ABSTRACT
Potassium nutrition has been used in controlling fungal and bacterial diseases of plants. However, this has not yet been tested on control of Fusarium (Fusarium oxysporum f.sp. cubense -Foc), a major fungal disease constraining apple banana production. This study was set up with a general objective to investigate the effects of potassium on tolerance to Fusarium wilt in apple bananas using chlorophyll content, stomatal conductance and severity of Fusarium wilt symptoms as indicators. A screen house pot experiment was set up using tissue culture plantlets of apple bananas cv Ndiizi. Treatments comprised of different sources of potassium (Manure, inorganic K and potassium solubilizing bacteria -KSB) in a full factorial combination. Manure and Muriate of potash (MOP) were applied at rates equivalent to 150 kg K/ha and KSB (Frateuria auranta) at a rate of 1 l/ha. Fusarium wilt was inoculated in half the pots at 8 weeks after planting. Measurements on chlorophyll content and stomatal conductance as well as growth parameters were taken on weekly basis from the time of inoculation. Fusarium wilt significantly reduced stomatal conductance and chlorophyll content of the plants. Application of integrated potassium management (KSB x MOP x Manure) significantly reduced Fusarium wilt symptom expression.
Key words: Chlorophyll content, stomatal conductance, Fusarium oxysporum sp. Cubense, manure, potassium.
Banana as a crop in Uganda is threatened by a combination of abiotic (soil nutrient, moisture or drought stress) and biotic (pests and diseases) constraints (Tinzaara et al., 2018). Potassium was highlighted by several authors (Taulya, 2013; Nyombi, 2013; Fratoni et al., 2017) as the most limiting nutrient in bananas. Regardless of cultivars, banana yields are significantly affected by banana weevil, nematodes, banana xanthomonas wilt, black sigatoka and banana bunchy top virus diseases (Blomme et al., 2017).
In addition to the general common diseases, apple bananas are affected by Fusarium wilt, Fusarium oxysporum spp. cubense (Foc). Foc exists as different pathogenic races such as Foc races 1, 2, 3 and 4 classified according to their ability to cause disease in different banana cultivars (Ploetz, 2015; Siamka and Zheng, 2018). Foc 1 is responsible for epidemics in apple bananas (AAB), kisubi (ABB), kayinja (ABB) and bogoya (Gros Michel) varieties, Foc 2 in cooking banana type bluggoe, Foc 3 in Heliconia spp. and Foc 4 in Cavendish cultivars (Viljoen et al., 2017). Fusarium wilt is a soil borne fungal disease and has remained one of the most important diseases in the country having wiped out susceptible cultivars on farms (PARM, 2016). It is a major factor that has lowered plantation longevity mainly for dessert bananas and production cannot meet the demands of the growing population due to up to 100% yield loss on some farms (Viljoen and Mostert, 2017).
The characteristic symptom of banana Fusarium wilt is chlorosis (yellowing) of older banana leaves (Ploetz, 2015). The yellowing most often begins at the leaf margins, from where it progresses to the leaf midrib. A second external symptom often linked to banana Fusarium wilt is the splitting of the pseudostem. The splitting is caused by the inability of dead leaf bases to expand as the plant grows, thus splitting open as the inner pseudostem swells (Viljoen et al., 2017).
Fusarium wilt induces characteristic internal symptoms in the rhizome and pseudostem, irrespective of the affected variety. When sliced longitudinally, affected pseudostems present reddish- to dark-brown lesions inside the leaf bases that form the pseudostem. Early infection in banana pseudostems is often yellow to dark red and limited to the xylem vessels. Diseased plants will have a characteristic yellow to dark-brown discoloration of the inner rhizome, which usually starts at the edges and progresses inwards (Ploetz, 2015; Viljoen et al., 2017). Plants affected with Fusarium wilt also have reduced plant biomass, leaf net photosynthetic rates and stomatal conductance (Wang et al., 2015). Fusarium wilt fungus blocks the xylem tissues thereby blocking movement of water and nutrients in the plant. Banana plants respond to constrained water uptake through closure of stomata (hence reduced stomatal conductance) and increased breakdown of chlorophyll thereby causing yellowing or chlorosis of leaves (Chávez-Arias et al., 2019).
Traditional methods such as chemical control using soil fumigants and fungicides have been tried to control the disease but are not economical and environmentally friendly (Siamka and Zheng, 2018). Other options tried were crop rotation using Chinese leek- Allium tuberosum (Zhang et al., 2013), pineapples (Wang et al., 2015) and ground cover (Deltour et al., 2017), biological control using Pseudomonas spp. and using resistant cultivars (Dale et al., 2017). Although resistant cultivars are an option, these have not yet been a success [in Uganda] as the banana crop improvement process takes a long time to achieve a locally acceptable resistant cultivar (Viljoen et al., 2017).
Plants have developed wide mechanisms to resist a variety of stressed conditions (Wang et al., 2013). Increasing evidence suggests that mineral nutrients play a critical role in plant stress resistance (Marschner, 2012). Out of all mineral nutrients, potassium plays a critical role in plant growth and metabolism and greatly contributes to the survival of plants under biotic and abiotic stresses (Taulya, 2013). Potassium-deficient plants tend to be more susceptible to infections than those with adequate supply (Wang et al., 2013). Reddy (2017) observed that application of potassium reduced susceptibility of bananas to Xanthomonas wilt. With adequate potassium, bananas increase in vigor and disease resistance (Silva et al., 2014). There has been a controversy of K-increasing resistance to fungal diseases in different plants (Andersen et al., 2018) but none of these studies has been done to evaluate the effect of K on Fusarium wilt tolerance in apple banana (cv.Ndiizi). Therefore, this study was set up to investigate the effects of integrated potassium management using inorganic fertilizer, manure and potassium solubilizing bacteria on apple banana tolerance to Fusarium wilt.
General objective
Evaluate the effects of integrated potassium management on Fusarium wilt tolerance in apple bananas.
Specific objectives
i) To determine the interactive effect of K on stomatal conductance and chlorophyll degradation in Foc affected apple bananas.
ii) To determine the incidence and severity of Foc 1 as influenced by mineral K or manure in apple bananas.
iii) To determine the effect of Foc 1 on the growth parameters of apple bananas.
Study area description
The study was carried out at Kawanda National Agricultural Research laboratories (NARL), 00º25’N and 32º31’E at an elevation of 1156 m above sea level. The area has two rainy seasons (March to May and September to December), with annual rainfall of 1250 mm. Daily minimum temperature is on average 15.3ºC and maximum temperature is on average 27.3ºC while the relative humidity is about 76.3% (Nyombi et al., 2009).
Experimental design
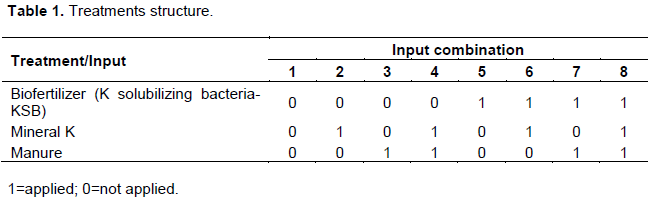
The experiment was set up in pots containing steam sterilized soil at 90ºC for 2 h in a screen house. A randomised complete design was used with full factorial combination of K-solubilizing biofertilizer, mineral K fertilizer and manure, in triplicate (Table 1).
Mineral K was applied using muriate of potash fertilizer-MOP containing 60% K2O at the rate of 150 kg K ha-1. MOP was procured from Githingi Elgon fertilizers company. Dry animal manure was applied at a rate that is equivalent to 150 kg K ha-1. Well composted cattle manure consisting of dung, urine and straw remains was used (Organic matter 5.88%, Nitrogen-3.0cmol/kg, potassium-0.21cmol/kg, Phosphorous-170ppm). The inputs were all incorporated at planting except for liquid biofertilizer containing Frateuria auranta (1x1013 CFU/ml) as a KSB which was sprayed in the soil at a rate of 1ltr/ha a week after application of muriate of potash. KinyPotash a liquid biofertiliser containing Frateuria was procured from Kinyara Sugar limited.
Pots of 10-liter capacity were perforated at the bottom and a 5-cm thick layer of sand added to permit rapid drainage. Each pot was then filled with 10 kg (dry weight basis) of soil and a composite soil sample was taken per treatment per replicate routine analysis of nutrient contents before application of the treatment inputs. The inputs were mixed with the soil (according to the treatment) before adding the soil to the pots to ensure uniformity of distribution.
Each pot was planted with a hardened tissue culture dessert apple banana (cv. Ndiizi) sucker and watered adequately. The plantlets were left to establish for 2 months before inoculating them with Foc race 1. Foc race1 was cultured on Potato dextrose media (Viljoen and Mostert, 2017). The grown fungus was then inoculated on millet seeds, and 25 g millet seeds containing spores x105 /ml was used to inoculate the soil. The inoculum was first tested for pathogenicity before application in the main experiment. Half the pots of each treatment were inoculated with Fusarium wilt and the other half was left uninoculated.
Data collection
(i) Effect of Foc on stomatal conductance and chlorophyll conten.
Stomatal conductance in mmol m?² s?¹ (Campbell and Norman, 1998) was measured weekly from the day of inoculation. This was done using a hand-held Leaf Porometer SC-1 from DecagonTM devices. Measurements were done at consistent time intervals between 9am to mid-day. Measurements were done on the 3rd youngest fully expanded leaf for two plants from the uninoculated group and 2 plants from the inoculated group in each nutrient input treatment per replication. For chlorophyll content, the same third youngest fully expanded leaf was considered for measurements. Chlorophyll meter Spad- 502 plus (from Konica MinoltaTM) was used. Readings were taken on five different points of the leaf lamina (avoiding the edges) and their average was recorded as the chlorophyll content reading of the entire leaf. Chlorophyll content was recorded in µmol m-2 (Süß et al., 2015)
(ii) Incidence and severity of Fusarium wilt on apple bananas
Plantlets were monitored on weekly basis for Foc exterior symptoms (yellowing of leaves). The number of plants showing symptoms was recorded per treatment. Fusarium wilt incidence was rated visually using the following scale: 0 = no wilt symptoms; 1 = 1 leaf wilted; 2 = 2 to 3 leaves wilted; 3 = 4 leaves wilted; 4 = all leaves wilted; and 5 = plant dead as described by Viljoen et al. (2017).
After a 4-months period, plants which were inoculated with Fusarium per treatment, per replicate were carefully uprooted from the pot and cut at the base to observe for internal symptoms described by Ploetz (2015). The corm dislocation was scored using the rhizome discoloration index (RDI) described by Viljoen et al., (2017). Rhizome discoloration scores were; 1-no internal symptoms, 2 -few internal spots, 3 -<1/3 discolored, 4 -1/3-2/3 discolored, 5 - >2/3 discolored and 6- entire inner rhizome discolored.
Disease severity was calculated according to the equation by Viljoen et al. (2017) Disease severity (%) = ∑ [(number of plants in disease scale category) x (specific disease scale category) / (total number of plants) x (maximum disease scale category)] x 100.
(iii) Growth monitoring
Growth was monitored by measuring different growth parameters (Füzy et al., 2019). Data were collected on pseudostem height (length from collar region to the vertex of the youngest unfurled pair of fully leaves measured using a metre ruler), pseudostem girth at base (measured using a measuring tape), length of 3rd functional leaf, with at the widest point of lamina were taken on weekly basis from time of inoculation on uninoculated and treated (inoculated) plants.
Data analysis
Data on disease incidence and severity, chlorophyll content, stomatal conductance and growth monitoring were subjected to analysis of variance and means separated by least significant difference (LSD) in GenStatTM at 95% confidence level.
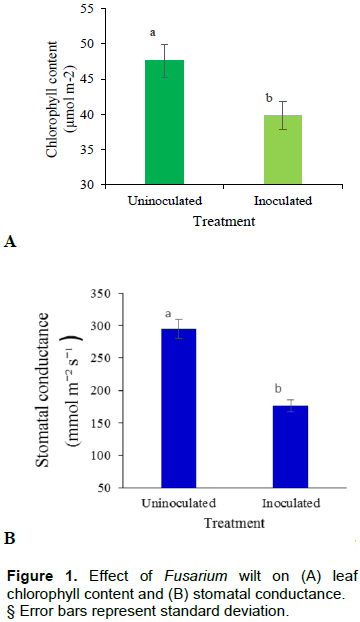
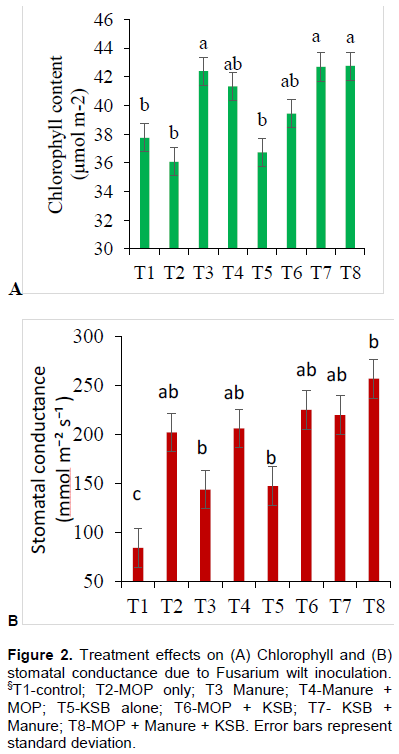
Uninoculated plants recorded significantly (p<0.001) higher chlorophyll content (µmol m-2) than those plants that were inoculated with Fusarium wilt. The same observation was noted with stomatal conductance; where the uninoculated plants had significantly higher stomatal conductance than the inoculated ones (Figure 1A and B). It was generally observed that, inoculating the plants with Fusarium wilt significantly reduced chlorophyll content and stomatal conductance across all treatments. Those treatments that receive manure as K source (sole or in combination) recorded, higher chlorophyll content than those treatment without. On the contrary, stomatal conductance was low in those treatments which received manure but was highest in MOP or KSB treatments (sole or combinations-T2, T4, T6, T8) Figure 2A and B.
There was a gradual decrease in both chlorophyll content and stomatal conductance due to effect of Fusarium wilt over time. Treatments T6 (MOP x KSB), T1 (no input) and T2 (MOP alone) were among the treatments that recorded the least readings by the 8th week after inoculation, while T8 (KSB x MOP x Manure) resiliently recorded higher reading (Figure 3A). Stomatal conductance also was negatively affected by Fusarium wilt inoculation as it gradually reduced over time (Figure 3B). As already noted above, same treatments with manure (sole or combinations) were the highly affected except for T8 (MOP x Manure x KSB).
Application of potassium significantly affected the expression of Fusarium wilt whether externally or basing on internal symptoms. Treatment T1 (no input) recorded significantly high (p<0.001) levels of corm discoloration (rotting) to all treatments. Out of a score of 6, T1 recorded a mean of 5.3 score which translated to about 88% of the corm being discolored. This was followed by T5 (KSB alone) which was also significant to all other treatments while treatment T8 (KSB x Manure x MOP), recorded the lowest corm discoloration score.
All plants in treatment T1 (no input) which were inoculated with Fusarium wilt exhibited pseudostem splitting (100%) which was significantly high (p<0.001) compared with treatments T7 (Manure x KSB) and T8 (Manure x KSB x MOP). A mean of 0.2 score equivalent to 20% of the plants in treatment T8 recorded pseudostem splitting. Similarly, T8 significantly supressed the expression of Fusarium wilt through wilting of leaves with only about 2 leaves per plant wilted w compared to 4 leaves per plant wilted in T1 (Table 2). Consequently, Fusarium wilt severity was highest in T1 followed by T5 while T8 and T4 (MOP x Manure) recorded the lowest (Figure 4).
Inoculation with Fusarium wilt on apple bananas significantly affected growth. This was manifested by significant reduction in girth, pseudostem height and leaf length of plants which were inoculated against the uninoculated ones. However, there were no significant differences observed on leaf width between the inoculated plants and the uninoculated.
Inoculating plants with Fusarium wilt significantly reduced chlorophyll content and stomatal conductance as compared with uninoculated plants (Figure 1A and B). There was a 16.1 % reduction in chlorophyll content caused by the effect of Fusarium wilt. On the other hand, stomatal conductance reduced by 40.2% between the inoculated and the uninoculated plants. This could be because Fusarium wilt causes a disruption in water and nutrient uptake which causes wilting; a situation that was not the case in healthy plants (Wang et al., 2015). Due to this reduced uptake of water and nutrients, banana plants responded to the constraint through closure of stomatal which was manifested in reduced stomatal conductance. In the same regard, reduced water uptake increased breakdown of chlorophyll thereby causing yellowing or chlorosis of leaves which was observed as reduced chlorophyll content (Chávez-Arias et al., 2019).
Chlorophyll contents were generally high in treatments where manure was applied (T3-Manure alone, T4-MOPx Manure, T7-KSBxManure and T8-KSBxMOPxManure, Figure 2A). This could be because application of manure supplied nitrogen which is a building block of the chlorophyll molecule (Kotapalli et al., 2017). Same treatments exposed to Fusarium wilt had significantly reduced chlorophyll content. Application of potassium solubilizing bacteria did not have any effect on chlorophyll content. However, with stomatal conductance, treatments which had application of potassium recorded higher readings than those where manure was applied (Figure 2B). Potassium being important in regulating the opening and closing of stomata, and therefore regulates carbon dioxide uptake (Kotapalli et al., 2017).
Higher reading of stomatal conductance where potassium was applied could also imply that plants were able to conserve more water in the tissues even in presence of disease stress (Galeano et al., 2019). K helps plants resist disease organism invasion by strengthening cellwall structure (Marscher, 2012). Plants with adequate K have thicker cellwalls than the deficient ones. This makes it harder for disease organisms to penetrate plant cells and establish an infection. In treatments where potassium levels were low, the cellwall structure could have been leaky hence nutrients out of the cell to the apoplast caused a fertile ground for the fungus to thrive (Galeano et al., 2019).
It was observed that chlorophyll content and stomatal conductance kept reducing in all treatments over time after inoculation with Fusarium wilt (Figure 3A and B).
Application of manure, Potassium solubilizing bacteria and Muriate of potash (T8) recorded higher chlorophyll contents and stomatal conductance than treatments where single nutrient source was used (T2, T5 and T3). This means that the supply of an individual nutrient is important, but it is also crucial for balanced supply of nutrients for improving plant health and development under varying environmental conditions (Li et al., 2018). The results of this study also agree with (Yanmei et al., 2018), potassium nutrition through use of biofertilizer was reported to reduce Fusarium wilt in China, although their study looked at using Bacilllus spp not Frateuria spp as a potassium solubilizing bacteria.
Potassium application irrespective of the source (MOP, Manure or KSB and their combinations) generally supressed the expression of Fusarium wilt in apple bananas. Although disease was able to express in all treatments, expression levels significantly varied across treatments (Table 2). This could have been because of improved nutrition provided by manure, MOP and KSB, and that were more vigorous to counter the disease effects (Dita et al., 2018). Application of nutrients, irrespective of the source (MOP or KSB or Manure or a combination) significantly suppressed wilting. Soil amendments by organic and mineral fertilizers can lead to bene?cial interactions between macro- and micro-nutrients; thus, they provide the optimum need for micronutrient requirements (René et al., 2017). Fertilizers have been reported to improve crop yield and quality and play a key role in the maintenance of soil productivity (Bayu, 2020).
Overall, treatment T1 (control) recorded the highest disease severity of over 60% followed by T5 (KSB alone) with 55% and lowest was obtained in T8 with about 22% severity level Figure (4). Therefore, it can be deduced that T8 (MOP x Manure x KSB) controlled about 38% of the Fusarium wilt in apple bananas. These results recorded lower control of Fusarium wilt using fertilizer when compared to the findings of Yanmei et al. (2018) where 60% of Fusarium wilt was suppressed by use of biofertilizer (Bacillus spp.) and 90% was suppressed by using KCl + biofertilizer.
There was a general reduction in disease severity with input combinations with manure (Figure 4). This could be attributed to the fact that manure supplies not only nitrogen which is a major nutrient for plant growth but could also have supplied beneficial microorganisms (such as the Bacillus spp. and Pseudomonas spp.) that are have been documented to supress Fusarium wilt in the soil (Köberl et al., 2017; Dita et al., 2018) recommended use of 5 t/ha of manure to reduce Fusarium wilt in soils. This is because; application of inorganic N to the soil through use of fertilizer (urea or ammonium nitrite) has a tendency to lower the pH of the soil which is associated with increasing incidences of Fusarium wilt. Low response to KSB in treatment T5 (KSB alone) could be attributed to the fact that the soil was sterilized and had low carbon source which acts as a source of food for potassium solubilizing bacteria (Vannier et al., 2019).
It was observed that Fusarium wilt significantly reduced pseudostem height, girth and leaf length (Table 3). Across all treatments, growth was observed to reduce 3 weeks after inoculation with Fusarium wilt. However, application of potassium mitigated the loss in growth vigor of the plants due to the disease. Similar findings were recorded by Reddy (2017), where potassium increased the resistance of plants to xanthomonas wilt.
Inoculation of apple banana with Fusarium wilt significantly reduced chlorophyll content and stomatal conductance. Treatments where manure was applied had higher chlorophyll content but lower stomatal conductance. A combination of manure and MOP (T4), KSB and Manure and MOP, Manure and KSB gave increased stomatal conductance and chlorophyll content than single input source.
As with other mineral nutrients, appropriate management practices of potassium relating to application can improve the uptake of potassium by plants and consequently increase crop production, while reducing disease incidence. The results of this study indicated that integrated application of potassium using MOP, Manure and KSB increased the capacity of plants to withstand the effects of Fusarium wilt. We recommend that these results be a benchmark to scale out the use of manure, MOP and potassium solubilizing bacteria on soils to study the long term effect of these inputs on Fusarium wilt tolerance in field grown apple bananas.
The authors have not declared any conflict of interests.
REFERENCES
|
Andersen EJ, Ali S, Byamukama E, Yen Y, Madhav P (2018). Disease Resistance Mechanisms in Plants. Department of Biology and Microbiology, South Dakota State University, Brookings, 57007 SD, USA.
Crossref
|
|
|
|
Bayu T (2020). Review on contribution of integrated soil fertility management for climate change mitigation and agricultural sustainability. Cogent Environmental Science 6(1):1823631.
Crossref
|
|
|
|
|
Blomme G, Dita M, Jacobsen KS, Pérez Vicente L, Molina A, Ocimati W, Poussier S, Prior P (2017). Bacterial diseases of bananas and enset: current state of knowledge and integrated approaches toward sustainable management. Frontiers in Plant Science 8:1290.
Crossref
|
|
|
|
|
Campbell GS, Norman JM (1998). An Introduction to Environmental Biophysics. Springer-Verlag: New York, Heidelberg, Berlin. Second Edition pp. 90-92.
|
|
|
|
|
Chávez-Arias CC, Gómez-Caro S, Restrepo-Díaz H (2019). Physiological, Biochemical and Chlorophyll Fluorescence Parameters of Physalis Peruviana L. Seedlings Exposed to Different Short-Term Waterlogging Periods and Fusarium Wilt Infection. Agronomy 9(5):213.
Crossref
|
|
|
|
|
Dale J, Paul A, Khanna KY, Harding R (2017). Transgenic Cavendish banana with resistance to Fusarium wilt tropical race 4. Nature Communications 8:1496.
Crossref
|
|
|
|
|
Deltour P, França SC, Pereira OL, Cardoso I, De Neve S, Debode J, Monica H (2017). Disease suppressiveness to Fusarium wilt of banana in an agroforestry system: influence of soil characteristics and plant community. Agriculture, Ecosystems and Environment 239:173-181.
Crossref
|
|
|
|
|
Dita M, Barquuero M, Heck D, Mizubuti SGE, Staver PC (2018). Fusarium wilt of bananas: current knowledge on epidemiology and research need towards disease management. Frontiers in Plant Science 9:1468.
Crossref
|
|
|
|
|
Fratoni M, Moreira, Adonis, Cardoso, Moraes, Almeida L, Pereira J (2017). Effect of Nitrogen and Potassium Fertilization on Banana Plants Cultivated in the Humid Tropical Amazon. Communications in Soil Science and Plant Analysis.
Crossref
|
|
|
|
|
Füzy A, Kovács R, Cseresnyés I, Parádi I, Szili Kovács T, Kelemen B, Rajkai K, Takács T (2019). Selection of plant physiological parameters to detect stress effects in pot experiments using principal component analysis. Acta Physiologiae Plantarum 41:56.
Crossref
|
|
|
|
|
Galeano E, Vasconcelos TS, Novais de Oliveira P, Carrer H (2019). Physiological and molecular responses to drought stress in teak (Tectona grandis L.f.). PLoS ONE 14(9):e0221571.
Crossref
|
|
|
|
|
Köberl M, Dita M, Martinuz A, Staver C, Berg G (2017). Members of Gammaproteobacteria as indicator species of healthy banana plants on Fusarium wilt- infested fields in Central America. Scientific Reports 7:45318.
Crossref
|
|
|
|
|
Li Y, He N, Hou J, Xu L, Liu C, Zhang J, Wang Q, Zhang X, Wu X (2018). Factors Influencing Leaf Chlorophyll Content in Natural Forests at the Biome Scale. Frontiers in Ecology and Evolution 6:64.
Crossref
|
|
|
|
|
Marschner P (2012). Mineral nutrition for higher plants. Third edition, ISBN: 978-0-12-384905-2.
|
|
|
|
|
Nyombi K, van Asten PJA, Leffelaar PA, Corbeels M, Kaizzi CK, Giller KE (2009). Allometric growth relationships of East Africa highland bananas (Musa AAA-EAHB) cv. Kisansa and Mbwazirume. Annals of Applied Biology 155(3):403-418.
Crossref
|
|
|
|
|
Nyombi K (2013). Towards sustainable highland banana production in uganda:opportunities and challenges. African Journal of Food, Agriculture, Nutrition and Development 13(2):7544-7561.
|
|
|
|
|
Platform for Agricultural Risk Management (PARM) (2016). Crop pests and Disease management in Uganda-Status and Investment needs. CABI.
|
|
|
|
|
Ploetz R (2015). Fusarium wilt of Bananas. Phytopathology Review; Phytopathology 105(12):1512-1521.
Crossref
|
|
|
|
|
Reddy PP (2017). Fertilizer Management. In: Agro-ecological Approaches to Pest Management for Sustainable Agriculture. Springer, Singapore.
Crossref
|
|
|
|
|
René PJ, Rietra J, Heinen M, Chistian O, Dimkpa , Prem , Bindraban (2017). Effects of Nutrient Antagonism and Synergism on Yield and Fertilizer Use Efficiency. Communications in Soil Science and Plant Analysis 48(16).
Crossref
|
|
|
|
|
Siamka SB, Zheng S (2018). Banana Fusarium wilt (Fusarium oxysporium f.sp cubense) control and resistance, in the context of developing wilt resistant bananas within sustainable production systems. Horticultural Plant Journal 4(5):208-218.
Crossref
|
|
|
|
|
Silva DRG, Spechar CR, Marchi G, Soares DA, Cancellier ED, Martins ES (2014). Yield, nutrient uptake and potassium use efficiency in rice fertilized with crushed rocks. African Journal of Agricultural Research 9(4):455-464.
Crossref
|
|
|
|
|
Süß A, Danner M, Obster C, Locherer M, Hank T, Richter K (2015). Measuring Leaf Chlorophyll Content with the Konica Minolta SPAD-502 Plus - Theory, Measurement, Problems, Interpretation. EnMAP Field Guides Technical Report, GFZ Data Services.
|
|
|
|
|
Taulya G (2013). East African highland bananas (Musa spp. AAA-EA) 'worry' more about potassium deficiency than drought stress. Field Crops Research 151:45-55.
Crossref
|
|
|
|
|
Tinzaara W, Ocimati W, Kikulwe E, Otieno G, Stoian D, Blomme G (2018). Challenges and opportunities for smallholders in banana value chains. Chapter 10.
Crossref
|
|
|
|
|
Vannier N, Agler M, Hacquard S (2019). Microbiota-mediated disease resistance in plants. PLOS Pathogens 15(6):e1007740.
Crossref
|
|
|
|
|
Viljoen A, Mostert G (2017). Green house inoculation of banana plantlets for fusarium wilt resistance.
|
|
|
|
|
Viljoen A, Mahuku G, Massawe C, Ssali RT, Kimunye J, Mostert G, Ndayihanzamaso P, Coyne DL (2017). Banana Pests and diseases. Field guide diagnostics and data collection. International Institute of Tropical Agriculture (IITA), Ibadan, Nigeria.
|
|
|
|
|
Wang B, Li R, Ruan Y, Ou Y, Zhao Y, Shen Q (2015). Pineapple-banana rotation reduced the amount of Fusarium oxysporum more than maize-banana rotation mainly through modulating fungal communities. Soil Biology and Biochemistry 86:77-86.
Crossref
|
|
|
|
|
Wang M, Zheng Q, Shen Q, Guo S (2013). The Critical Role of Potassium in Plant Stress Response. International Journal of Molecular Sciences 14(4):7370-7390.
Crossref
|
|
|
|
|
Yanmei QZ, Zhinghong Z, Chunmao L (2018). Effects of the combination of biofertilizer and potassium fertilizer on the control of banana Fusarium wilt. Journal of Plant Protection 43(3).
|
|
|
|
|
Zhang H, Mallik A, Zeng RS (2013). Control of panama disease of banana by rotating and intercropping with Chinese chive (Allium tuberosum Rottler): role of plant volatiles. Journal of Chemical Ecology 39(2):243-252.
Crossref
|
|