve people livelihoods.
Socio-cultural aspect and different uses in rural areas
In North America, pumpkin is considered the fruit of health and is used in crops association with beans and corn (OECD, 2016). In Islam, pumpkin fruit in general represents the tree that God grew to give food and medicine to Jonas (PROTA, 2018). They further stated that the exegetes affirmed that the term "Yaqtin" refers to pumpkin. Some of them attribute tremendous advantages to these plants including the speed of its growth, the shade provided by its leaves due to their large size and flexibility, the nutritional quality of its fruits, the possibility of eating them cooked or raw, the usefulness of its peels and the fact that wolves do not dare get closer to it (PROTA, 2018). These authors further reported that the novelist Agatha Christie, in the humorous prologue to The Labors of Hercules, wanted to retire and devote herself to improving the taste of pumpkin.
C. moschata is used in food, animal feed and medicine. In human food, it constitutes vegetables whose leaves, fruits and seeds are consumed (PROTA, 2018). In Zimbabwe, “Musatani” landraces are used especially as leafy vegetables (Ndoro et al., 2007). According to these authors, during the rainy season the leafy vegetables of the pumpkin are consumed 3 to 4 times in a week. The different uses of the varieties of pumpkin in Benin (townships of Couffo and Borgou) are human consumption, sale, medicinal use and occultism (Ezin et al., 2021). According to these authors, the fruits of the pumpkin are used in the preparation of several recipes (simple sauce, egusi sauce and cooked ekpin) to satisfy the food and nutritional needs of the populations. Pumpkin seeds represent a nutritionally balanced source of protein (Vinayashree and Vasu, 2021). They contain all the essential amino acids except lysine and threonine. This abundance of pumpkin in nutrients will be greatly beneficial for pre-school children (FAO/WHO, 2007). The extracted oil from C. maxima seeds could be potentially utilized as a preservative and functional ingredient in foods and cosmetics (Montesano et al., 2018). The vines and fruits are used as fodder for domestic animals (Noguera, 2002). Cucurbita species are used in traditional medicine; as anthelmintic (Chou and Huangfu, 1960; Lozoya, 1994; Schabort, 1978); to treat prostate (Duke and Ayensu, 1985); as nerve tonic and to soothe burns, inflammation (Chopra et al., 1956); and as anti-hyperglycemic agent (Andrade-Cetto and Heinrich, 2005). In Benin, the leaves of "Ekpin", a variety of the pumpkin, are used to fight against fever and external hemorrhoids (Ezin et al., 2021). The content of active phytochemicals in Cucurbitaceae species must be further exploited for both preventive and therapeutic purposes (Salehi et al., 2019).
Economic importance
In 2019, the main importing countries of fruits of the genus Cucurbita, all species combined in the world were: the United States of America (544.993 t), France (166.127 t) and Germany (108.909 t) (FAOSTAT, 2019). Additionally, the financial value of each of these imports was: US$438.532.000, US$164.113.000, and US$134.634.000, respectively. In terms of exports, Mexico (538.038 mt), Spain (449.193 mt) and New Zealand (84.626 mt) are the largest exporters in the world in 2019. The costs of these exports are US$424.439.000, US$ 405.414.000 and US$ 41.433.000, respectively.
According to FAOSTAT (2019), South Africa (1.710 mt), Libya (1.339 mt) and Namibia (481 mt) are the major importing countries of Cucurbita fruits in Africa. The costs of these importations are: US$601.000, US$652.000 and US$320.000, respectively. The main exporting countries in Africa are: Morocco (45.943 t), South Africa (18.909 t) and Tunisia (2.367 t) with export costs of: US$38.494.000, US$9.036.000 and US$1.306.000, respectively.
ECOLOGICAL REQUIREMENTS
Ecological conditions
The tropics are a favorable area for the cultivation of pumpkin (PROTA, 2018). According to these authors, it can be grown up to 1800 m altitude. It requires daytime temperatures above 20°C and nighttime temperatures above 14°C for good growth. It shows a slight response to short days. The production of pumpkin does not an abundance of water. C. moschata is fairly drought tolerant, but sensitive to frost and salinity. Excess moisture during the rainy season stimulates the development of fungal and bacterial diseases, causing leaf dieback, wilting and fruit rot. Pumpkin can be grown in almost any reasonably fertile, well-drained soil with a neutral to slightly acidic pH (5.5 - 6.8). Overall, the ecological requirements will depend on the soil, climatic conditions, pumpkin varieties, and management practices.
Plant growth and development
In extensive cropping systems, pumpkin is produced in crop combinations with corn or sorghum (PROTA, 2018). This production occurs on termite mounds, garbage heaps, and livestock pens. Commercial production of improved cultivars requires pure cultivation. This production system is not well developed in African countries.
C. moschata is propagated by seed with a sowing spacing of 2 m × 2 m. The seed requirement at sowing is 2 to 7 kg/ha. High planting density provides soil coverage and faster weeds control. After sowing, there is the emergence of seedlings between 5 and 7 days. On fertile soil, the stems continue to grow as long as they can root at the nodes; they can exceed a length of 20 m. During the vegetative phase, for an optimal growth, the recommended fertilizer applications are: 50-100 kg/ha of N, 20-40 kg/ha of P and 40-80 kg/ha of K. Irrigation should be applied in dry conditions (50 mm/week). Sometimes plants are spiked to control growth and promote branching. Flowering is more or less continuous and starts 35 to 60 days after seedling emergence. Mendlinger et al. (1992)stated that the collected accessions of pumpkin reached 50% flowering in the range of 57.3 to 88.3 days. In Nigeria, according to Agbagwa et al. (2007), flowering occurred 56 days after C. moschata sowing. However, the different variations observed in days to 50% flowering could be mainly due to intra-species variability. The ratio of male to female flowers is approximately 20:1 (PROTA, 2018). According Agbagwa et al. (2007), the ratio of male to female flowers is 9:1. The highest and lowest male to female flower ratio in Bangladesh was 12.11:4.08 and 10.24:3.27, respectively (Islam et al., 2016). This ratio is influenced by environmental factors (temperature and day length) and chemical regulators (ethylene and gibberellin) (OECD, 2016). Male sex expression is favored by long days and high temperatures (PROTA, 2018). According to these authors, one to two fruits develop per vine. The fruit ripens 60-120 days after sowing. Based on agro-morphological evaluation of pumpkin genotypes in northern Bangladesh, fruit ripening occurred in the range of 103 to 123 days after sowing (Ahamed et al., 2011). Recommended good cultural practices call for mulching the soil, weeding, rouging, pest and disease control measures, wrapping the fruit in paper to protect it from fruit flies, and hand pollination to increase fruiting.
Diseases and pests
C. moschata is attacked by many pathogens and pests. Downy mildew (Pseudoperonospora cubensis) often becomes a devastating disease under very high humidity (OECD, 2016). The lower leaf surface becomes covered with a light purplish layer and the leaf dies completely. Good cultural practices such as crop rotation, good drainage are an integral part of the various control methods for late blight. Erysiphe cichoracearum causes a disease known as powdery mildew that occurs when moisture is low (PROTA, 2018). Symptoms are usually seen first on older leaves. Infested leaves die, plants prematurely senesce by reducing photosynthesis and thus declining yield (Agrios, 2005). Alternaria (Alternaria cucumerina) is a fungal disease that defoliates and kills the plant within a few weeks (Blancard et al., 1994). Symptoms are represented by small, round, whitish necrotic spots. Leaves turn gray or yellow and dry out. Attacks of this disease can be limited by using healthy seed, disposing of crop residues, and rotating crops. Didymella bryoniae is a fungus that causes black rot or gum disease (Davis, 2008). Symptoms of this disease include fruit discoloration and branching wilt. The use of healthy seeds, preventive fungicide (mancozeb) and crop rotation are part of the control methods. Fusarium (Fusarium oxysporum) causes yellowing of leaves followed by wilting of the entire plant (OECD, 2016). The use of healthy seeds, elimination of crop residues, crop rotation and rational use of nitrogen fertilizers can control this pathogen. Anthracnose (Colletotrichum lagenarium) causes defoliation and lesions on the fruit (Bar-Nun and Mayer, 1990).
Many viruses have been reported on C. moschata. These include cucumber mosaic virus (CMV), papaya ring spot virus type W (PRSV-W), squash mosaic virus (SqMV) and watermelon mosaic virus (WMV-2) (OECD, 2016). Cucumber mosaic virus manifests as leaf yellowing, ring spots, stunting, and deformation of leaves, flowers, and fruit. Aphids, seeds, and weeds are the primary vectors of CMV (OECD, 2016). PRSV-W is transmitted through aphids. Leaves and plants become mottled and branching are stunted (Brunt, 1996). SqMV is transmitted non-persistently by several insects (Brunt, 1996); by seeds and mechanically (OECD, 2016). Symptoms of WMV-2 are stunted fruits and yellowing leaves. Plants stop producing marketable fruit one to two weeks after infection (OECD, 2016). To prevent the spread of virus diseases, control of the main vectors of these viruses (aphids, whiteflies, and thrips) is necessary (PROTA, 2018).
CHARACTERISTICS OF THE VARIETIES
There are several varieties of pumpkin, only some varieties will be listed in this paper (Table 5).

Harvest and post-harvest activity
Harvest is performed over a period of 2 to 6 months after planting (PROTA, 2018). Yield is highly dependent on species, variety, and cultural conditions. The fruit weight of C. moschata varies between 1 and 10 kg, respectively; the fruit yield is low under extensive cultivation (5 t/ha) (Whitaker and Davis, 1962). The use of improved cultivars can yield 30 t/ha, the average yield is 15 t/ha. The yield of leaves is 20 t/ha for a harvest period of 2 months. Excessive harvesting of young leaves significantly reduces fruit yields. Seed yield is variable and can reach up to 300 to 500 kg/ha (Sharma and Lal, 1998).
In Africa, the deficiency of micronutrients in the daily diets is leading to adulteration of food, malnutrition and nutrition insecurity and unsafety. Pumpkin is regarded as a reservoir of micronutrients but not sufficiently exploited owing to many factors such as the lack of knowledge of the food virtues of this vegetable by the consumers and the waste due to post-harvest losses. The leaves of pumpkin are highly perishable and the fruits cannot be stored for long periods. In this context, fermentation of pumpkin leaves seems to be essential vital processing method for increasing the shelf life of this vegetable (Misci et al., 2021). According to the work of these authors, fermentation could help to preserve the leaves of Cucurbita spp. by preventing contamination by altering microorganisms and by enhancing the nutritional value of the final product. This method consists of tying the leaves in bundles and making them moist under a jute bag. The drying of the leaves constitutes a means of conservation. The dried leaves are stored in containers and used in times of shortage. This innovation packages will improve upon the food and nutritional security. Post-harvest preservation will allow the pumpkin leaf vegetable to be available for consumption. Its affordability will allow it to be accessible to different segments of the population. The fruits can be stored for long periods. The "Butternut" variety can be stored for at least one month and the large pumpkin for several months (Whitaker and Davis, 1962).
They further illustrated that storage is at 10 to 16°C and 70% humidity. In cold storage, cold damage occurs at temperatures below 10°C. Drying the flesh of the pumpkin is a preservation method for its later use in soups and stews (PROTA, 2018).
Seeds are removed after the fruit is eaten. These seeds can be used as planting materials or in human food. Seeds are sold shelled or unshelled (Souley et al., 2018). The different operations in order to obtain pumpkin powder are: sorting, sprinkling, hulling and grinding (Salifou et al., 2015). Manual hulling is tedious; mechanized hulling is now practiced in several countries including Nigeria and Sudan. Hulled seeds are more susceptible to storage pathogens, particularly Aspergillus, which release toxic aflatoxins in hot and humid conditions (PROTA, 2018).
Processing and valorization
Inventory of processing products and derivatives
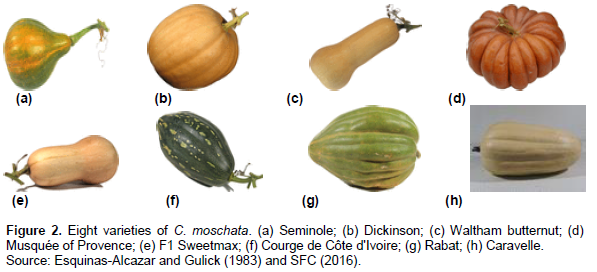
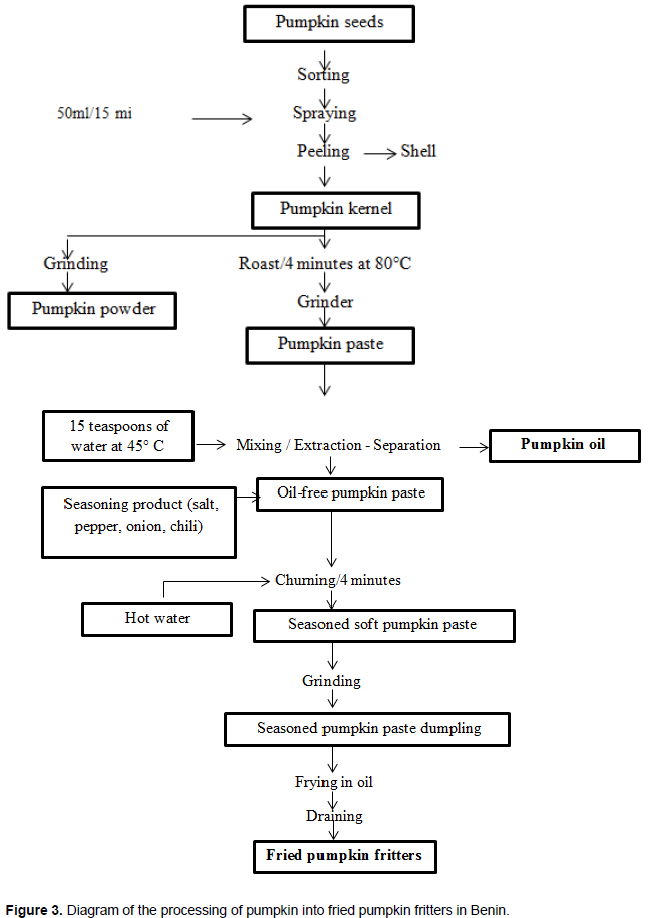
C. moschata has been playing an important role in the food and nutritional security of rural communities since ancient times (Belkebla and Makhloufi, 2016). The purpose of processing is to add value to agricultural products and increase their post-harvest shelf lives. In the agri-food field, many pumpkin-based products and preparations are marketed as fruit jams, syrups (Nawirska et al., 2009). These authors also showed that pumpkin is also used as an additive in various food products. Pumpkin fruits are processed into soups, stews (Durante et al., 2014); juices and marinades (Aydin and Gocmen, 2015). According to Zargar et al. (2014), their pulps can be incorporated into chicken sausages for the purpose of enriching them with plant-based dietary fiber. A combination of pumpkin and apple leads to marmalade (Figure 2) (Rakotovao, 1999). Pumpkin seeds can be used as substitutes for Egussi (Citrulus lanatus, L. siceraria, and Cucumeropsis edulus) (PROTA, 2018). According to the work of Salifou et al. (2015), three derivative products are obtained from these seeds. These include pumpkin powder; pumpkin oil and fried pumpkin fritter (Figure 3).
OPPORTUNITIES AND CHALLENGES FOR C. MOSCHATA IN AFRICA
Opportunities
C. moschata is under the responsibility of the Southern African Developing Countries Plant Genetic Resources Center (SPGRC) based in Zambia (PROTA, 2018). Algeria, Egypt, Malawi, South Africa, Nigeria, Kenya, Cameroon, Congo and Niger are among the African countries that value pumpkin production. Studies addressing various issues related to production improvement precede the promotion of pumpkin. However, pumpkin is not included in Benin's agricultural policy despite being an extraordinary vegetable with the potential to be used as a medicinal as well as a nutritious multifunctional food. Pumpkin peels and flesh contain essential minerals as well as phytochemicals (β-carotene, total flavonoids, and total phenolic) that can help with antiaging and the immune system (Hosen et al., 2021). The consumption of Pumpkin enhances vitamin A intake, fortifies diets, and diversifies foods by including animal-derived foods (Buzigi et al., 2021).
According to the work of Du et al. (2011), C. moschata can be cultivated in different agro-ecological zones and presents a great morphological variability (color and shape of the fruit). The collection of south Africa and other African countries could be the starting point for future breeding programs in Africa, which could contribute to reduce hunger, malnutrition and generate income both in rural and urban areas for the producers and especially women (Ntuli et al., 2017). In Africa, the production of C. moschata is very low and is for local consumption (Andres, 2004). It is adapted to different abiotic conditions (climate and soil) (Gwanama et al., 2000). In many developing countries, indigenous foods, which are often more nutritious than modern foods traded on the world market, are neglected and forgotten (CDB, 2008). In a context of climate change and loss of biodiversity, pumpkin appears as a potential candidate for food security of populations. This choice is justified by its adaptability to several environmental conditions and by its richness in nutritive elements. Thanks to its different uses, the pumpkin creates a food diversity that contributes to a balanced and nutritious diet. Its production and transformation contribute to the creation of employment. In Niger in the Guidimouni basin, labor costs represent 6% of operational expenses on an area of 2500 m2 (Souley et al., 2018). The various costs associated with importing and exporting pumpkin are an indicator of the availability of demand and supply in the market (FAOSTAT, 2019). In Niger, the price of 100 pumpkins varies from 60000 to 130000 FCFA depending on periods of abundance and shortage (Souley et al., 2018). On the Africa market, supply exceeds demand, as consumers are not aware of the nutritional and therapeutic virtues of pumpkin.
Challenges
According to Zargar et al. (2014), a major challenge will be to increase agricultural production in the coming years so that food and nutritional security is a reality for the local population and to meet consumer demands for quality agricultural products. In this situation of health crisis, the effective use of pumpkin and its derivatives could contribute to food security; improve the income of producers and other links involved in the value chain. Among the challenges to be taken up for the promotion and valorization of the pumpkin in Africa are:
(1) The promotion of research and development addressing the issues of seed quality, production management, pests and diseases, weeds and soil fertility, varietal improvement rich in nutrients and vitamins to meet the needs of consumers,
(2) Establishing agronomic production systems to maximize productivity,
(3) Establishing production expansion strategies, efficient storage and processing technologies,
(4) Put in place different innovative farming systems and technological packages for the popularization of the benefits of pumpkin to producers and consumers,
(5) The establishment of a well-structured and developed value chain.
(6) Availability of genetically improved varieties
(7) Genetic erosion of some varieties
(8) Government policies to promote the crop through research funding
(9) Including it in African’ diet and nutrition programs and policies
(10) Dire need to popularize the crop and its utilization at all levels
CONCLUSION
Pumpkin is a nutrient-rich, high-yielding, low-cost vegetable. It can help to alleviate food and nutritional insecurity. The transformation processes make it possible to have pumpkin-based products with a good organoleptic and sensory quality and respecting the sanitary standards. It is a significant source of income for producers. But despite all these assets, it is still a marginalized crop in research and development programs and in the food habits of the populations. The immediate consequences of these actions are, on one hand, the absence of selection programs for yield, disease resistance and the implementation of processing technologies in the agri-food, medicinal and cosmetic fields. On the other hand, the risks of genetic erosion of traditional varieties of pumpkin. In this context, the richness of this species must be studied with the support of various ethnobotanical, agronomical and morphological identification tools. These results could contribute to understand not only the crucial role that farmers play in the management and exploitation of the diversity of these species, but also the organization of this diversity in a perspective of varietal improvement. Many challenges remain to be lifted in order to make pumpkin production profitable in Africa.
CONFLICT OF INTERESTS
The authors have not declared any con?ict of interests.
ACKNOWLEDGEMENTS
The authors sincerely thank Gazali SANNI, David CARRENA and Nelson VODOUNON for their scientific contributions during this study.
REFERENCES
|
Achigan-Dako EG, Pasquini MW, Assogba-Komlan F, N'danikou S, Yédomonhan H, Dansi A, Ambrose-Oji B (2010). Traditional vegetables in Benin. Cotonou, Benin: CENAP pp. 128-129. |
|
|
Adams GG, Imran S, Wang S, Mohammad A, Kok S, Gray DA, Channell GA, Morris GA, Harding SE (2011). The hypoglycaemic effect of pumpkins as anti-diabetic and functional medicines. Food Research. International 44(4):862-867. |
|
|
Adebayo O, Farombi A, Oyekanmi A (2013). Proximate, mineral and anti-nutrient evaluation of pumpkin pulp (Cucurbita pepo). Journal of Applied Chemistry 4(4):25-28. |
|
|
Agbagwa IO, Nsukwu BC, Mensah SI (2007). Floral biology, breeding system and pollination ecology of Cucurbita moschata (Duch. ex Lam) Duch. ex poir varieties (Cucurbitaceae) from parts of the Niger Delta, Nigeria. Turkish Journal of Botany 31(5):451-458. |
|
|
Agrios G (2005). How plants defend themselves against pathogens. Plant Pathology 5:207-248. |
|
|
Ahamed K, Akhter B, Islam M, Ara N, Humauan M (2011). An assessment of morphology and yield characteristics of pumpkin (Cucurbita moschata) genotypes in northern Bangladesh. Tropical Agriculture Research and Extension 14(1):8-10. |
|
|
Andrade-Cetto A, Heinrich M (2005). Mexican plants with hypoglycaemic effect used in the treatment of diabetes. Jersey Evening Post 99(3):325-348. |
|
|
Andres T (2004). Diversity in tropical pumpkin (Cucurbita moschata): a review of infraspecific classifications. Dans A. Lebeda and H. Paris (dirs), Progress in Cucurbit. Genetics and Breeding Research pp. 107-112. |
|
|
Aruah CB, Uguru MI, Oyiga BC (2010). Variations among some Nigerian Cucurbita landraces. African Journal of Plant Science 4(10):374-386. |
|
|
Aydin E, Gocmen D (2015). The influences of drying method and metabisulfite pre-treatment on the color, functional properties and phenolic acids contents and bioaccessibility of pumpkin flour. LWT - Food Science and Technology 60(1):385-392. |
|
|
Bar-Nun N, Mayer AM (1990). Cucurbitacins protect cucumber tissue against infection by Botrytis cinerea. Phytochemistry 29(3):787-791. |
|
|
Belkebla A, Makhloufi N (2016). Optimization of the extraction of phenolic compounds and development of a yogurt based on squash. Thesis. University A. Mira - Bejaia, Algérie. |
|
|
Blancard D, Lecoq H, Pitrat M (1994). A colour atlas of cucurbit diseases: observation, identification and control. London, Engleterre: Manson Publishing pp. 15-30. |
|
|
Brunt AA (1996). Plant viruses online: descriptions and lists from the VIDE database. Moscow, Etats-Unis: University of Idaho. pp. 30-45. |
|
|
Buzigi E, Pillay K, Siwela M (2021). Potential of pumpkin to combat vitamin A deficiency during complementary feeding in low- and middle-income countries: Variety, provitamin A carotenoid content and retention, and dietary reference intakes. Critical Reviews in Food Science and Nutrition pp. 1-10. |
|
|
Canto-Aguilar MA, Parra-Tabla V (2000). Importance of conserving alternative pollinators: assessing the pollination efficiency of the squash bee, Peponapis limitaris in Cucurbita moschata (Cucurbitaceae). Journal of Insect Conservation 4(3):201-208. |
|
|
CDB (2008). Biodiversity and Agriculture: Protecting biodiversity and ensuring food security. Montréal, Canada: Em Dash Design pp. 1-56. |
|
|
Chopra RN, Nayar SL, Chopra IC (1956). Glossary of Indian medicinal plants. CSIR 89:250-265. |
|
|
Chou HC, Huangfu M (1960). Pumpkin (Cucurbita moschata) seed in the treatment of acute schistosomiasis. Chinese Medical Journal 80(2):115-120. |
|
|
Cutler HC, Whitaker TW (1968). A new species of Cucurbita from Ecuador. Annals of the Missouri Botanical Garden pp. 392-396. |
|
|
Dar RA, Sharma J (2011). Genetic variability studies of yield and quality traits in tomato (Solanum lycopersicum L.). International Journal of Plant Breeding and Genetics 5(2):168-174. |
|
|
Davis RM (2008). Diseases in UC IPM pest management guidelines: Cucurbits. Agriculture Natural Research Publication 3445:45-65. |
|
|
Du X, Sun Y, Li X, Zhou J, Li X (2011). Genetic divergence among inbred lines in Cucurbita moschata from China. Scientia Horticulturae 127(3):207-213. |
|
|
Duke JA, Ayensu ES (1985). Medicinal plants of China. Reference Publications: Algonac, MI. pp. 19-35. |
|
|
Durante M, Lenucci MS, Mita G (2014). Supercritical carbon dioxide extraction of carotenoids from pumpkin (Cucurbita spp.). International Journal of Molecular Sciences 15(4):6725-6740. |
|
|
Esquinas-Alcazar JT, Gulick P (1983). Genetic resources of Cucurbitaceae: a global report. IBPGR pp. 95-110. |
|
|
Ezin V, Gbemenou UH, Sanni GBTA, Ahanchede A (2021). Ethnobotanical study of pumpkin (Cucurbita moschata Duchesne) landraces in Benin. CABI Agriculture and Bioscience 2(1) :1-9. |
|
|
FAO/WHO (2007). Protein and amino acid requirements in human nutrition. Report of a Joint WHO/FAO/UNU Expert Consultation, WHO technical report series no. 935. Geneva, Switzerland: World Health Organization. |
|
|
FAOSTAT (2019). Available online: |
|
|
Fu C, Shi H, Li Q (2006). A review on pharmacological activities and utilization technologies of pumpkin. Plant Foods for Human Nutrition 61(2):70-77. |
|
|
González E, Montenegro Mariana A, Nazareno Monica A, Lopez de Mishima Beatriz A (2001). Carotenoid composition and vitaamin A value of an Argentinaian squash (Cucurbita moschata). Archivos Latinoamericanos de Nutrición 51(4):395-399. |
|
|
Gwanama C, Labuschagne M, Botha A (2000). Analysis of genetic variation in Cucurbita moschata by random amplified polymorphic DNA (RAPD) markers. Euphytica 113(1):19-24. |
|
|
Holland B, McCance RA, Widdowson EM, Unwin I, Buss D (1991). Vegetables, herbs and spices: Fifth supplement to McCance and Widdowson's The Composition of Foods (Vol. 5). Royal Society of Chemistry. |
|
|
Hosen M, Rafii MY, Mazlan N, Jusoh, M, Oladosu Y, Chowdhury MFN, Muhammad I, Khan MMH (2021). Pumpkin (Cucurbita spp.): A Crop to Mitigate Food and Nutritional Challenges. Horticulturae 7:352. |
|
|
Islam M, Mohsin G, Rahman M, Hasanuzzaman M, Biswas B (2016). Genetic Divergence in Pumpkin (Cucurbita moschata). Advances in Plants and Agriculture Research 4(5):1-4. |
|
|
Jacobo-Valenzuela N, Jose De Jesus Zazueta-Morales J, Gallegos-Infante JA, Aguilar-Gutierrez F, Camacho-Hernandez IL, Rocha-Guzman NE, Gonzalez-Laredo RF (2011). Chemical and physicochemical characterization of winter squash (Cucurbita moschata D.). Notulae Botanicae Horti Agrobotanici 39(1):34-40. |
|
|
Janick J, Paull RE (2008). The encyclopedia of fruit and nuts. CABI. |
|
|
Jaswir I, Shahidan N, Othman R, Has-Yun Hashim YZ, Octavianti F, Bin Salleh MN (2014). Effects of season and storage period on accumulation of individual carotenoids in pumpkin ?esh (Cucurbita moschata). Journal of Oleo Science 63(8):761-767. |
|
|
Jeffrey C (1990). Biology and Utilization of the Cucurbitaceae. Ithaca, Etats-Unis: Cornel University Press pp. 3-9. |
|
|
KEW (2021). Available online: |
|
|
Kiramana JK, Isutsa DK (2017). First detailed morphological characterisation of qualitative traits of extensive naturalized pumpkin germplasm in Kenya. International Journal for Digital Society 6(7):500-525. |
|
|
Kulczy?ski B, Gramza-Micha?owska A (2019a). The profile of carotenoids and other bioactive molecules in various pumpkin fruits (Cucurbita maxima Duchesne) cultivars. Molecules 24(18):3212-3224. |
|
|
Kulczy?ski B, Gramza-Micha?owska A (2019b). The profile of secondary metabolites and other bioactive compounds in Cucurbita pepo L. and Cucurbita moschata pumpkin cultivars. Molecules 24(16):2945-2967. |
|
|
Lira R, AndrèsTC, Nee MC (1995). In Systematic and Ecogeographic Studies on Crop Genepools. Estudios taxono'micos y ecogeograficos de las Cucurbitaceae latino-americanas de importancia economica: Cucurbita, Sechium, Sicana y Cyclanthera. Edited by Lira R, México DF and Rome, Italia: International Plant Genetic Resources Institute, Instituto deo Biologia pp. 1-115. |
|
|
Lozoya X (1994). Two decades of Mexican ethnobotany and research in plant drugs. Ethnobotany and the Search for New Drugs pp. 130-152. |
|
|
Mbogne JT, Youmbi E, Ibouraïman B, Ntsefong GN (2015). Agromorphological, chemical and biochemical characterization of pumpkin (Cucurbita maxima and Cucurbita moschata,Cucurbitaceae) morphotypes cultivated in Cameroun. Research in Plant Sciences 3(1):12-17. |
|
|
Mendlinger S, Chweya J, Benzioni A, Seme A, Ventura M, Lungaho C, Okoko V (1992). Collections, evaluations and breeding of African edible vegetables. Annual report: BeerSheva, Israel pp. 25-92. |
|
|
Merrick LC (1990). Systematics and evolution of a domesticated squash, Cucurbita argyrosperma, and its wild and weedy relatives. In Biology and utilization of the Cucurbitaceae. Ithaca, USA: Cornell University Press pp. 77-95. |
|
|
Misci C, Taskin E, Dall'Asta M, Fontanella MC, Bandini F, Imathiu S, Sila D, Bertuzzi, T, Cocconcelli PS, Puglisi E (2021). Fermentation as a tool for increasing food security and nutritional quality of indigenous African leafy vegetables: the case of Cucurbita sp. Food Microbiology 99:103820-103831. |
|
|
Montesano D, Blasi F, Simonetti MS, Santini A, Cossignani L (2018). Chemical and nutritional characterization of seed oil from Cucurbita maxima L.(var. Berrettina) pumpkin. Foods 7(3):30-44. |
|
|
Nawirska A, Figiel A, Kucharska AZ, Sokó?-??towska A, Biesiada A (2009). Drying kinetics and quality parameters of pumpkin slices dehydrated using different methods. Journal of Food Engineering 94(1):14-20. |
|
|
Ndoro OF, Madakadze RM, Kageler S, Mashingaidze AB (2007). Indigenous knowledge of the traditional vegetable pumpkin (Cucurbita maxima/moschata) from Zimbabwe. African Journal of Agricultural Research 2(12):649-655. |
|
|
Nepi M, Pacini E (1993). Pollination, pollen viability and pistil receptivity in Cucurbita pepo. Annals of Botany 72(6):527-536. |
|
|
Noguera FA (2002). Historia natural de Chamela, UNAM. pp. 35-80. |
|
|
Norshazila S, Irwandi J, Othman R, Zuhanis Y (2014). Carotenoid content in dierent locality of pumpkin (Cucurbita moschata) in Malaysia. International Journal of Pharmacy and Pharmaceutical Sciences 6(3):29-32. |
|
|
Ntuli NR, Madakadze RM, Zobolo AM (2017). Variation in morphology and yield traits of Cucurbita landraces in northern KwaZulu-Natal, South Africa. South African Journal of Plant and Soil 34(5):389-397. |
|
|
OECD (2016). Squashes, pumkins, zucchinis and gourds (Cucurbita species). Safety Assessment of Transgenic Organisms in the Environment. Paris, France: OECD Publishing pp. 84-130. |
|
|
PROTA (2018). Available online: |
|
|
Provvidenti R (1990). Viral diseases and genetic sources of resistance in Cucurbita species. Biology and Utilization of the Cucurbitaceae. Ithaca, USA: Cornell University Press pp. 427-435. |
|
|
Rahman MM, Juahir H, Islam MH, Khandaker MM, Ariff TM, Nik WMN (2019). Prophetic vegetable Pumpkin, Its impressive health benefits and total analysis. Bioscience Research 16(4):3987-3999. |
|
|
Rakotovao AM (1999). Contribution to the valorisation of pumpkins and apples in marmalade. Engineer Thesis, Antananarivo University, Madagascar. |
|
|
Robinson RW, Decker-Walters D (1997). Cucurbits. Cab international. pp. 5-20. |
|
|
Roura S, Del Valle C, Aguero L, Davidovich L (2007). Changes in apparent viscosity and vitamin C retention during thermal treatment of butternut squash (Cucurbita moschata Duch) pulp: efect of ripening stage. Journal of Food Quality 30(4):538-551. |
|
|
Salehi B, Sharifi-Rad J, Capanoglu E, Adrar N, Catalkaya G, Shaheen S, Jaffer M, Giri L, Suyal R, Jugran AK (2019). Cucurbita plants: From farm to industry. Applied Sciences 9(16):3387-3408. |
|
|
Salifou A, Alidou C, Tchobo FP, Soumanou MM (2015). Caractérisation physique et valorisation des amandes de trois espèces de courge (Citrulus lanatus, Lagenaria siceraria et Cucumeropsis edulus) produites au Bénin. BRAB 78:37-43. |
|
|
Sanjur OI, Piperno DR, Andres TC, Wessel-Beaver L (2002). Phylogenetic relationships among domesticated and wild species of Cucurbita (Cucurbitaceae) inferred from a mitochondrial gene: Implications for crop plant evolution and areas of origin. Proceedings of the National Academy of Sciences 99(1):535-540. |
|
|
Schabort JC (1978). Cucurbitacin 19-hydroxylase in Cucurbita maxima. Phytochemistry 17(6):1062-1064. |
|
|
SFC (2016). French Society of Cucurbitaceae. (Accessed 8th August 2020). Available online: |
|
|
Sharma B, Lal T (1998). Improvement and cultivation: Cucurbita and Benincasa. In: Nayar, N.M. & More, T.A. (Editors). Cucurbits. Science Publishers Inc., Enfield NH, United States pp. 155-168. |
|
|
Souley ML, Tchokanaka A, Ousmane S, Bello CA (2018). Fiche technico-économique pour la culture de la courge Bagobira. Chambre Régionale d'Agriculture de Zinder (Niger) 5:1-3. |
|
|
Stephenson AG, Devlin B, Horton JB (1988). The effects of seed number and prior fruit dominance on the pattern of fruit production in Cucurbits pepo (Zucchini squash). Annals of Botany 62(6):653-661. |
|
|
US Environmental Protection Agency (1999). Notice of filing a pesticide petition to establish a tolerance for a certain pesticide chemical in or on food. Federal Register 64(195):47-78. |
|
|
Vidal MdG, Jong DD, Wien HC, Morse RA (2010). Pollination and fruit set in pumpkin (Cucurbita pepo) by honey bees. Brazilian Journal of Botany 33(1):106-113. |
|
|
Vinayashree S, Vasu P (2021). Biochemical, nutritional and functional properties of protein isolate and fractions from pumpkin (Cucurbita moschata var. Kashi Harit) seeds. Food Chemistry 340:128177-128186. |
|
|
Weeden N (1984). Isozyme studies indicate that the genus Cucurbita is an ancient tetraploid; Cucurbit genetics cooperative pp. 15-50. |
|
|
Whitaker TW, Davis GN (1962). Cucurbits : Botany, cultivation, and utilization. New York, Etats-Unis: Interscience Publishers pp. 162-170. |
|
|
Whitaker TW (1974). Cucurbita, handbook of genetics. Boston, USA: Springer: pp. 135-144. |
|
|
Whitaker T, Robinson R (1986). Squash breeding. Breeding vegetable crops 584:209-242. |
|
|
Wilson HD, Doebley J, Duvall M (1992). Chloroplast DNA diversity among wild and cultivated members of Cucurbita (Cucurbitaceae). Theoretical and Applied Genetics 84(7-8):859-865. |
|
|
Yadav M, Jain S, Tomar R, Prasad G, Yadav H (2010). Medicinal and biological potential of pumpkin: An updated review. Nutrition Research Reviews 23(2):184-190. |
|
|
Zargar FA, Kumar S, Bhat ZF, Kumar P (2014). Effect of pumpkin on the quality characteristics and storage quality of aerobically packaged chicken sausages. SpringerPlus 3(1):1-10. |
|
|
Zhou CL, Mi L, Hu XY, Zhu BH (2017). Evaluation of three pumpkin species: correlation with physicochemical, antioxidant properties and classi?cation using SPME-GC-MS and E-nose methods. Journal of Food Science and Technology 54(10):3118-3131. |
|
|
Zomlefer WB (1994). Guide to flowering plant families. London, Royaume-Uni: University of North Carolina Press pp. 50-80. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0