ABSTRACT
The influence of the terminal bud treatment and genotype on the cutting of plagiotropic semi-lignified kola tree cuttings was studied with the aim of vegetative propagation. Two dressing modes cuttings (B1: Presence of terminal bud and B2: Absence of terminal bud) were tested on three genotypes (D9L20A3, 315 and 323) in a split-plot design with the genotype in the large plot and the cutting dressing mode in the small plot. The experimental unit consists of twenty cuttings. Six months after transplanting, no significant difference of cutting dressing mode on the survival rate was noted. The survival rate was 76.1±11.7% with terminal bud and 70±12.17% without terminal bud for an overall mean of 73.06±12.1%. However, it appears that the way in which kola plant cuttings are dressed, including the removal of the apical bud from semi-lignified plagiotropic cutting, boosts root development and growth despite the predisposition of some kola plant genotypes to rooting (genotype 315). The cuttings dressing method including terminal bud suppression favours root formation at the cuttings, taproot length growth, fresh and dry root biomass compared to cuttings with a terminal bud.
Key words: Cola nitida, cuttings, genotype, terminal bud.
Despite its economic importance, kola nut production in Côte d'Ivoire faces several difficulties. Indeed, the kola nut has a slow germination and the seedlings enter into production late (5 to 6 years after planting). In order to shorten the time of entry into production, the cutting of the kola tree has been initiated. Unfortunately, in the nursery, the survival rate of the plants is low for the species and the growth of plants from cuttings is very slow. It is necessary to wait 12 to 18 months to obtain plants suitable for transplanting in the field. In addition, the root quality of these plants is poor. It is therefore necessary to propose methods to improve the survival rate of cuttings, to accelerate the growth and root development of kola plants from cuttings. Previous research on several tropical tree species, including kola tree, has indicated a wide range of factors such as genotype, substrate type, leaf area, length of cuttings and rhizogenic substances; which influence the rooting of cuttings (Paluku et al., 2018) in the nursery. These factors also include apical dominance (Charrier, 1969). This apical dominance is the control exerted by the apical portions of the shoot over the outgrowth of the lateral buds and also over root. The classical explanations for correlative inhibition have focused on hormone/nutrient hypotheses (Cline, 1991). The terminal bud is one of the seats of production of high concentration of hormone such as auxin (Normanly, 2010). The auxin hormone and its polar movement, originating in young shoot organs like terminal bud (Aloni et al., 2003, 2006), play a crucial role in many aspects of root growth, development and differentiation. Auxin regulates the development of the primary and lateral roots (Blilou et al., 2005; Raven et al., 2005). The aim of our study is to improve kola rooting system of plant from cutting by suppression of terminal bud. Could this elimination of the terminal bud on the cuttings, contrary to current practices, promote the root development of the cuttings in kola and improve survival rates in the nursery? In the present study, this aspect will be tested in order to propose a method of dressing the cuttings and an optimal method of cuttings of the kola under tunnel.
Study site and characteristics
Experiments were conducted in April 2019 at Centre National de Recherche Agronomique (CNRA) Man Station, located in the Tonkpi Region, West of Côte d’Ivoire western (7° 19,130'N; 8° 19,452'W). This six-month trial ended in October 2019. Rainfall in the Man area is monomodal. The dry season generally runs from October to March and the rainy season from April to September. The site received an average annual rainfall in 2018 of 1632 mm. The temperature in 2019 ranged from 23 to 27°C.
Plant
The improved kola variety of Centre National de Recherche Agronomique (CNRA) was used for the experiment. 360 kola cuttings of tree genotype were used for experiment. We took 120 cuttings per genotype. These three genotypes were identified by the following codes D9L20A3, 315 and 323. These genotypes were selected on the basis of their productivity.
Technical material
The technical equipment used for this study consists of pruning shears for taking and dressing the cuttings and a decameter for measuring the circumference and height of the cuttings. White plastic bags, hermetically sealed with a stapler and stored in glacial containers were used to preserve the cuttings during transport. The growing medium used for this trial was topsoil (black soil). Bags measuring 30 cm high by 15 cm in diameter were used for transplanting the cuttings. IVORY × 80% WP Fungicide (a.i.: Maneb, Manufacturer: ARYSTA Lifescience) was used for preventive treatment as soon as the cuttings were transplanted.
The method of cuttings used was tunnel cutting (Koko et al., 2011). The reinforcement of the tunnel to shelter the pots (bags) containing the cutting was made of 2.4 m arches connected by bamboo slats. The tunnel was covered with 100 µ thick and 2.6 m wide transparent plastic sheeting, which was veneered on the ground at the sides and ends with stones and bamboo. The tunnels were placed under a nursery shelter which consists of a 2 m high palm frame that allows about 50% of the total light to pass through (Figure 1).
Description of the experiment
Experimental design
The experimental design used was a factorial block arranged in a split-plot with two factors and three replicates. One factor was the terminal bud treatment. Cutting were either with presence or absence of terminal bud (Figure 2). The other factor was the genotype with three modalities (D9L20A3; 315; 323). The genotype was in the large plot and terminal bud treatment in the small plot. A total of six treatments were studied in this trial. Twenty pots each containing one cutting of the same genotype was used per treatment. A total of 120 cuttings from the same genotype were used for this experiment (40 cuttings/genotype/replicate). A total number of 360 cuttings were used for the three genotypes.
Preparation of materials and setting of cuttings
In each tunnel, the pots were filled with previously homogenised topsoil. Three hundred and sixty cuttings from 3 genotypes (120 cuttings/genotype) were taken early in the morning from semi-lignified plagiotropic twigs. The size of the cuttings ranged from 10 to 12 cm. They have 4 leaves cut in half. Cuttings were set about 3 cm deep in the pots.
Conduct of the experiment
The arrangement of the pots and the phytosanitary treatment were carried out one day before the cuttings were tunnelled. For the phytosanitary treatment, the fungicide IVORY 80% WP (a.m. maneb, Manufacturer: ARYSTA Lifescience) was used (70 g of fungicide in 2 L of water applied in the pots). Cuttings were watered in the morning every 2 days with approximately 100 ml/pot. For the measurement of growth parameters, on the 20 pots of each treatment, the number of live plants was recorded at six months. The number of life cuttings at the time of data collection was used to estimate survival. Root development (root length and number of roots), number of new leaves, height of the seedling and aerial and root biomass were assessed after six months. A cutting was considered to have rooted if it had a root of at least 1 cm (Atangana et al., 2006). A rooted cutting was assessed for number of roots by counting, whereas root lengths were measured using a ruler. Dry biomass was assessed using an electronic scale after air drying for two weeks.
Statistical analyses of the data
For the parameters examined, a comparison of the means between the different factors and the different treatments was carried out using the analysis of variance (ANOVA). When a significant difference is observed between treatments for a given factor, the ANOVA is supplemented by post-hoc tests, in particular the Newman-Keuls test, to identify significant differences between the means at the 5% threshold. For all these tests, the STATISTICA 7.1 software was used. The survival rate (SR) was calculated according to the following formula:
Survival rate = 100 × (number of live plants/initial number of plants).
Impact of terminal bud suppression and genotype on cutting survival rate
The ANOVA of the factors "Terminal bud treatment: Presence or Absence of the terminal bud" and "Genotype" on the survival rate of cuttings showed no significant effect of the two factors (Table 1). The survival rate of kola cuttings (Table 2) for the three genotypes (D9L20A3, 315, 323) ranged from 51.7 % to 86.7%. The mean survival rate was 73.06±12.1% for all clones in this trial.
Impact of terminal bud removal and genotype type on the development of the aerial system of the kola cuttings (diameter, height and number of new leaves)
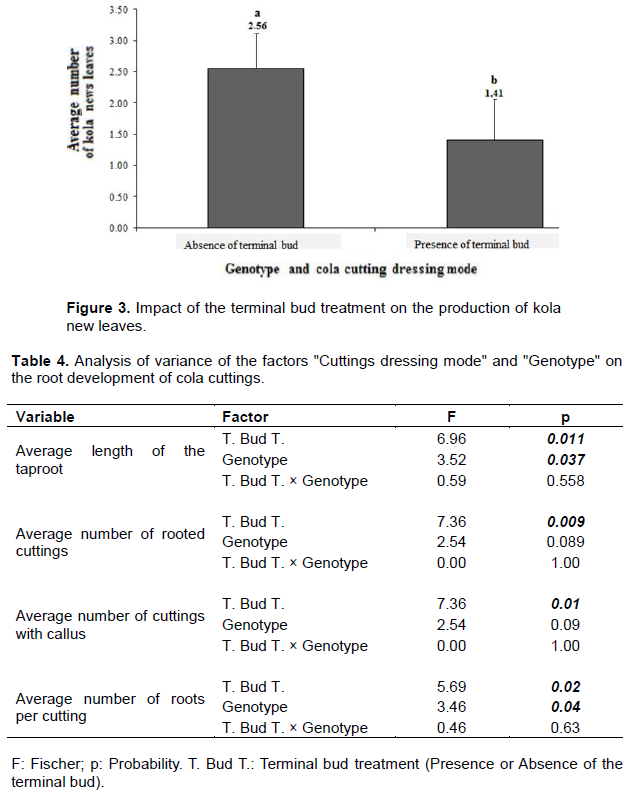
The impact of terminal bud removal and genotype type on the development of kola cuttings aerial system including collar diameter, height and number of new leaves was assessed through an analysis of variance (Table 3). In this study, terminal bud removal had a significant effect (p=0.027) only on the number of new leaves formed. In fact, cuttings with no terminal bud produced more leaves than kola tree cuttings whose terminal bud remained intact with respectively 2.56 and 1.41 new leaves produced on average (Figure 3).
Impact of terminal bud removal and genotype type on the development of the kola root system
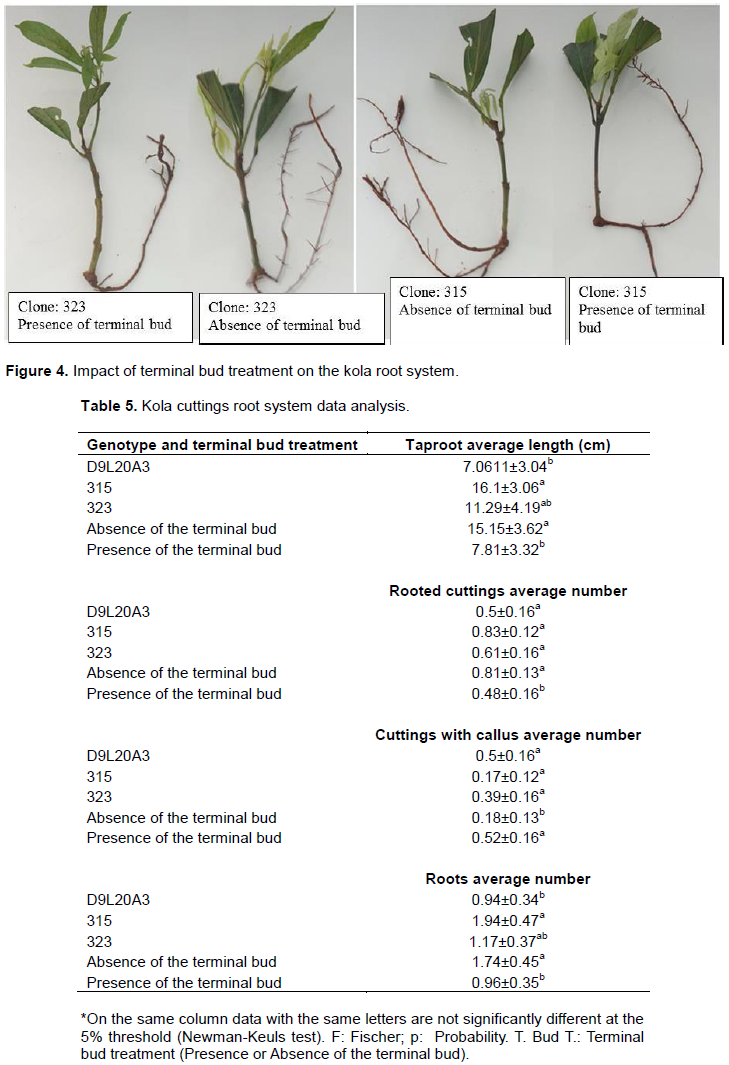
Kola cuttings root development was strongly affected by the removal or not of the terminal bud during tunnel cutting (Table 4 and Figure 4). Parameters such as the average number of rooted cuttings (p=0.009) and the average number of cuttings with callus (p=0.01) was affected. The terminal bud treatment including terminal bud removal favours rooting of cuttings (0.81±0.13 versus 0.48±0.16) (Table 5). Conversely, callus formation is preponderant in cuttings that have preserved their terminal bud (0.52±0.16 versus 0.18±0.13). For parameters such as mean taproot length (p=0.011; p=0.037) and mean number of roots per cutting (p=0.02; p=0.04), an effect of both factors was noted. The removal of the terminal bud stimulates the growth of the taproot (15.15±3.62 versus 7.81±3.32). However, it should be noted that genotype 315 is predisposed to the production of long taproot (16.1±3.06 cm after six months). As for the average number of roots produced per cutting, it was the same observation. The absence of terminal bud would increase root production (p=0.02); however, the genotype's aptitude for root production should not be minimized (p=0.04). Genotype 315 has the highest average number of roots produced per cutting after six months of cutting (1.94±0.47).
Impact of terminal bud treatment and genotype on dry aerial and root biomass of kola
Fresh root biomass and dry root biomass were the two parameters significantly impacted by terminal bud
removal (p=0.04 and p=0.039, respectively) (Table 6). Data analysis of kola cuttings root biomass (Table 7) revealed that fresh (0.63±0.19 g) and dry (0.4±0.12 g) root biomass was significantly greater in the case of terminal bud removal of cuttings than fresh (0.35±0.15 g) and dry (0.22±0.09 g) kola cutting biomass with terminal buds.
The survival rate of kola cuttings for the three genotypes (D9L20A3, 315 and 323) ranged from 51.7% to 86.7%. The survival rate was 76.1±11.7% with terminal bud and 70±12.17% without terminal bud for an overall mean of 73.06±12.1% regardless of the clone used. This survival rate is higher than the first rate of about 41% previously obtained by Séry et al. (2019) for dry season tunnel cutting of kola. This difference is the result of several factors including the rainy season period (April to October) favourable to cuttings as opposed to the dry season (Ricez, 2008) and the ability of clones (D9L20A3, 315 and 323) to tunnel cuttings (Séry et al., 2019; Koko et al., 2011). In this trial, the impact of terminal bud and genotype suppression could not be demonstrated on the survival rate but on the development of the aerial system of kola cuttings; in particular on the number of new leaves formed, root development and dry root biomass. Indeed, cuttings with no terminal bud produced more leaves than kola cuttings with an intact terminal bud. The same applies to the root development of kola cuttings, which is strongly affected by the removal or not of the terminal bud during tunnel cutting of the kola tree. The apical bud and young leaves are the site of production of high concentration of phytohormone such as auxin (Indole Acetic Acid) (Normanly, 2010) then it is transported by the cellular route by diffusion or using membrane proteins (Kramer and Bennett, 2006) or via phloem vessels (Davies, 2010) to reach the places of function in all parts of the plant including the root system. This hormone controls apical dominance. It inhibits the development of axillary buds, stems and roots at high concentrations. As a result, the suppression of the terminal bud reduces the concentration of auxin in these organs and removes the inhibition exerted by it (William-G Hopkins, 2003). Root elongation is particularly sensitive to auxin (Gaspar et al., 2003; Pacurar et al., 2014). At very low concentrations (10-8 M or even less), it causes growth of excised or intact roots. The way in which cuttings are dressed, including the removal of the terminal bud thus favours the rooting of cuttings (Charrier, 1969). This hypothesis is supported by the fact that in cuttings that have preserved their terminal bud there is late root production with a preponderance of cuttings with callus. This is probably due to high concentrations of auxins produced by the apical bud.
For parameters such as the average length of the taproot and the average number of roots per cutting, in addition to the effect of apical bud suppression, the effect of genotype was also noted. In fact, some genotypes were found to be predisposed to root production, notably clone 315. Root formation is a complex physiological process that is influenced by various endogenous and exogenous factors such as the genetic composition of mother plants, thus of the genotype, their physiological state, and several other environmental factors (Makouanzi et al., 2014). Fresh root biomass and dry root biomass were therefore positively impacted by the removal of the terminal bud.
The authors have declared any conflict of interests.
The authors are grateful to the Fonds Interprofessionnel pour la Recherche et le Conseil Agricoles (FIRCA) for funding the project "Improvement of kola productivity in Côte d'Ivoire" under which this study was carried out.
REFERENCES
|
Aloni R, Aloni E, Langhans M, Ullrich CI (2006). Role of auxin in regulating Arabidopsis ?ower development. Planta 223:315-328.
Crossref
|
|
|
|
Aloni R, Schwalm K, Langhans M, Ullrich CI (2003). Gradual shifts in sites of free-auxin production during leaf-primordium development and their role in vascular differentiation and leaf morphogenesis in Arabidopsis. Planta 216:841-853.
Crossref
|
|
|
|
|
Atangana AR, Tchoundjeu Z, Asaah EK, Simons AJ, Khasa DP (2006). Domestication of Allanlackia floribunda: Amenability to vegetative propagation. Forest Ecology and Management 237:246-251.
Crossref
|
|
|
|
|
Blilou I, Xu J, Wildwater M, Willemsen V, PaponovI, Friml J (2005). The PIN auxin ef?ux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433:39-44.
Crossref
|
|
|
|
|
Charrier A (1969). Contribution à l'étude de la morphogenèse et de la multiplication végétative du cacaoyer (Théobroma cacao L.). Café Cacao Thé 13: 97-115.
|
|
|
|
|
Cline MG (1991). Apical dominance. Botanical Review 57:318-358.
Crossref
|
|
|
|
|
Davies PJ (2010). Regulatory factors in hormone action: level. location and signal transduction. In: Davies PJ (ed) Plant Hormones. Springer. Netherlands. pp. 16-35.
Crossref
|
|
|
|
|
Gaspar TH, Kevers C, Faivre-Rampant O, Crèvecoeur M, Penel C, Greppin H, Dommes J (2003). Changing concepts in plant hormone action. In Vitro Cellular and Developmental Biology-Plant 39:85-10.
Crossref
|
|
|
|
|
Koko L, Koffi N, Konan A (2011). Multiplication végétative du cacaoyer (Theobroma cacao L.) par la technique de bouturage direct sous tunnel plastique. Journal of Applied Bioscience 46: 3124-3132.
|
|
|
|
|
Kramer EM, Bennett MJ (2006). Auxin transport: a field in flux. Trends Plant Science 11:382-386.
Crossref
|
|
|
|
|
Makouanzi G, Bouvet J-M, Denis M, Saya A, Mankessi F, Vigneron P (2014). Assessing the additive and dominance genetic effects of vegetative propagation ability in Eucalyptus- influence of modeling on genetic gain. Tree Genet Genomes 10:1243-1256.
Crossref
|
|
|
|
|
Normanly J (2010). Approaching cellular and molecular resolution of auxin biosynthesis and metabolism. Cold Spring Harbor Perspectives in Biology 2:1-17.
Crossref
|
|
|
|
|
Pacurar DI, Perrone I, Bellini C (2014). Auxin is a central player in the hormone cross-talks that control adventitious rooting. Physiologia Plantarum 151:83-96.
Crossref
|
|
|
|
|
Paluku A, Okungo A, Bwama M (2018). Bouturage de Cola acuminata (P. Beauv.) Schott and Endl.: Influence du substrat, de la longueur et de la surface foliaire sur l'enracinement de boutures à Kisangani. RD Congo. Journal of Applied Bioscience 123:12354-12362.
Crossref
|
|
|
|
|
Raven PH, Evert RF, Eichhorn SE (2005). Biology of plants, 7th edn, New York: Freeman.
|
|
|
|
|
Ricez T (2008). Etude des modes de régénération à faible Coût de Prosopis africana et Detarium microcarpum en forêt classée de Dinderesso. Master II 'Bioressources en régions tropicales et méditerranéennes'. Université Paris XII. p. 60.
|
|
|
|
|
Séry DJM, Bonsson B, Gnogbo R, Gbédié N, Ouattara Y, Légnate H et Kéli ZJ (2019). Influence du génotype et du nombre de feuilles sur la croissance en pépinière des boutures du colatier (Cola nitida [Vent.] Schott et Endlicher.). International Journal of Biological and Chemical Sciences pp. 3144-3156.
Crossref
|
|
|
|
|
William GH (2003). Physiologie végétale. Bruxelles: De Boeck P 514.
|
|