Full Length Research Paper
ABSTRACT
A field experiment was conducted on twelve sorghum genotypes against one local and two standard checks at Haro Sabu Agricultural Research Center (HSARC) sub sites for three consecutive years (2016-2018) using randomized completely block design (RCBD) to evaluate and select high yielding sorghum genotypes and to assess the impact of genotype by environmental interaction on grain yield and yield components across diverse growing environments of western Oromia. Eight agronomic traits and three economically important disease reaction were collected depending on the crop descriptor. Pooled over locations analysis of variance detected significance difference among tested genotypes for all collected traits. Genotype by environment interactions (G×E) significantly affected all recorded traits excluding days to heading and thousand seed weight. Genotype and genotype by environment interaction (GGE) bi-plot analysis revealed that G3, G11 and G12 as ideal genotypes in terms of yielding ability and stability and were promoted as candidates for possible release and use as genetic resource in future breeding programs.
Key words: Sorghum bicolor L., Genotype by environment interactions (G×E), Genotype and genotype by environment interaction (GGE) bi-plot, stability.
INTRODUCTION
Among grain crops cereals are the major food crops in Ethiopia, both in terms of the land coverage and volume of production (CSA, 2016). Sorghum is the most widely grown food crop in Ethiopia. It thrives in a range of agro-climatic zones including high and low altitudes. High altitude sorghums grow satisfactorily at altitudes as high as 2,300 m where mean temperatures range from a minimum of 14 to 26°C. Sorghum (Sorghum bicolor L.) is an important drought tolerant rain fed cereal largely cultivated for food, feed and fodder by subsistence farmers in Ethiopia (Ayana et al., 2000). The national average production of sorghum is 2.5 tonha-1 (CSA, 2016).Of the total national sorghum production (432,3299.8 ton), Oromia region shares production (1,884,630.1 ton) of sorghum which is almost about half of the total annual production of the country (CSA, 2016). In sub Saharan Africa and south Asia sorghum is consumed as staple food and is also used in the production of a variety of by-products like alcohol, edible oil, and sugar (Wang et al., 2008). It is used as food, feed, beverage, and its stalk was used for construction of fences in Western Ethiopia and surrounding vicinities. It has a dense and deep root, has ability to reduce transpiration through leaf rolling and stomatal closure among others which in turn makes the crop to survive dry spelling periods. Hence sorghum has become a strategic crop in the face of climate unpredictability across different sorghum growing environments. In spite of all these advantages, sorghum has been a neglected crop, both at national as well as global level and the sorghum crop production is still very low (Stemler et al, 1977).
Among figurative problems, a biotic and biotic factor, the effect of genotype by environment interaction and stability of released varieties across the growing environments are the major one (Tesso et al., 2004; Girma et al., 2010). It is recalled that genotype and genotype by environment interaction (GGE) bi-plot is the most recent approach for analysis of G×E and ever more being used in G×E studies in plant breeding research (Yang et al., 2007). The GGE bi-plot model was used extensively in quantitative genetics and plant breeding (Yang et al., 2007). Additionally, the additive main effects and multiplicative interactions (AMMI) model are also defined as powerful tools for effective analysis and interpretation of multi environment data structure in breeding programs. In most cases plant breeders faces instability of yield when genotypes were grown in different environments due to G×E. Therefore, multi -environment trials (MET) are required to identify specific and the general adaptability pattern of genotypes. The aim of the present study was, therefore, to examine the stability and yielding performance of sorghum genotypes s and to identify stable and high yielding cultivar for wider cultivation.
MATERIALS AND METHODS
Twelve sorghum genotypes were tested against one local and two standard checks (Table 1) were evaluated for three (2016-2018) cropping seasons at Haro Sabu agricultural research center on station, Hawa-Galan Farmers Training Center (FTC), Kombo FTC, and Guliso FTC of Western Oromia, Ethiopia (Table 2). The trial was planted in randomized completed block design (RCBD) with three replications. Each plot consists of six rows (with four harvestable rows) having 3 m plot length with inter-row and intra-row spacing of 0.75 and 0.15 m, respectively and 2 m spacing was used between each block. A seed rate of 25 kgha-1 and recommended fertilizer was applied. All other agronomic practices were performed as per the recommendation for the crop.
Data collection method
Five plants were selected randomly before heading from each row (four harvestable rows) and tagged with thread and all the necessary plant based data were collected from these sampled plants.
Plant-based: Plant height, head height and head weight.
Plot based: Days to heading, days to physiological maturity, lodging percentage, thousand seed weight, grain yield and three economically important insect pest and disease reaction like stalk borer (Chilo Partellus), anthracnose (Colletotrichum sublineolum) and leaf blight (Exserhilum turcicum) were scored.
Statistical analysis
AMMI method as described in Zobel et al. (1988) was used to analyze adaptability and phenotypic stability using the following statistical model:
Where, Yij is the yield of the ith genotype in the jth environment; 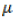 is the grand mean; gi and ej are the genotype and environment deviations from the grand mean, respectively; λk is the eigen value of the PCA analysis axis k; αik and γij are the genotype and environment principal component scores for axis k; n is the number of principal components retained in the model and εij is the error term.
is the grand mean; gi and ej are the genotype and environment deviations from the grand mean, respectively; λk is the eigen value of the PCA analysis axis k; αik and γij are the genotype and environment principal component scores for axis k; n is the number of principal components retained in the model and εij is the error term.
AMMI stability value of the ith genotype (ASV) was calculated for each genotype according to the relative contribution of IPCA1 to IPCA2 to the interaction SS as follows (Purchase et al., 2000):
Where, SSIPCA1/SSIPCA2 is the weight given to the IPCA1 value by dividing the IPCA1 sum of squares by the IPCA2 sum of squares. Based on the rank of mean grain yield of genotypes (RYi) across environments and rank of AMMI stability value (RASVi) a selection index called Genotype Selection Index (GSI) was calculated for each genotype, which incorporates both mean grain yield (RYi) and stability index in single criteria (GSIi) as suggested by Farshadfar (2008):
GSIi = RASVi + RYi
The analysis of GGE Bi-plot method was carried out for grain yield, according to the following model (Yan and Tinker, 2006).
Ÿij = λ1 γi1 δj1+ λ2 γi2 δj2+ ρij
where λ1and λ2 are singular values of the first and second Principal Components (PC) associated with the matrix of the effects of genotypes added to effects of genotype × environment interactions; γi1 and γi2 are eigenvectors of the first and second PC associated with the effect of the genotype i; δj1 and δj2 are eigenvectors of the first and second PC associated with the effect of the environment j; ρij is the residual of the model associated with the genotype i in the environment j.
Bi-plots of the scores associated with two first PC were generated to better understanding the interrelationship among genotypes and/or environments, as proposed by Yan and Tinker (2006). Analysis of variance was carried using statistical analysis system (SAS) version 9.2 software (SAS Inst., 2008). AMMI analysis and GGE bi-plot analysis were performed using GenStat 15th edition statistical package (GenStat, 2012).
RESULTS AND DISCUSSION
Combined analysis of variance
All agronomic and yield component parameters were affected by environmental effect whereas all except grain yield were affected by cropping seasons. Days to maturity and head weight of genotypes were not affected across a cropping seasons and it agrees with the findings of Worede et al. (2020). Except days to heading and thousand seed weight all agronomic and yield components among tested genotypes were statistically affected across an environmental set. Statistically significant (P < 0.01) difference were documented for grain yield among genotypes, environments and G×E and it’s in concordance with the finding of Filho et al. (2014) and Worede et al. (2020)(Table 3).
Yield performance of sorghum genotypes across environments
The mean performance of the tested sorghum genotypes for grain yield showed fluctuation over growing seasons and environments (Table 4). It’s also noted that some genotypes consistently performed in a set of tested environments whereas some of them are irregular across locations. The highest grain yield was recorded from G11 (5.56-ton ha-1) genotype at Haro Sabu (2018) whereas the lowest was from G8 (1.27-ton ha-1) at Haro Sabu (2016). The combined over locations showed G12 as the highest yielder. In contrary, the local check included in this study was the low yielder among all tested genotypes that might be stem due to the genetic potential of the genotype (Mengistu et al., 2013). The disparity in yield rank of genotypes across the growing environments displays the prevalence of G×E interactions (Purchase et al., 2000; Yang et al., 2007).
Agronomic performance
Delayed days to heading and days to physiological maturity were recorded from genotypes G11 (132.5) and G12 (131.04) whereas G5 (169.17) and G6 (169.17) were early to days to heading and days to physiological maturity suggesting a great flexibility for developing improved varieties suitable for various agro-ecologies with variable length of growing period. G1, G4, G8, G9 and G10 were high in terms of plant height indicating that these genotypes might be susceptible to root and/or stem lodging (Mengistu et al., 2019). Contrariwise, G3, G11 and G12 genotypes were medium in terms of plant height indicating that the possibility to develop resistant variety against lodging problems. Moreover, G3, G11and G12 were recorded the highest grain yield and they had 23.33, 21.72 and 24.3% yield advantage over the best standard check G2 (Table 5). Those genotypes that had better grain yield among tested genotypes had correspondingly low scores to economically important insect pest and disease reactions. Maximum anthracnose disease reaction score was recorded from G2 and G6. Likewise, maximum leaf blight disease reaction was recorded from G2 and G10. Conversely, G3, G11 and G12 genotypes were better tolerant to stalk borer, anthracnose and leaf blight (Table 6).
Additive main effects and multiple interaction (AMMI) model
AMMI analysis of variance (ANOVA) with the appropriate AMMI model was indicated in Table 7. The analysis of variance (ANOVA) indicated highly significant differences (P ≤ 0.01) for environments, genotypes and importantly G×E.
The genotype, environment and genotype by environment interaction explained 23.1, 69.5 and 7% of the total variation indicating the prevalence of considerable environmental variation. The interactive principal component axis (IPCA-1) and IPCA-2 axis of the G×E were highly significant (P ≤ 0.01). The first principal component managed over 68.6% of the G×E sum squares while the second principal component revealed 18.6% of the interaction, and the remaining 12.8% is due to residual (noise) and it is difficult to interpret and thus need to be discarded. Considerable percentage of G×E was explained by the first two IPCA axes. Different authors suggest the importance of apprehending most of the G×E sum squares in the first axis, to attain accurate information (Purchase et al., 2000; Kaya et al., 2002).
AMMI stability value (ASV) and genotype selection index (GSI)
G3, G11 and G12 were the highest yielder genotypes with relatively moderate ASV (Table 8). G9 and G5 showed the lowest ASV accompanied with the lowest grain yield. However, stability alone cannot be considered in production agriculture and hence identifying genotypes with high grain yield coupled with consistent stability across growing environments has paramount importance (Farshadfar, 2008). In this regard, GSI was utilized to further identify stable genotypes with better yield performance. Accordingly, G3, G11, G12, and G5 were considered as most stable genotypeswhereas; G7 was the least stable genotypes.
Genotype and genotype by environment interaction (GGE) biplot analysis
The polygon is drawn by joining the cultivars (G3, G4, G8, G10, G7 and G12) that are located farthest from the biplot origin so that all other cultivars are contained in the polygon. These vertex cultivars are the highest-yielding cultivar in all environments that share the sector with it. Vertex cultivars in which any environments fell in their sectors were the poor performing genotypes. Genotypes such as G1, G5 and G6 located at the origin would rank the same in all environments and is not responsive to the change in environments. G3, G11 and G12 genotypes were the best yielder among tested genotypes and relatively stable genotypes across various environments (Figure 1). G1, G5, G8 and G9 genotypes were inferior in yield performance and stable genotypes and G7 was the most unstable genotypes.
Genotype-focused scaling considers stability and mean grain yield concurrently and environments as well as genotypes that fall in the central (concentric) circle of genotype-focused scaling are considered as an ideal environments and stable genotypes, respectively (Gauch and Zobel, 1997). Genotype G3, G11 and G12 fell in and around the center of concentric circle and therefore, ideal genotypes (Figure 2). Contrariwise, G8, G9 and G10 are located far from ideal genotypes and thus they are undesirable genotypes.
CONCLUSION AND RECOMMENDATIONS
The genotypes were significantly influenced by environment, genotype and their interaction. GGE biplot and GSI index incorporating with the ASV and the yield capacity of the different genotypes in a single non-parametric index were found to be useful for discriminating genotypes with superior and stable grain yield. Depending on yield performance and reasonable stability G3, G11 and G12 genotypes were the best in the test environments and they can be used as candidates for possible release and for use as parents in future breeding programmes.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGMENTS
The authors greatly appreciate Oromia Agricultural Research Institute for financial support. Haro Sabu Agricultural Research Center staff members are warmly appreciated for technical and administrative support.
REFERENCES
|
Ayana A, Bekele E, Bryngelsson T (2000). Genetic variation in wild sorghum (Sorghum bicolor ssp. verticilliflorum (L.) Moench) germplasm from Ethiopia assessed by random amplified polymorphic DNA (RAPD). Hereditas 132(3):249-254. |
|
|
Central Statistical Agency (CSA) (2016). Area and production of crops in the Meher season. Statistical Bulletin.Central Statistical Agency (CSA), Addis Ababa, Ethiopia. |
|
|
Farshadfar E (2008). Incorporation of AMMI Stability Value and Grain Yield in a Single Non-Parametric Index (Genotype Selection Index) in Bread Wheat. Pakistan Journal of Biological Sciences 11:1791-1796. |
|
|
Filho JA, Tardin, FD, Daher RF, Barbé TC, Paula CM, Cardoso MJ, Godinho VPC (2014). Stability and adaptability of grain sorghum hybrids in the off-season. Genetics and Molecular Research 13(3):7626-7635. |
|
|
Gauch H, Zobel RW (1997). Identifying mega-environments and targeting genotypes. Crop Science 37(2):311-326. |
|
|
GenStat (2012). GenStat Software, Version 15th Edition. GenStat.co.uk. |
|
|
Girma M, Amsalu A, Ketema B (2010). Combining ability for yield and its components in Ethiopian sorghum (Sorghum bicolor (L.) Moench) landraces. East African Journal of Sciences 4(1):34-40. |
|
|
Kaya Y, Palta C, Taner S (2002). Additive main effects and multiplicative interactions analysis of yield performances in bread wheat genotypes across environments. Turkish Journal of Agriculture and Forestry 26(5):275-279. |
|
|
Mengistu G, Lule D, Desalegn K, Daba C, Feyisa H, Gerema G, Mamo K (2013). Registration of "Chemeda and Gemedi" Sorghum Varieties. East African Journal of Sciences 7(1):61-62. |
|
|
Mengistu G, Shimelis H, Laing M, Lule D (2019). Assessment of farmers' perceptions of production constraints, and their trait preferences of sorghum in western Ethiopia: Implications for anthracnose resistance breeding. Acta Agriculturae Scandinavica, Section B-Soil and Plant Science 69(3):241-249. |
|
|
Purchase JL, Hatting H, Deventer CS (2000). Genotype ×environment interaction of winter wheat (Triticum aestivum L.) in South Africa: II. Stability analysis of yield performance. South African Journal of Plant and Soil 17(3):101-107. |
|
|
SAS Institute Inc. (2008). Statistical analysis Software version 9.2, Cary, NC: SAS Institute Inc. USA. |
|
|
Stemler ABL, Harlan JR De Wet JMJ (1977). The sorghums of Ethiopia. Economic Botany 31(4):446. |
|
|
Tesso T, Claflin LE. Tuinstra MR (2004). Estimation of combining ability for resistance to Fusarium stalk rot in grain sorghum. Crop Science 44(4):1195-1199. |
|
|
Wang D, Bean S, McLaren J, Seib P, Madl R, Tuinstra M, Shi Y, Lenz M, Wu X, Zhao R (2008). Grain sorghum is a viable feedstock for ethanol production. Journal of Industrial Microbiology and Biotechnology 35(5):313-320. |
|
|
Worede F, Mamo M, Assefa S, Gebremariam T, Beze Y (2020). Yield stability and adaptability of lowland sorghum (Sorghum bicolor (L.) Moench) in moisture-deficit areas of Northeast Ethiopia. Cogent Food and Agriculture 6(1). |
|
|
Yang W, Kang MS, Ma B, Woods S, Cornelius PL (2007). GGE biplot vs AMMI analysis of genotype-by-environment data. Crop Science 47(2):643-653. |
|
|
Yan W, Tinker NA (2006). Biplot analysis of multi-environment trial data: Principles and applications. Canadian Journal of Plant Science 86(3):623-645. |
|
|
Zobel RW, Wright MJ, Gauch HG (1988). Statistical analysis of a yield trial. Agronomy Journal 80(3):388-393. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0