Disturbances of terrestrial origin are important determinants in the growth, carbonate production and well- being coral reefs. In this study, coral growth (density, linear extension and calcification rates) and corallite (diameter and density) variables were determined for selected species from four Kenyan protected reef lagoons, with one receiving a pulse of sediment discharge. Variable coral growth responses were observed, with some species showing higher growth rates while others exhibited low growth rates within the sediment impacted reef. Species corallite characteristics also showed variable responses, though corallite diameters were found to be larger in the sediment impacted reef with low corallite densities generally being observed in the mangrove-fringed reef. Coral bulk density was found to be poorly correlated to calcification and extension rate but calcification and extension rates were found to be highly correlated (R values 0.84 to 0.98). The variable responses observed in the current study have important implications and applications in coral reef health and management by providing information on the susceptibility of different corals species to local stressors, and together with coral distribution and composition data this information may be useful in the formulation of suitable and scientific based reef management strategies and also in predicting reef performance under future climate change scenarios.
Key words: Calcification, corals, climate change, density, linear extension, sediments, terrestrial disturbances.
Coral reefs, the most complex and diverse ecosystems of the world, are the product of interaction between calcium carbonate constructive and destructive processes with environmental conditions. Coral reefs are known to be the most prolific biomineralising ecosystems in nature with calcification rates of ~2-6 kg CaCO3 m-2 yr-1 as testament to the evolutionary success of the association between corals and the dinoflagellates algae, zooxanthellae (Barnes and Devereux, 1984). However, coral reefs are increasingly threatened by local and global environmental disturbances leading to progressive declines in reef-building potential and ultimately to states of net reef erosion. Changes in water quality due to modification of catchment areas through various human activities (Fabricius et al., 2011; Golbuu et al., 2011; Nakajima et al., 2013; Chen et al., 2015) are considered major factors in the degradation of coral reef worldwide. Increased human activities such as farming, coastal development and deforestation have elevated the rate of soil erosion and sediment input into rivers with subsequent increase in levels of turbidity, sediments and nutrients in coral reef areas (GESAMP, 2001; Rotmann and Thomas, 2012; D’Olivo et al 2014; Kumara et al., 2015). Recently, the number of reports on reef degradation due to land based stressors has increased (Crabbe and Carlin, 2007; Padilla-Gami?o et al., 2012; Shantz and Burkepile, 2014; Prouty et al., 2014) with model estimates indicating that 22% of coral reefs worldwide (Bryant et al., 1998) and ~50% in countries with widespread land clearing are threatened by inland pollution and erosion.
Disturbances of terrestrial origin affect regeneration, recovery and calcium carbonate production processes of coral reefs (McCulloch et al., 2003) and have been recognized as significant controlling forces in the growth and skeletal properties of reef corals (Padilla-Gamino et al., 2012; Bartley et al., 2013; Kumara et al., 2015). Further, although sediments and turbidity play a significant role in marine geochemical processes and food webs, studies have shown that high sediment levels and turbidity have deleterious effects on coral reefs at the colony level (Golbuu et al., 2011) including reduced recruitment, photosynthesis, tissue thickness and increased mucus production. At the community level, high sediment levels lead to reduced coral cover, richness and diversity, coral colony abundance as well as increased disease incidences and mortality (Kuta and Richardson, 2002; Ruiz-Moreno et al., 2012).
Previous studies have provided insights into the effects of sediments on coral growth (Carricart-Gavinet and Merino, 2001; McDonald and Perry, 2003; Larsson et al., 2013; Kumara et al., 2015) with results suggesting complex, variable and even contradictory findings. Coral subjected to high sediment levels have shown declines in skeleton density, extension and calcification rates (Crabbe and Smith, 2005; D’Olivio et al., 2013; Nakajima et al., 2013; Larsson et al., 2013), though higher extension rates have also been measured in corals under terrestrial influence (Carricart-Ganivet and Merino, 2001; Edinger et al., 2000; Cruz-Pi?ón el al., 2003). Still, other studies reported no effect on coral growth parameters as a result of high sediment concentrations (Edinger et al., 2000; Torres and Morelock, 2002; Golbuu et al., 2011). These contrasting findings may be of particular significance in future survival, existence and conservation of reefs (Kleypass et al., 1999; Kru?i? et al., 2012; Chen et al., 2015; Klein et al., 2015). Therefore, there is need for further studies to highlight these conflicting findings, this increase our understanding on the effects of sediments on coral growth and also provide information useful in the management of sediment disturbed reefs. This study was undertaken to investigate the response of coral growth rates (linear extension, density and calcification) and skeletal charac-teristics (corallite diameter or size and density) of scleractinian corals from Kenyan reef lagoons to increased sediment levels and the implication and application to coral reef management and conservation.
Description of study areas
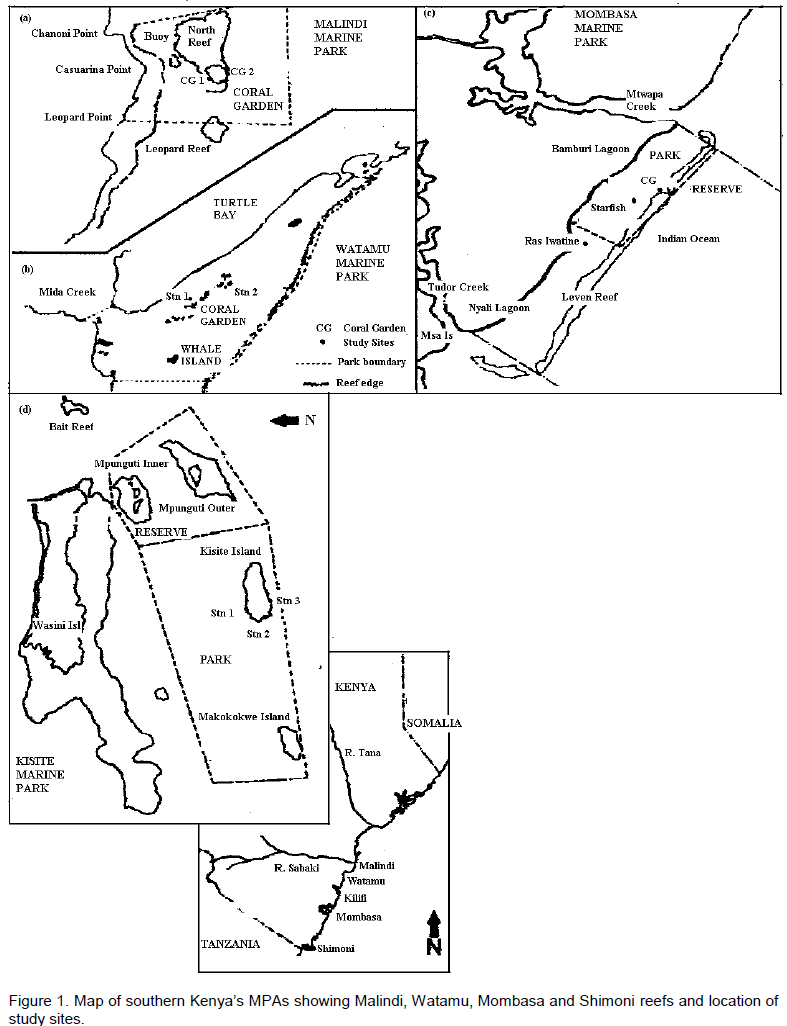
Four reef lagoons (Figure 1a to d) were chosen due to their accessibility, type of exploitation, influence of river discharge in addition to previous studies conducted. The study was undertaken in Mombasa, Watamu, Malindi and Shimoni’s Kisite Marine National Parks (MNP), all reefs receiving protection from fishing and shell collection for over 15 years. Malindi reefs experience a pulse of land derived discharge during the short rain period (Sept-Nov, North East Monsoon, NEM season) from the nearby Sabaki River, potentially supplying sediments and nutrients. Watamu reef forms part of the Watamu Marine National Park and Reserve including the expansive (360 km2), highly ramified, groundwater sustained Mida mangrove creek to its west (van Katwijk et al., 1993). Mombasa MNP is a long stretch of lagoonal fringing reef ~ 6 km long and 1 km from the shore situated in the Bamburi area about 5 km north of Mombasa town. This reef experiences water exchange with the Tudor and Mtwapa mangrove creeks on either end of the reef and the ocean via a depression (channel) through the reef. The fourth reef is in the Shimoni area within the Kisite Marine National Park in the southernmost part of the Kenyan coast close to the Tanzania border. The park is ~ 8 km offshore from the small town of Shimoni and on the seaward side of Wasini Island. This reef was chosen because of its remote location hence minimal influence from any anthropogenic disturbances. Three study sites were chosen from Malindi and Shimoni and two sites for each of the smaller (area) Mombasa and Watamu reefs.
Measurement of environmental factors
Measurements of environment parameters were conducted twice a month over a 3 year period between 2005 and 2007. Seawater surface temperature (SST) and salinity were taken (in triplicate) and measured in the field with an automated temperature-salinity probe. Triplicate current speed measurements were taken in the field from a rubber boat using a hand-held current meter. Chlorophyll a, nutrients and total suspended sediments (TSS) measurements and analyses were done on three 1-L water samples collected from each site. Chlorophyll a and nutrients (nitrates and phosphates) were determined by filtering water samples through 0.45 µm pore size filters into acid-washed vials and chlorophyll a and nutrients (nitrates and phosphates) determined through measurements of absorbance, spectrophotometrically. Total suspended sediments (TSS) were quantified by filtering triplicate 1 L water samples from each site through pre-weighed 0.45 µm pore size glass filters, filters then oven dried overnight at 60 ?C and TSS concentration obtained gravimetrically.
Sedimentation (sediment deposition or trapped sediments) rates were measured using replicate sediment traps deployed and retrieved biweekly from the study reefs. Each set of sediment traps consisted of four cylindrical plastic cups, 13.5 cm long and 7 cm in diameter tied to a PVC pipe firmly fixed to the bottom of the reef, the open end of the cups being ~ 50 cm from the sea bottom. Each reef had three sets of traps except Mombasa which had only two traps. After collection sediments were washed with freshwater, small animals and plant material removed and sediments oven dried at 60°C overnight, weighed and sedimentation rates computed in g cm-2day-1. Organic content in TSS and deposited sediment was determined as loss in weight after combusting 3 replicate (5 g) sediment samples in a furnace at 500°C for 4 h. Acid-insoluble fraction was determined by digesting five 5 to 10 g sediment samples with dilute (5%) hydrochloric acid (HCl) and weighing the residue after drying, assuming non-carbonate (acid insoluble) fraction to entirely be of terrestrial origin. All data are averages of sample collection and measurements conducted over 3 months during each of the SE (March to October) and NE, (October to March) monsoon (McClanahan, 1988) periods.
Linear extension rates
Coral species for extension rate studies were selected based on their common occurrence in the study reefs as well as ease of identification. Linear extension rates of selected corals were studied by measuring the diameters of labeled massive forms, height/length of branching corals and distance between the edge of encrusting forms and fixed points (not less than three) around the colony (lateral growth) using calipers. Ten different individual coral colonies or branches were chosen for each coral species and labeled with cable ties numbered with holes (1 to 10) and changes in extension measured after 2 months. At the end of the experimental period, algae were scraped off the numbered end of the cable tie to reveal the identity of the coral and the height, distance from fixed point or diameter of coral to the nearest mm and later converted to annual linear extension rates (mm yr-1).
Skeletal density and calcification rates
Five sample corals per species were collected from each site and small pieces (3 to 5 g) cut from each for density and calcification determination. Bulk density was determined by suspending a small basket from a spring balance using a thin copper wire into an aquarium filled with distilled water. Coral samples kept immersed in water from collection and throughout the experiment were weighed in air (wet) and then in distilled water and later dried in an oven to constant weight. Bulk density was measured by determination of total enclosed volume by weighing samples while preventing the filling of skeletal voids with the weighing medium (Bucher et al., 1998). After measurement of the dry weight, a water-proof coating was applied by quickly dipping samples in molten paraffin wax (at 105 to 110°C), waxed coral pieces were then weighed in air and then in water and the bulk density calculated as
wt in air ÷ [(waxed wt in air - waxed wt in water) × water density]
Coral calcification rates (g CaCO3 cm-2 yr-1) were then calculated as the product of bulky density (g cm-3) and extension rate (mm yr-1) for each individual coral sample and averaged for each coral species.
Corallite characteristics
Corallite (diameter and density) characteristics were studies as described by Sabater and Yap (2004). Briefly, a stereo microscope equipped with a micrometer eyepiece and a net micrometer was used.
Corallites were selected from three randomly chosen parts of a 5 g coral piece (with branch tips being avoided), counted and their diameters measured. Measurements of corallite diameter were estimated by measuring the distance between the two opposite walls of each corallite. Corallites were counted within an area bounded by the net micrometer of the microscope eyepiece for estimation of the corallite density, the magnification employed depending on corallite sizes. Corallite density (number of corallites per unit area, #/unit area) was then computed from the value of corallite counts and its corresponding area.
Data analysis
The study was intended to investigate seasonality in coral growth, however loss of samples during the rough SEM season prevented this, therefore, all analyses were conductor on pooled data. Averages for environment data, growth parameters and corallite characteristics were compared between reefs using one-way ANOVA and post-hoc multiple comparison tests undertaken using STATISTICA version 6.0. Transformation of data was performed where necessary after tests for homogeneity and homoscedasticity. Tukey’s HSD test was used to find which means were different after detecting significant differences. Relationships between the three growth parameters (extension rates, density and calcification) were then investigated for all the data from the four reefs together as well as for each individual reef.
Environmental factors
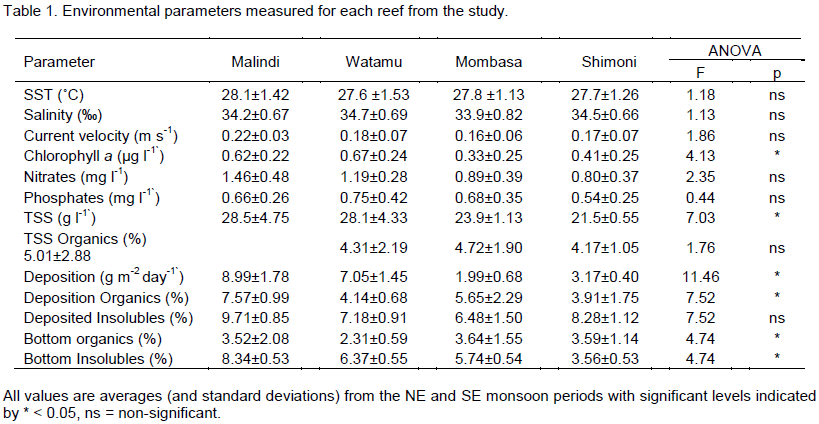
No significant differences in temperature, salinity and current velocity were detected between study reefs though current velocity was found to be high in Malindi compared to the other sites (Table 1). Chlorophyll a concentration was found to be significantly higher in Malindi and Watamu compared to Mombasa and Shimoni, however, phosphates and nitrates concentration did not differ between reefs. Significant high total suspended sediment (TSS) and sedimentation (deposition) rates were measured in Watamu and Malindi compared to Shimoni and Mombasa. No significant differences between reefs were detected for TSS organic content, however, Watamu reef exhibited low bottom sediment organic content compared to the rest of the study reefs. The percent organic content of trapped sediments (sediment deposition) in Malindi was found to be significantly higher relative to all other reefs. Additionally, acid insoluble residue content for bottom and deposited sediments was found to be high in Malindi compared to all other reefs (MLD>WTM=MSA>SHM).
Linear extension rates
Only one species, Pocillopora damicornis was observed to exhibit significant higher extension rates in the Malindi sediment impacted reef compared to all other reefs (Table 2). On the other hand, Acropora robusta, Porites rus, Porites cylindrica, Pocillopora eydouxi, Galaxea fascicularis and Echinopora gemmacea, all showed higher extension rates in Shimoni reference reef compared to Malindi sediment-impacted reefs. The following species exhibited no differences in extension rates between reefs, Porites lutea, Favia sp, Favites sp and Alveopora fenestrata. Extension rates for Acropora humilis, A. fenestrata and P. eydouxi were found to be higher in Mombasa compared to all other reefs. No differences in extension rates were detected between the reference and sediment impacted reefs for Acropora humilis, Acropora sp, Montipora digitata and Pavona decusatta.
Bulk density and calcification rates
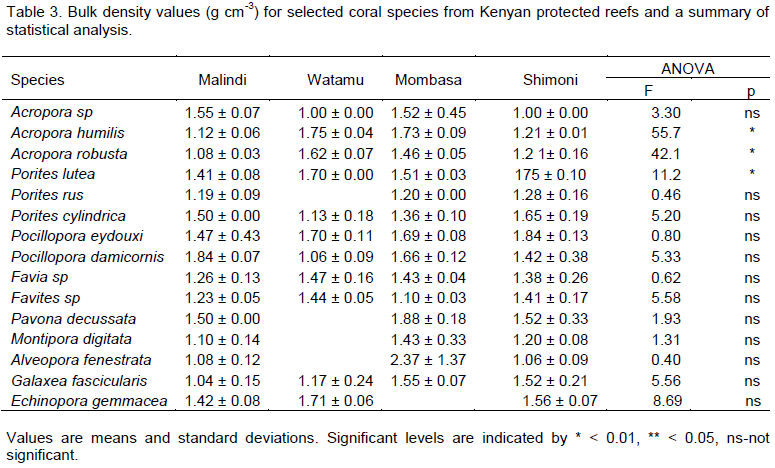
Bulk density data revealed low values for all species in Malindi (except Acropora sp, P. cylindrica, P. damicornis
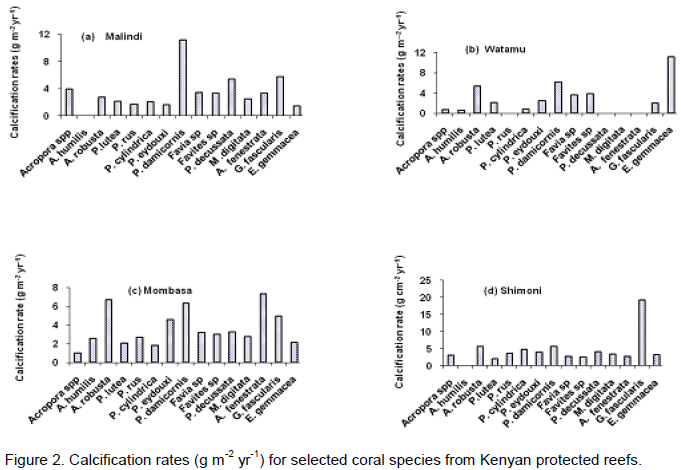
and A. fenestrata) compared to Shimoni but only the difference for A. humilis, A. robusta and P lutea, were significant (Table 3). Acropora sp, A. fenestrata and P. damicornis showed non-significant higher bulk densities in Malindi compared to the Shimoni reference reef. Bulk density for A. humilis, A. robusta, P. lutea, Favia sp, Favites sp and E. gemmacea were found to be higher in Watamu compared to other reefs. Generally, high bulk densities were measured for corals from Mombasa reef relative to other reefs including the Shimoni reference reef. Coral calcification rates were found to be around 3.5 gm-2yr-1 (Figure 2). Acropora sp, P. damicornis, Favia sp and Favites sp, P. decusatta, M. digitata and A. fenestrata had higher calcification rates in sediment exposed Malindi reef than in the Shimoni reference reef. By contrast, A. robusta, E. gemmacea, P eydouxi, P. rus, P. cylindrica and G. fascicularis showed higher calcification rates in Shimoni compared in Malindi. A. humilis and P. lutea showed no differences in calcification rates between reefs in the present study. On average calcification rates were found to be higher in Mombasa and lower in Watamu compared to the rest of the reefs.
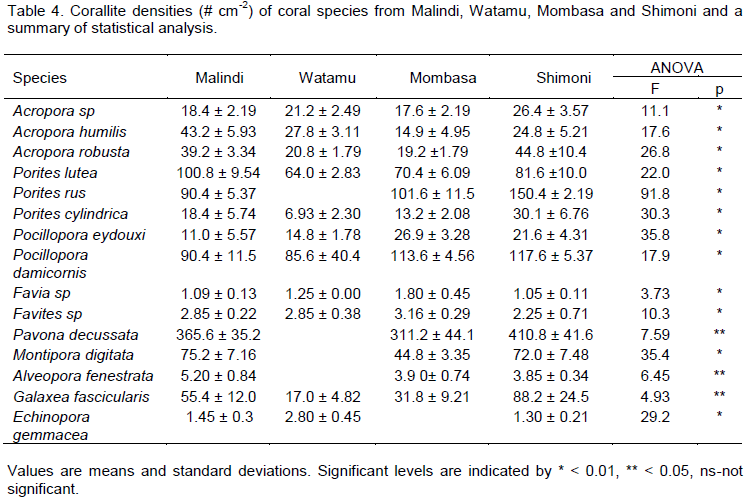
Corallite characteristics
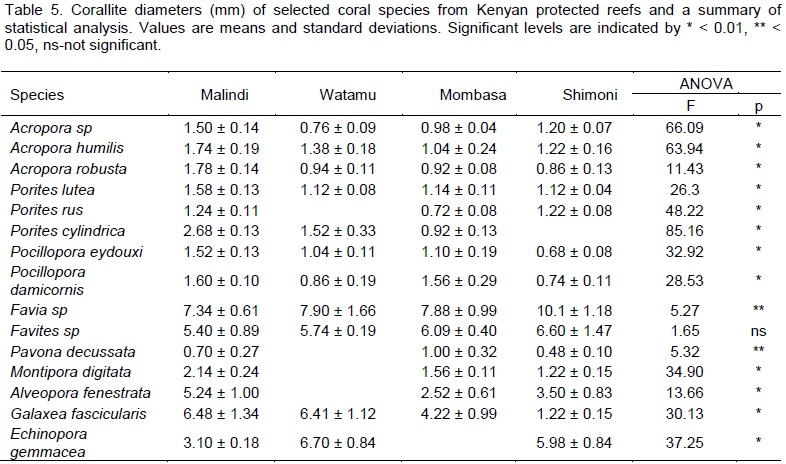
All species showed significant differences in corallite density between reefs but only A. humilis, P. lutea and A. fenestrata had significantly higher corallite density (numbers of corallites per unit area) in Malindi relative to all other reefs (Table 4) whereas Acropora sp, P. rus, P. cylindrica, P. eydouxi and P. damicornis, exhibited significant higher corallite density in Shimoni compared to the rest of the study reefs. High corallite density was measured for P. eydouxi, Favia sp and Favites sp in Mombasa compared to all other study reefs. Low corallite densities were generally found in Watamu for all species relative to all other reefs except Acropora sp, A. humilis, A. robusta and E. gemmacea. Corallite diameter studies revealed larger corallites for Acropora sp, A. humilis, A. robusta, P. lutea, P. eydouxi, P. damicornis, M. digitata, A. fenestrata in Malindi compared to all other study reefs (Table 5) and were generally low in Mombasa reefs. Favia sp and Favites sp had significantly larger corallite diameters in Shimoni relative to all reefs while P. decussata had larger corallites in Mombasa compared to all other reefs.
Correlation between growth parameters
Relationships between the growth parameters were investigated with simple regression analysis for all data from the four reefs combined (Figure 3) as well as for each individual reef (Figure 4). For the combined data the relationships were variable being strong between calcification and extension rates, but bulk density was again found to be poorly correlated with the other two coral growth variables. For individual reefs, bulk density was weakly correlated to the other two growth parameters, however, calcification rate and linear extension were strongly correlated. This strong relationship between calcification rate and linear extension in all the studied reefs suggests that variations in calcification rates are more closely related to extension rate variations rather than with variations in bulk density.
The influence of the Sabaki River on Malindi reef system’s water quality was evident from the high sediment, chlorophyll a and nutrient levels measured in the present study results. Sedimentation rates observed in Malindi (9.0 g m-2 day-1) were lower than those measured using tiles (Obura, 1995), comparable to those from tubular traps (McClanahan and Obura, 1997) in the same site and close to values suggested by Rodgers (1990) to be detrimental to reef-building corals (10 g/m2/day). However, observed suspended sediment (TSS) concentrations were higher than those previously measured by other workers (Brakel, 1984; van Katwijk et al., 1993; McClanahan and Obura, 1997) suggesting possible sediment increase with time. This increase may be attributed to changes along the Sabaki catchment areas due to increased population and agricultural activities, inappropriate farming methods and poor or failed soil conservation measures (Dunne, 1979; Bartley et al., 2013; Prouty et al., 2014). Changes in land use practices and population growth have previously been implicated in increasing the transport of sediments into the marine environment (Bartley et al., 2013; Prouty et al., 2014) with subsequent detrimental effects on coral reefs. In an earlier study, van Katwijk et al. (1993) reported presence of R. Sabaki sediments in Watamu reefs; however this was not in agreement with satellite and field study results (Brakel, 1984; McClanahan and Obura, 1997) we therefore suggest that high sedimentation rates observed in Watamu are likely the result of bottom sediment re-suspension especially during rough SEM conditions. As a nutrients proxy, elevated levels of chlorophyll a concentration measured in Malindi and Watamu relative to Mombasa and Shimoni may be due to (1) Sabaki River discharge enhancing phytoplankton productivity, (2) disturbed sediments releasing nutrients into the water column (3) upwelling off southern Somali and northern Kenya coast and (4) seapage of nutrient-rich ground water and runoff from Mida mangrove creek for the case of Watamu (McClanahan, 1988; Ohowa, 1996). High chlorophyll a (=increased phytoplankton) interferes with light penetration and exacerbates the negative effects of elevated sediment concentrations and this should be of concern to Watamu and Malindi reefs in light of predicted impacts of global climate change events.
High sediment organic and acid insoluble fractions from Malindi confirm terrestrial influence. Although, stress levels on corals have been linked directly to the organic content of sediments (Bartley et al., 2013), nevertheless, we observed a thriving coral community in Malindi possibly due to East Africa’s high tidal range (~4 m), site hydrodynamics, likely presence of sediment acclimatized/tolerant coral species and tolerable organic fraction levels. The above first two factors enhance flushing rates and therefore minimize accumulation and detrimental effects of sediments on reef communities. However, continued increases in land-based pollutants posse future concerns to the ecology of reefs and are of great significance for the Malindi reef areas in terms of park revenue and local community earnings since the highest turbid conditions occur during the NEM period (peak tourist and fishing season). There is therefore need to reverse or mitigated these conditions through robust soil conservation measures and land use practices upstream of the Sabaki as well as understanding the response of corals to sedimentation.
Combined reef growth data from all species revealed three contrasting groups: those with linear extension and calcification rates (1) positively (A. fenestrata, Favites sp, Favia sp, P. lutea, P. damicornis and P. decusatta) or (2) negatively (Acropora sp, A. humilis, Acropora robusta, P. cylindrica, P. eydouxi and G. fascicularis) affected by sediment concentrations and (3) those with growth parameters un-affected by sediment levels. The variable response of corals to sediments levels observed in the current study is similar to that found in previous studies (Bucher and Harrison, 2000; Carricart-Ganivet et al., 2000; Cruz-Pinon et al., 2003; Crabbe and Smith, 2005; Golbuu et al., 2011) and confirms the species-specificity of coral response to sediment exposure and other disturbances. This has been linked to individual coral species life history strategies of adaptation/tolerance to increased sedimentation and turbidity levels (Obura, 1995; Edinger et al., 2000; Fabricius, 2005) including physiological (mucus production, trophic level, P:R ratio), mechanical (cilliary action) as well as morphological modifications (corallite size and density, geometry). For example, Wooldridge (2014) concluded that in sediment-laden waters, reduced light penetration lowers photo-synthesis and disrupts autotrophy forcing species with thick tissue layers such as Porites to become heterotrophic as well as utilize energy reserves. Further, Padilla-Gamino et al. (2012) showed that some corals such as Porites rus change whole-colony morphologies from a plate-like to branching forms depending on the light conditions. This morphological and physiological plasticity is important in enabling a number of coral species to diversify strategies for energy acquisition and sediment rejection in order to facilitate persistence in turbid environments.
At a first glance the pattern of increased extension rate in the sediment-exposed reefs seemed anomalous since high sediment (and nutrient) levels have been associated with lowered extension rates. Such increases may be attributed to elevated zooxanthellae activity and increased heterotrophy that enhance coral metabolism and growth (Edinger et al., 2000; Fabricius, 2005). For example, P. damicornis has been reported to feed readily on sediments and therefore able to compensate for the low photosynthesis-respiration ratio at elevated turbidity levels (Anthony, 2000) explaining the high prevalence and enhanced growth rates of this species in the sediment impacted Malindi site in the present study. However, continued increase in sediments or nutrients influx has been implicated in diminishing extension owing to enhanced levels of phytoplankton and sediments interfering with light penetration (Edinger et al., 2000) and therefore lowering photosynthesis and calcification rates. Enhanced coral extension rates in stressful environments has also been associated with the utilization of stored (tissue) energy reserves (lipids, carbohydrates), however this capacity is not equally effectual in all coral species. The observed response pattern (increased extension rate) has been suggested to be a metabolic response pattern of corals with compromised health status and reduced probability to persist in the future as a reef-building and reproductive entities (Hughes and Grottoli, 2013; Woodridge, 2014). Such coral species would therefore be highly vulnerable to synergistic effects of local and global climate stressors.
Our results also show diminished linear extension rates in Watamu relative to the other mangrove fringed reef (Mombasa) and in Malindi compared to all other reefs. Observed reduction in coral growth parameters are similar to earlier findings (Rodgers, 1990; Edinger et al., 2000; Crabbe and Smith, 2005) and can be associated with reduced light penetration (due to increased sediment and phytoplankton concentration) and metabolic stress associated with energy expenditure in sediment rejection (ciliary action, polyp distension and mucus production), indicating low tolerance to increased sediments levels. We suggest that differences in extension rates observed between the two mangrove-fringed reefs likely resulted from different levels of nutrients in Mombasa relative to Watamu due to (1) volume of water reaching respective reef from creeks, (2) the distance of Mombasa reefs from the Tudor and Mtwapa creeks or (3) diluting effect due to mixing of lagoonal and oceanic waters through the depression in the Mombasa reef (Ohowa, 1996; Mwashote et al., 2005). The observed of lack of a coral extension rate response to elevated sediment level in some species (P. lutea, Favia sp. Favites sp and A. fenestrata) is unclear but may either be due to (1) counteracting effects of nutrients (enhanced extension rates) and sediments (reduced extension) canceling out each other, (2) individual coral species adaptations and tolerance to sediment levels, (3) lack of genetic capability to utilize particulate organic matter or (4) presence of low energy reserves available for growth (Veron, 2000; Padilla-Gamino et al., 2012). Moreover, Anthony (2006) suggested that no-growth response to high sediment levels show that adverse impacts of sediments operate at the community or population level rather than at the individual level. The present study results thus confirm earlier reports of variable coral growth responses in polluted environments (Anthony and Fabricius, 2000; Fabricius, 2005) caused by changes in coral trophic status, morphological adaptations (Padilla-Gamino et al., 2012) and sediment tolerance, with some species becoming mixotrophic in high turbidity conditions while others remain phototrophic gaining only a small proportion of energy from particulate feeding.
Further, in the present study corals growing in the turbidreef showed larger diameters and calcification rates similar to the other findings (Todd et al., 2001; Todd, 2008). Increase in corallite size (diameter) has been associated with the efficient removal/rejection of sediment, though larger corallites have also been implicated in causing low reproductive output (Lauzinger et al., 2003). Large corallites also mean low corallite and skeleton density but such re-modeling interferes with the coal’s ability to feed, compete for space and resist hydro-dynamic forces, therefore such modifications may not be ecologically ‘cost’ effective. Diminished corallite diameters measured in the mangrove-fringed reefs suggest that nutrients may have a negative effect on corallite size or that the reduction in corallite size is a strategy to survive in unfavourable conditions. Todd et al. (2001) contend that coral genotypes with phenotypic advantages enable corals to survive in environmental conditions normally perceived as unfavourable. Our results are preliminary and further studies and experi-ments are therefore needed to confirm and further understand how coral species respond to sediment and turbidity, especially with the use of reciprocal transplants experiments (RTEs).
Bulk density values in the current study ranged between 1.00 and 1.88 g cm-3. Normally, coral density is restrained between an upper limit of 2.94 g cm-3 (pure aragonite) and a lower threshold value necessary to offer corals structural resistance against mechanical and hydrodynamic damage. Consistent low skeletal bulk density values were generally observed in the sediment exposed consisted with previous results (Carricart-Ganivet and Merino, 2001; Cooper et al., 2008). The observed low bulk density in polluted waters may be linked to either one or a combination of the following: (1)Heterotrophy and nutrient uptake increasing extension rates with the same or lower calcification rates (Carricart-Ganivet and Merino, 2001), (2) The uptake of terrestrial organic matter increasing the growth of soft tissue (Fabricius, 2005) with concomitant increase in porosity to the detriment of skeletal density or (3) Trapped particles within coral skeleton acting as crystallization inhibitors in the calcium deposition process (Belda, 1993; Atkinson et al., 1995).
Although incorporated sediments were not measured in the present study, evidence of sediment incorporation in corals from the Malindi area has previously been reported (Fleitmann et al., 2007). Surprisingly, bulk density values in the mangrove-fringed reefs were generally found to be higher than the offshore reef despite low density and high extension rates being associated with increased levels of nutrients in inshore waters (Belda et al., 1993; Atkinson et al., 1995). Here, we suggest that temperature differences between reef could be the likely cause of this observation,
similar to the observations of Carricart-Ganivet (2004).
Calcification is an energy dependent process and maximum calcification occurs when conditions are optimum for energy uptake (Carricart-Ganivet, 2007; Allemand et al., 2011). However, in stressful environments energy allocation for calcification is considerably reduced (Allemand et al., 2011; Cabra-Tera et al., 2013; Wooldridge 2014) by diversion to sediment rejection and removal mechanisms leading to diminished coral calcification rates. Average coral calcification rates measured in the present study were comparable to those from previous studies (Carricart-Ganivet and Merino, 2000; De’ath, 2009; Carricart-Ganivet, 2011). Diminished calcification rates in turbid reefs result from increased energy expenditure required in sediment removal and rejection mechanisms through cilliary action and mucus production, with subsequent reduction in energy allocation to the calcification process (Kumara et al., 2015). However, high calcification rates were measured for Acropora sp, P. damicornis, P. decussata, G. fascicularis and A. fenestrata in the sediment impacted reef implying sediment tolerance and heterotrophic capabilities in these species. Corals have been shown to employ different coral growth strategies in disturbed environments with some investing increased calcification in linear extension while others use increased calcification to construct denser skeletons (Cruz-Pi?ón et al., 2003; Carricart-Ganivet, 2007).
The present study results also show that high extension rates do not necessarily translate into high calcification rates contrary to the findings of Carricart-Ganivet and Merino (2001). For example, in Watamu, the fastest growing coral was P. darmicornis (58.1 mm yr-1) with a calcification rate of 6.2 g cm-2 yr-1, a value lower than for E. gemmacea (11.2 g cm-2 yr-1) with extension rates of 54.7 mm yr-1. Also, in Shimoni P. eydouxi had extension rates of 21.6 mm yr-1 and a calcification rate of ~4.0 g cm-2 yr-1 compared to growth rates of 26.1 mm yr-1 and calcification rates of 2.8 g cm-2 yr-1 for A. fenestrata. Individual species differences in density coupled with the fact that calcification rate is a function of linear extension rate and density may be the likely cause of these observed findings. Further, species-specific abilities to utilize energy reserves and particulate matter as an energy source may also contribute to these variable and contrasting coral species responses. Low calcification rates measured in turbid waters have also been attributed to the cost of reproduction (egg and larval development) especially in brooding corals (Cabra-Tena, 2013), mode of sediment removal (Larsson et al., 2013) and reduced light capture (for photosynthetic rates) due to diminished photosynthetic rates.
Coral growth, reef health and reef management implications
The present study documents variable coral species growth responses (increased and decreased growth characteristics) to the influence of sediments and turbidity. Coral growth strategy of enhanced or diminished extension rates in sediment impacted reefs has important implications and applications in coral reef health and management by providing information on the performance of corals in polluted environments (Cabra-Tena et al., 2013). Such information may be useful in the formulation of coral reef management and conservation strategies. Rapid extension rates have been linked to the instability of the coral-algae relationship and therefore a proxy for bleaching sensitivity as well as an indicator of the vulnerability to bleaching, reproductive capacity and likelihood of disease attack during stress (Wooldridge, 2014). This is of major importance in inshore polluted reefs considering future predicted increases in global environmental factors. Local stress factors will likely interact with ocean acidification and/or SST potentially shifting the calcification-erosion balance leading to coral reef degradation, habitat loss and decline in reef structure, ecosystem function and services (Ruiz-Moreno et al., 2012; Wisshak et al., 2013). This will have severe consequences on the reef ecology and livelihood of coastal communities. Moreover, the lack of expected coral extension rates response observed in the current study (Rodgers, 1990; Edinger et al., 2000) does not necessarily point towards absence of impact since extension rates may only be evident long after other signs of environmental stress and serious reef degradation have already occurred. Further, since low density skeletons and high bioerosion rates have been associated with inshore terrigenous influenced reefs (Tribollet et al., 2002; Tribollet and Golubic, 2005), with individual corals employing different growth strategies in response to water quality changes (Carricart-Ganivet, 2007; Golbuu et al., 2011; Nakajima et al., 2013) any options for the management of reefs experiencing local disturbances must take these aforementioned facts into consideration.
The strong and significant impacts of sediments loading on coral reefs thus require appropriate mitigation and regulation measures in watershed areas as part of the strategies for the management and protection of reef ecosystems. This way the potential compounding effects of anthropogenic and climate change stressors (Maina et al., 2011) are likely to be minimized or alleviated. For example, increased nutrients and sediments have been linked to coral disease outbreaks (Pollock et al., 2014) and may negatively impact recovery and resilience from climate change impacts (Ateweberhan et al., 2013) and on the other hand, anthropogenic stressors may increase the severity of climate change impacts (Wiedenmann et al., 2012). Atewerbahan et al. (2013) reported that recovery from bleaching is severely retarded by local environmental parameters and that reef organisms having calcification already compromised by local environmental conditions cease to grow or dissolve altogether as a result of increased pH. Thus the variable responses of corals to disturbances are key ingredients in the management of coral reefs by helping to understand how important coral characteristics are likely to change in response to current and future environmental stresses and how management interventions might assist in safeguarding coral reefs against long term negative changes. Given the mounting evidence of increased frequency and severity of climate change in future and the increased vulnerability of coral reefs to increases in chronic local stressors (Maina et al., 2011; Ateweberhan et al., 2013;), management and alleviation of local stressors, particularly sedimentation will play a critical role in enhancing reef resilience and acclimation under future climate change scenarios (Ateweberhan et al., 2013). Further, considering the economic and ecological importance of coral reefs, studies on water quality parameters coupled with experiments on interactive effects of climate related stressors and local environmental factors will be required in order to shed more light on response of corals to environmental change and for sustainable and scientifically inspired coral reef management options.