ABSTRACT
A good planting medium is required for raising tree seedlings in the nursery, however Guinea savanna soils are generally poor in nitrogen and organic matter. This poses a challenge in accessing fertile soil for tree nurseries in Northern Ghana. The experiment was conducted in the Nyankpala campus to explore the potential of plant biochar as growth media for raising tree seedlings. Growth media prepared from six different biochar formulations (Groundnut Husk Biochar; Rice Husk Biochar; Wood Biochar; Groundnut Husk Biochar + Soil; Rice Husk Biochar + Soil; Wood Biochar + Soil) and control (untreated topsoil) were each replicated in three seed boxes. Seed boxes were arranged in a Completely Randomized Design with 50 Khaya senegalensis seeds sown in each box. Percentage seed emergence did not vary significantly between treatments (p > 0.05) although Groundnut Husk Biochar recorded a marginally higher emergence (65.71%). Similarly, Groundnut Husk Biochar recorded a significantly higher plant height (10.23 cm) in the second week after planting (p < 0.05) as well as mean number of leaves (6.02) in the sixth week after planting (p < 0.05). In general, Groundnut Husk Biochar had the greatest effect on initial growth performance of K. senegalensis and could therefore be explored as a growth medium for raising tree seedlings in Northern Ghana.
Key words: Biochar, growth media, Khaya senegalensis, plant height, soil.
Biochar is a carbon-rich product produced through thermal decomposition of biomass in a closed container with limited or no supply of oxygen at a temperature below 700°C (Nartey and Zhao, 2014; Lehmann, 2007). Biochar may be produced from a wide range of organic feedstock materials such as plant residue, wood biomass, and organic waste from municipal and industrial sources (BÅ™endová et al., 2012). But, the quality and quantity of biochar produced can be influenced by feedstock properties (Zhao et al., 2013) and conditions of the carbonization process (Steiner, 2016; Gaskin et al., 2008). For instance, higher lignin content of feedstock is associated with higher biochar yield. Biochar is well known for its potential contribution to climate change mitigation due to the high residence time of the carbon in biochar (Hammes et al., 2009). This makes it a good reservoir of carbon lost in decomposition (Laird et al., 2010). Biochar has also been identified as an effective adsorbent of organic pollutants in waste water (Liu and Zhang, 2009). Aside the environmental benefits, the high stability and water retention capacity of biochar makes it suitable for soil amendment (Lehmann, 2007). It improves both physical and chemical properties of soils when used as soil amendment (Jha et al., 2010; Chan et al., 2008; Glaser et al., 2002).
The incorporation of biochar has been reported to influence soil structure, texture, porosity, particle size distribution and density thereby enhancing its water holding capacity (Amonette and Joseph, 2009). Bulk density and water holding capacity of sandy soils can be enhanced by the micro and macropores in biochar structure (Novak et al., 2012). Biochar can convert the labile carbon into aromatic structures of relatively low decomposition rates (Kuzyakov et al., 2009). This carbonized form makes biochar suitable for addressing nutrient leaching and carbon depletion in poor soils (Ding et al., 2016).
Despite the potential of biochar as a soil amendment (Thies and Rillig, 2009) few trails have been conducted in the savanna to ascertain its ability to improve soil condition for raising seedlings which is a great challenge to nursery managers in northern Ghana. Savanna soils are generally low in organic matter and nitrogen (FAO, 2005) which limits its ability to support the growth of tree seedlings in the nursery requiring management options to improve soil fertility (Predotova et al., 2010). This study explored the possibility of using biochar produced from plant residues as growth media for tree seedlings in the Guinea savanna zone of Ghana. This will provide valuable information to nursery managers in the zone who are expected to produce large quantities of seedlings to feed the Ghana government reforestation programme.
Study area
The experiment was conducted at the tree nursery of the Faculty of Natural Resources and Environment (FNRE) of the University for Development Studies in the Tolon District (Figure 1). The site is located at latitude 9°25' N to 10° 40' N and longitude 0° 58' N to 1° 12' W with an altitude of 183 m above sea level (SARI, 1997). The area falls within the Guinea savanna agro-ecological zone which records a unimodal rainfall pattern with a mean annual rainfall of 1,034.4 mm (SARI, 2004). The mean maximum temperatures (35°C) are recorded in March and April whilst the lowest temperatures (22°C) often occur in December (SARI, 2016). The vegetation is generally grassland interspersed with some woody species. Among some of the dominant woody species indigenous to the Guinea savanna include Vitellaria paradoxa (shea), Adansonia digitata (baobab), Parkia biglobosa (dawadawa), Pterocarpus erinaceus (rosewood) and others (SARI, 2004)
Biochar production
Rice husk and groundnut husk feedstock were obtained from a commercial rice mill at Nyankpala. However, wood shavings feedstock was obtained from the Nyankpala market for the experiment. The various feedstocks were charred in a modified oil barrel following the procedure described in Steiner et al. (2018). The biochar was produced on a simple top-lit updraft gasifier where a modified oil barrel was perforated with holes at the bottom to facilitate the free flow of primary air from the bottom. Also, larger L-shaped holes of 6 × 6 cm were perforated at the top sidewalls of the barrel which enabled the flow of secondary air. After which a cut was made on the top of the lid to create an opening of 20 cm with a 1 m tall chimney attached to the lid. Gasifiers were then produced in triplicates for the carbonization of the three different feedstocks. The biochar of the different plant residues was each ground into finely uniform particles before they were used as growth media.
Seed viability test
Khaya senegalensis seeds obtained from the Tamale Forestry Services Division (FSD) were sorted and tested for viability prior to the experiment. One hundred seeds were randomly selected for the viability test using the floating test (Baatuuwie et al., 2019). The seeds recorded a percentage germination of 85%.
Experimental design
Six different biochar formulations were used as experimental treatments with untreated topsoil as control; 100% groundnut husk biochar (GHB), 100% rice husk biochar (RHB), 100% wood biochar (WB), GHB + soil at a ratio of 1: 1, RHB + soil at a ratio of 1:1, WB + soil at a ratio of 1:1. Soil was collected from the FNRE mango plantation. Each treatment was replicated in three plastic seed boxes of sizes 0.45 × 0.25 m half-filled with the treatment growth medium and arranged in a complete randomized design, fifty (50) K. senegalensis seeds were then broadcasted in each seed box, after which they were gently covered with a thin layer of the same treatment growth medium. The seed boxes were kept under a shade net in the FNRE tree nursery. Each seed box was watered twice daily (morning and evening) with 1500 ml of water from the day of sowing to the end of the experimental period (six weeks). Weeds were removed regularly by hand to prevent competition.
Seed emergence was recorded by counting the number of seeds germinated daily per seed box for a period of thirty days after sowing. Growth parameters and chlorophyll content were recorded once every two weeks (second, fourth and sixth weeks after planting). However, plant girth was not recorded in the second week after planting because seedlings were still fragile. A TYS-B portable chlorophyll meter was used for measuring the leaf chlorophyll content, measuring tape for plant height and calipers for collar diameter (girth). Leaves were counted manually.
Data analysis
All data collected were recorded and classified in Microsoft Office Excel 2007. Mean plant height, leaf chlorophyll content, number of leaves and plant girth were subjected to one-way analysis of variance using Minitab version 17. Means were separated using Fishers least significant differences (LSD) and considered significant at α = 0.05.
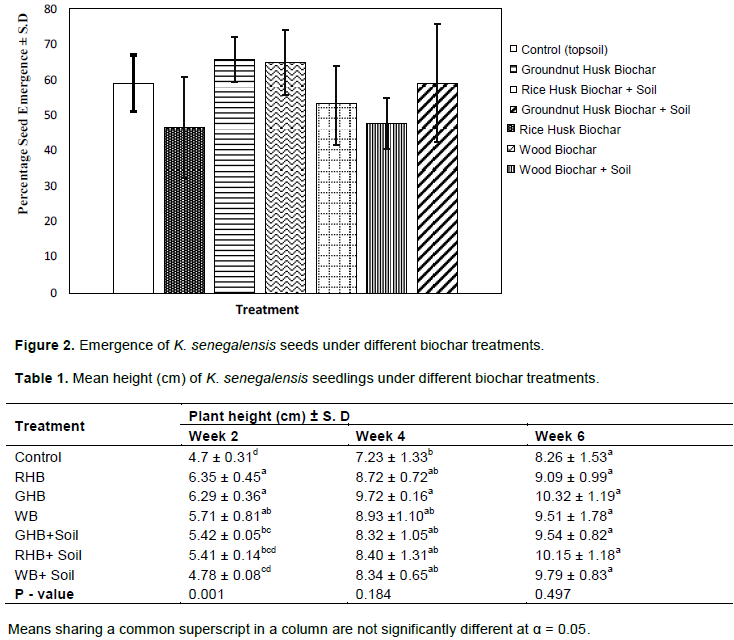
Effect of biochar on K. senegalensis seed emergence
Percentage seed emergence did not vary significantly between treatments (P = 0.65), but seed emergence under GHB was marginally higher (65.71%) than all other treatments. The RHB recorded the least percentage seed emergences (46.67%) (Figure 2). The relatively higher percentage emergence (65.71%) recorded under GHB than all other treatments might be due to the presence of Karrikins in GHB. Karrikins in biochar is reported to trigger germination of dormant seeds and regulate plant development (Kochanek et al., 2016). Biochar is also known to enhance soil pH (Novak et al., 2009), therefore the ability of a plant biochar to positively influence the pH of a growth media could aid in overcoming the inhibitory factors to germination.
Effect of biochar on initial growth performance of K. senegalensis
There was a significant difference in plant height (P = 0.001) between treatments in the second week after planting. However, plant height did not differ significantly between treatments in the 4th (P = 0.18) and 6th (P = 0.49) weeks after planting (Table 1). GHB recorded a marginally higher mean seedling height (10.32 cm) among all other treatments whilst the least (8.26 cm) was recorded in the control at the end of the experiment.
The control recording the least plant height (4.7 cm) in the sixth week after planting (Table 1) affirms the fact that savanna soils are poor in nitrogen and organic matter which barely support plant growth without major amendments (FAO, 2005). Therefore, biochar associated growth media might have some elevated levels of nitrogen and other nutrients for seedling growth. This is an indication that biochar properties might have influenced physical and chemical properties of the growth media providing favorable conditions for the K. senegalensis seedlings. On the contrary, plant height did not differ significantly in the 6th week after planting but GHB recorded a marginally higher plant height (10.32 cm) than all other treatments. This could be an outcome of the high adsorption capacity of GHB which enabled accumulation of soil ions for plant root access (Saleh et al., 2011). Mean number of leaves per plant did not differ significantly between treatments in the second (P = 0.47) and fourth weeks (P = 0.84) after planting (Table 2). However, there was a significant difference between treatments (P = 0.047) in the sixth week after planting with seedlings growing on GHB recording the highest mean (6.02) number of leaves whilst RHB + Soil recorded the least (4.97).
The significantly higher number of leaves (6.02) recorded on GHB in the sixth week after planting might be attributed to the porous nature of GHB which created an atmosphere for microbial colonization and perhaps facilitated nutrient absorption and adsorption. GHB is reported to exhibit both monolayer and multilayer adsorption properties which enhances the adsorption of chemical elements such as ammonium ions (Clough et al., 2013). This makes the GHB surface heterogeneous in nature with the ability to adsorb all kind of plant nutrient elements. Lee et al. (2016), equally indicated the porous nature of GHB as a property through which it adsorbs dissolved ions such as calcium.
Plant girth did not differ significantly between treatments (P = 0.730) in the sixth week after planting although RHB had the largest plant girth (1.99 ± 0.1210) (Table 3). Contrary to the fact that most growth parameters were higher under GHB, the largest plant girth was recorded under RHB (1.99 cm). This could be due to the high ash content of rice husk (60 to 70%) attributed to the active uptake of silicon in rice (Carter et al., 2013). Again, rice husk provides a readily soluble form of lime (Nattaporn et al., 2013) which can increase nutrient availability for plant root uptake facilitating stem development.
Chlorophyll content of K. senegalensis leaves did not vary significantly between treatments in the second week after planting (P = 0.092). However, chlorophyll content (mg m-2) differed significantly between treatments in the 4th (P = 0.002) and 6th (P = 0.0001) weeks after planting. Generally, in the 6th week after planting, seedlings of the control had the highest chlorophyll index (49.92 ± 6.26) whilst the WB recorded the least (24.57 ± 0.15) chlorophyll content (Table 4).
Chlorophyll content deviated from the trend for all other measured parameters with control (topsoil) recording a significantly higher chlorophyll content (49.92 ± 6.26) than all other treatments (P = 0.0001) in the 6th week after planting (Table 4). Perhaps the black colour of biochar served as a “black body” which absorbed sunlight thereby reducing the amount of solar radiation available for chlorophyll formation.
Biochar prepared from different plant residues have varied effects on seed emergence and plant growth when used as growth media. GHB had the greatest effect on seedling emergence, plant height and number of leaves. However, in terms of plant girth, RHB mixed with soil produced seedlings with the largest stems. The topsoil (control) without any amendment had a unique influence on K. senegalensis seedlings, producing seedlings with high chlorophyll content. In general, GHB had the greatest effect on plant growth and could be used by nursery managers for raising tree seedlings in the Guinea savanna zone of Ghana.
The authors have not declared any conflict of interests.
REFERENCES
|
Amonette JE, Joseph S (2009). Characteristics of biochar: micro chemical properties. In. Lehmann J, Joseph S (Eds.), Biochar for environmental management science and technology. UK, Earthscan pp. 33-66.
|
|
|
|
Baatuuwie BN, Nasare LI, Smaila A, Issifu H, Asante WJ (2019). Effect of seed pre-treatment and its duration on germination of Detarium microcarpum (Guill. and Perr.). African Journal of Environmental Science and Technology 13(8):317-323.
Crossref
|
|
|
|
|
BÅ™endová K, Tlustoš P, Szákováa J, Habart J (2012). Biochar properties from different materials of plant origin. Proceedings 4th International Symposium on Trace Elements in the Food Chain, Friends or Foes, 15-17 November, 2012, Visegrád, Hungary.
|
|
|
|
|
Carter S, Shackley S, Sohi TB, Suy HS (2013). The impact of Biochar application on soil properties and plant growth of pot grown lettuce (Lactuca sativa) and Cabbage (Brassica chinensis). Agronomy 3(12):404-418.
Crossref
|
|
|
|
|
Chan KY, Van Zwieten L, Meszaros I, Downie A, Joseph S (2008). Agronomic values of green waste biochar as a soil amendment. Australian Journal of Soil Research 45(8):629-634.
Crossref
|
|
|
|
|
Clough TM, Condron, LM, Kammann C, Muller C (2013). A review of biochar and soil nitrogen dynamics. Agronomy 3:275-293.
Crossref
|
|
|
|
|
Ding Y, Liu Y, Liu S, Li Z, Tan X, Huang X, Zeng G, Zhou L, Zheng B (2016). Biochar to improve soil fertility. A review. Agronomy for Sustainable Development 36(2):36.
Crossref
|
|
|
|
|
Food and Agriculture Organization of the United Nations (FAO) (2005). Fertilizer use by crop in Ghana.
|
|
|
|
|
Gaskin JW, Steiner C, Harris K, Das KC, Bibens B (2008). Effect of low-temperature pyrolysis conditions on biochar for agricultural use. American Society of Agricultural and Biological Engineers 51(6):2061-2069
Crossref
|
|
|
|
|
Glaser B, Lehmann J, Zech W (2002). Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal - A review. Biology and Fertility of Soils 35:219-230.
Crossref
|
|
|
|
|
Hammes K, Schmidt MWI, Johannes L, Czimczik C, Laird D, Sohi S (2009). Stability of biochar in soil. In: Lehmann J, Joseph S (Eds.), Biochar for environmental management science and technology. UK, Earthscan pp.169-182.
|
|
|
|
|
Jha P, Biswas AK, Lakaria BL, Subba RA (2010). Biochar in agriculture prospects and related implications. Current Science 99(9):1218-1225.
|
|
|
|
|
Kochanek J, Long RL, Lisle AT, Flematti GR (2016). Karrikins identified in biochars indicate postfire chemical cues can influence community diversity and plant development. PLoS ONE 11:0161234.0.
Crossref
|
|
|
|
|
Kuzyakov Y, Subbotina I, Chen H, Bogomolova I, Xu X (2009). Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labelling. Soil Biology and Biochemistry 41:210-219.
Crossref
|
|
|
|
|
Laird DP, Fleming B, Wang R, Horton D, Karlen H (2010). Biochar Impact on Nutrient Leaching from a Midwestern Agricultural Soil. Geoderma 158:443-449.
Crossref
|
|
|
|
|
Lee JW, Hawkins B, Kidder MK, Barbara RE, Buchanan AC, Day D (2016). Characterization of biochars produced from peanut hulls and pine wood with different pyrolysis conditions. Bioprocess 3:15.
Crossref
|
|
|
|
|
Lehmann J (2007). Bio-energy in the black. Frontiers in Ecology and the Environment 5(7):381-387.
Crossref
|
|
|
|
|
Liu Z, Zhang F (2009). Removal of lead from water using biochars prepared from hydrothermal liquefaction of biomass. Journal of Hazardous Materials 167(3):933-939.
Crossref
|
|
|
|
|
Nartey OD, Zhao B (2014). Biochar preparation, characterization, and adsorptive capacity and its effect on bioavailability of contaminants: An Overview. Advances in Materials Science and Engineering 2014(715398):1- 2.
Crossref
|
|
|
|
|
Nattaporn P, Gilkes R, Wanpen, W, Apinya D, Timtong D (2013). The Effects of Pyrolysis Conditions on the Chemical and Physical Properties of Rice Husk Biochar. International Journal of Material Science 3(3):98-103.
|
|
|
|
|
Novak JM, Busscher WJ, Watts DW, Amonette, JE, Ippolito, JA, Lima IM, Gaskin J, Das KC, Steine C, Ahmedna M, Rehrah D, Schomberg H (2012). Biochars impact on soil-moisture storage in an ultisol and two aridisols. Soil Science 177(5):310-320.
Crossref
|
|
|
|
|
Novak, JM, Busscher DL, Laird M, Ahmedna DW, Novotny EH, Hayes MHB, Madari BE, Bonagamba TJ, Azevedo ER, de, Souza AA, de Song G, Nogueira CM, Mangrich AS (2009). Lessons from the Terra Preta De Indios of the Amazon region for the utilisation of charcoal for soil amendment. Journal of the Brazilian Chemical Society 20(6):1003-1010.
Crossref
|
|
|
|
|
Predotova M, Gebauer J, Diogo RVC, Schlecht E, Buerkert A (2010). Emissions of ammonia, nitrous oxide and carbon dioxide from urban gardens in Niamey, Niger. Field Crops Research Journal 115(1):1-8.
Crossref
|
|
|
|
|
Saleh ME, Mahmoud AH, Rateb KA (2011). Influence of rice husk biochar application and irrigation water salinity on growth and nitrogen use efficiency by wheat. Alexandria Journal of Agricultural Research 56(1):125-131.
|
|
|
|
|
Savannah Agricultural Research Institute (SARI) (1997). Soil survey. Nyankpala pp. 9-22.
|
|
|
|
|
Savannah Agricultural Research Institute (SARI) (2004). Annual Report for the year 2004. Nyankpala P 89.
|
|
|
|
|
Savannah Agricultural Research Institute (SARI) (2016). Annual Report for the year 2016 Nyankpala.
|
|
|
|
|
Steiner C (2016). Considerations in biochar characterization. In: Guo M, He Z, Uchimiya SM (Eds.), Agricultural and environmental applications of biochar: advances and barriers, SSSA special publication, Vol 63. Soil Science Society of America, Inc., Madison, pp. 87-100.
Crossref
|
|
|
|
|
Steiner C, Bellwood-Howard I, Häring V, Tonkudor K, Addai F, Atiah K, Abubakari, A, Kranjac-Berisavljevic G, Marschner B, Buerkert A (2018). Participatory trials of on-farm biochar Production and use in Tamale, Ghana. Agronomy for Sustainable Development 38:1-12.
Crossref
|
|
|
|
|
Thies JE, Rillig MC (2009). Characteristics of biochars: biological properties. In. Lehmann J, Joseph S (Eds.), Biochar for environmental management. Earthscan, London pp. 85-105.
|
|
|
|
|
Zhao L, Cao MO, Zimmerman A (2013). Heterogeneity of biochar properties as a function of feedstock sources and production temperatures. Journal of hazardous Materials 256-257:1-9.
Crossref
|
|